Back to Journals » International Medical Case Reports Journal » Volume 9
Treatment of pyoderma gangrenosum with thalidomide in a myelodysplastic syndrome case
Authors Malkan UY , Gunes G, Eliacik E, Haznedaroglu IC
Received 21 November 2015
Accepted for publication 12 January 2016
Published 16 March 2016 Volume 2016:9 Pages 61—64
DOI https://doi.org/10.2147/IMCRJ.S101000
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ronald Prineas
Umit Yavuz Malkan, Gursel Gunes, Eylem Eliacik, Ibrahim Celalettin Haznedaroglu
Department of Hematology, School of Medicine, Hacettepe University, Ankara, Turkey
Abstract: Thalidomide may be used as a treatment option for pyoderma gangrenosum (PG) and myelodysplastic syndrome (MDS). Herein, we aimed to report a patient who was treated well with thalidomide and whose diagnosis was PG with MDS. A 61-year-old man with painless ecchymotic lesions in his right upper extremity was admitted to the hospital in Isparta, Turkey, in January 2015. The lesions were diagnosed as PG. In his anamnesis, it was found that he was diagnosed with MDS 6 years ago and had been treated with cyclosporine at 2×100 mg for 5 years, which was stopped in January 2015. Aspiration from liver lesion revealed the presence of Mycobacterium tuberculosis, so antituberculosis treatment was started. Bone marrow investigation revealed MDS-refractory anemia with excess blasts (7%). For lesions in bilateral upper extremities, thalidomide treatment was started at 50 mg/d. After 1 month from the initiation of thalidomide treatment, the lesions in upper extremities had disappeared. In the literature, there are some reports of patients with PG who were successfully treated with thalidomide. Our patient is a complicated case who simultaneously has MDS, PG, and tuberculosis infection. The reason for thalidomide usage in our patient was the need of immune modulation without immune suppression. Our patient has tolerated the drug well, and excellent response was obtained after 1 month of initiation of thalidomide treatment. To conclude, thalidomide is a very effective drug acting as an immune modulator, which is useful in the clinical management of both MDS and PG.
Keywords: pyoderma gangrenosum, thalidomide, myelodysplastic syndrome
Introduction
Pyoderma gangrenosum (PG) is an occasional neutrophilic dermatosis. Therapy could be difficult because of painful skin lesions. Incidence of the disease is ~5/1,000,000.1 Inflammatory bowel diseases, hematological malignancies, or rheumatologic diseases have been diagnosed in majority of patients.2–4 Myelodysplastic syndromes (MDSs) may be associated with PG.3–5 The mechanism of PG is not yet fully understood; however, disorders of immune system seem to have an important role.5 Small traumas may aggregate PG. There were different types of PG lesions.6 The main pattern is a painful ulcerative lesion. The clinical course can be very aggressive depending on the underlying disease. Steroids, tacrolimus, and cyclosporine may be used in the treatment. Thalidomide may be used as a treatment option in some patients.4,7,8 Herein, we aimed to report a patient who was diagnosed as PG with MDS and responded well to thalidomide treatment.
Case report
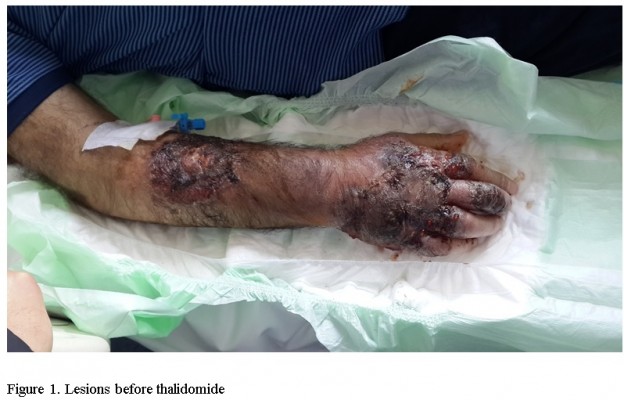
A 61-year-old man with painless ecchymotic lesions in his right upper extremity was admitted to the hospital in Isparta, Turkey, in January 2015. He was consulted to dermatology clinic, and he was given amoxicillin treatment. Punch biopsy was taken from the lesions that have revealed PG. Steroid treatment was given at 40 mg prednisolone for 2 days. The lesions were regressed immediately. He also had symptom of fever. Abdominal tomography was performed, which revealed lesions as 32×22 mm in liver and 35 mm diameter in spleen that were considered as abscess. Necrotic cells were observed from percutaneous samples, which were taken from abscess. He was given antibiotics; however, the lesions appeared again. Because immature myeloid cells were seen in blood film, he was referred to our hospital in April 2015. In his anamnesis, it was found that he was diagnosed as MDS 6 years ago and had been treated with cyclosporine at 2×100 mg for 5 years, which was stopped in January 2015. On admission, his body temperature was 38.1°C. The laboratory tests showed: hemoglobin, 9.2 g/dL; white blood cells, 5.1×103/μL; and thrombocytes, 10×103/μL. Exogenous platelet apheresis replacement was performed. He had ecchymotic lesions in both his extremities (Figure 1). Magnetic resonance imaging was performed for lesions that favor tuberculosis infection. Aspiration from liver lesion revealed the presence of Mycobacterium tuberculosis, so antituberculosis treatment was started. Bone marrow investigation revealed MDS-refractory anemia with excess blasts (7%). For lesions in bilateral upper extremities, thalidomide treatment was started at 50 mg/d. Lesions began to regress, and the patient tolerated thalidomide well, so the dose was increased to 100 mg/d. After 1 month from the initiation of thalidomide treatment, the lesions in upper extremities had disappeared (Figure 2).
  | Figure 1 Lesions before thalidomide. |
  | Figure 2 Lesions after thalidomide. |
All of the ethical considerations were strictly handled in accordance with the Helsinki Declaration. As a standard of care/action of the hospitals of Hacettepe Medical School, it was confirmed based on patient records that the study participant gave written informed consent at the time of hospitalization and relevant diagnostic/therapeutic standards of care.
Discussion
First-line treatment in PG often contains high-dose steroids, and if there is resistance to steroids, many immunosuppressive agents, such as cyclophosphamide, cyclosporine A, clofazamine, azathioprine, and chlorambucil, could also be used.9 In the literature, there are some reports of patients with PG who were successfully treated with thalidomide. A 3-year-old refractory PG patient was successfully treated with thalidomide 100 mg/d.10 The time required to achieve treatment response could be different between cases. For example, in one case, PG was treated with thalidomide after 6 months of initiation of therapy.11 However, a refractory PG patient with penis involvement just healed in 5 days after the initiation of thalidomide with 100 mg/d.12 Another PG patient responded to treatment after 10 weeks of thalidomide usage. In addition, thalidomide is effective against rheumatic diseases associated with PG. Two patients with Behcet’s syndrome and PG were successfully treated with thalidomide.13,14 An important concern about thalidomide usage in PG is the duration of treatment. In a PG patient, excellent treatment response was observed for 2 years with thalidomide 100 mg/d; however, after discontinuation of drug, relapse occurred.15
There are several reports on PG in the English literature.16,17 The thalidomide activity mechanism is still not fully understood, but it is known that the result of usage of thalidomide exposes antiangiogenic effect and increases the oxidative stress.18 Thalidomide increases the levels of interleukin (IL) 2, IL-4, and IL-5; however, it decreases the levels of tumor necrosis factor α, IL-6, IL-10, and IL-12.19 It also modifies the secretion of interferon γ.19 Thalidomide promotes proliferation and migration functions of keratinocytes. Thus, it is a very useful agent in skin lesions because it enhances reepithelialization.20 Thalidomide could also be used as a treatment option for MDS.21
Our patient is a complicated case who simultaneously has MDS, PG, and tuberculosis infection. The reason for thalidomide usage in our patient was the need of immune modulation without immune suppression. We did not prefer steroid and other immunosuppressive drugs because of M. tuberculosis infection. So, thalidomide was considered as the best option in our patient. Unlike previous cases, we have started the thalidomide treatment with 50 mg/d, and 1 week later, we have increased the dose to 100 mg/d. Our patient has tolerated the drug well, and excellent response was obtained after 1 month of initiation of thalidomide treatment. The plan for our patient is to continue the drug as long as the patient tolerates because there is a risk of relapse in case of discontinuation.
Conclusion
To conclude, thalidomide is a very effective drug acting as an immune modulator, which is useful in the clinical management of both MDS and PG.
Disclosure
The authors have no conflicts of interest in this work, including specific financial interests, relationships, and/or affiliations relevant to the subject matter or materials included.
References
Ruocco E, Sangiuliano S, Gravina AG, Miranda A, Nicoletti G. Pyoderma gangrenosum: an updated review. J Eur Acad Dermatol Venereol. 2009;23:1008. | |
Von den Driesch P. Pyoderma gangrenosum: a report of 44 cases with follow-up. Br J Dermatol. 1997;137:1000. | |
Bennett ML, Jackson JM, Jorizzo JL, Fleischer AB Jr, White WL, Callen JP. Pyoderma gangrenosum. A comparison of typical and atypical forms with an emphasis on time to remission. Case review of 86 patients from 2 institutions. Medicine (Baltimore). 2000;79:37. | |
Binus AM, Qureshi AA, Li VW, Winterfield LS. Pyoderma gangrenosum: a retrospective review of patient characteristics, comorbidities and therapy in 103 patients. Br J Dermatol. 2011;165:1244. | |
Ahronowitz I, Harp J, Shinkai K. Etiology and management of pyoderma gangrenosum: a comprehensive review. Am J Clin Dermatol. 2012;13:191. | |
Powell FC, Hackett BC, Wallach D. Pyoderma gangrenosum. In: Goldsmith LA, Katz SI, Gilchrest BA, et al., editors. Fitzpatrick’s Dermatology in General Medicine. Vol. 1. 8th ed. New York: McGraw-Hill Companies, Inc.; 2012:371. | |
Kim TH, Oh SY, Myung SC. Pyoderma gangranosum of the penis. J Korean Med Sci. 2009;24:1200. | |
Federman GL, Federman DG. Recalcitrant pyoderma gangrenosum treated with thalidomide. Mayo Clin Proc. 2000;75:842. | |
Ehling A, Karrer S, Klebl F, Schaffler A, Muller-Ladner U. Therapeutic management of pyoderma gangrenosum. Arthritis Rheum. 2004;50:3076–3084. | |
Venencie PY, Saurat JH. Pyoderma gangrenosum in a child. Treatment with thalidomide. Ann Pediatr. 1982;29:67–69. | |
Hecker MS, Lebwohl MG. Recalcitrant pyoderma gangrenosum: treatment with thalidomide. J Am Acad Dermatol. 1998;38:490–491. | |
Farrell AM, Black MM, Bracka A, Bunker CB. Pyoderma gangrenosum of the penis. Br J Dermatol. 1998;138:337–340. | |
Munro CS, Cox NH. Pyoderma gangrenosum associated with Behcet’s syndrome – response to thalidomide. Clin Exp Dermatol. 1988;13:408–410. | |
Rustin MH, Gilkes JJ, Robinson TW. Pyoderma gangrenosum associated with Behcet’s disease: treatment with thalidomide. J Am Acad Dermatol. 1990;23:941–944. | |
Buckley C, Sarkany I, Bayoumi AH. Pyoderma gangrenosum with severe pharyngeal ulceration. J R Soc Med. 1990;83:590–591. | |
Wollina U. Pyoderma gangrenosum – a systemic disease? Clin Dermatol. 2015;33(5):527–530. | |
Wollina U, Tchernev G. Pyoderma gangrenosum: pathogenetic oriented treatment approaches. Wien Med Wochenschr. 2014; 164(13–14):263–273. | |
Kim JH, Scialli AR. Thalidomide: the tragedy of birth defects and the effective treatment of disease. Toxicol Sci. 2011;122(1):1–6. | |
Singhal S, Mehta J. Thalidomide in cancer. Biomed Pharmacother. 2002;56(1):4–12. | |
Nasca MR, O’Toole EA, Palicharla P, West DP, Woodley DT. Thalidomide increases human keratinocyte migration and proliferation. J Invest Dermatol. 1999;113:720–724. | |
Musto P. Thalidomide therapy for myelodysplastic syndromes: current status and future perspectives. Leuk Res. 2004;28:325–332. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
