Back to Journals » Clinical Ophthalmology » Volume 13
Treatment of central serous chorioretinopathy with topical NSAIDs
Authors Bahadorani S, Maclean K, Wannamaker K, Chu ER, Gresores N, Sohn JH, Diaz-Rohena R, Singer MA
Received 18 January 2019
Accepted for publication 4 June 2019
Published 15 August 2019 Volume 2019:13 Pages 1543—1548
DOI https://doi.org/10.2147/OPTH.S202047
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Sepehr Bahadorani,1 Kyle Maclean,1 Kendall Wannamaker,1 Edward Rickie Chu,1 Nathan Gresores,2 Jeong-Hyeon Sohn,1 Roberto Diaz-Rohena,1 Michael A Singer2
1Department of Ophthalmology, University of Texas Health Science Center at San Antonio, San Antonio, TX, USA; 2Medical Center Ophthalmology Associates, San Antonio, TX, USA
Purpose: Central serous chorioretinopathy (CSCR) is a common retinopathy that is often observed until resolution. The purpose of this study is to evaluate the effects of topical nonsteroidal anti-inflammatory drugs (NSAIDs) on timing of CSCR recovery.
Methods: An IRB-approved retrospective review was conducted on patients that had been diagnosed with a new-onset, symptomatic case of CSCR. Patients were either observed only (13 untreated eyes) or treated with topical bromfenac or nepafenac (14 eyes) over an average of about a 4–5 week follow-up period.
Results: There was no statistical significance between central macular thickness (CMT) and visual acuity of treatment and control groups at the initial presentation. However, at the follow-up visit, CMT reductions in the treatment group were significantly higher than in the control group (p<0.006).
Conclusion: Use of topical NSAIDs in the treatment of acute CSCR leads to a faster rate of reduction in the subretinal fluid volume over a follow-up period of a few weeks.
Keywords: central serous chorioretinopathy, CSCR, CSC, NSAIDs, bromfenac, nepafenac, subretinal fluid, SRF
Introduction
Central serous chorioretinopathy (CSCR) is a common retinopathy that is characterized by spontaneous serous detachment of the neurosensory retina in the macular region. Visual symptoms in patients with CSCR are often related to the localization of serous retinal detachment in the macular area, presenting with blurred vision, relative central scotoma, metamorphopsia, and reduced contrast sensitivity. This condition appears to affect males more often than females, and perhaps more so in mid-life.1,2 The precise etiology of CSCR is unknown but it is suggested that this disease may develop due to increased permeability of choroidal vessels with subsequent increased tissue hydrostatic pressure that overcomes the RPE barrier function and lead to the accumulation of subretinal fluids.3 Another possible explanation is that excess steroid use or endogenous cortisol production may lead to impaired RPE function, choroidal vascular autoregulation, hypercoagulability and augmented platelet aggregation. Indeed, risk factors to CSCR include steroid use, conditions that are associated with increased cortisol production (i.e., pregnancy and Cushing's disease), type A personality, psychological stress, hypertension, and coagulation disorders.4,5
Most cases of CSCR are self-limiting and regress spontaneously within 3–4 months. Therefore, observation with or without the removal of risk factors is one of the mainstays of therapy for acute CSCR. However, chronic cases pose a risk of progressive vision loss and thus, different treatment options such as photodynamic therapy (PDT), micro-pulse laser, intravitreal injection of anti-vascular endothelial growth factor (anti-VEGF) agents, and systemic anti-corticosteroid medications have been used. However, these treatments can have unwanted complications, while their effectiveness in the treatment of CSCR show either controversial or low-quality evidence of improvement.6–9 Therefore, it is not surprising that there is not yet a solid identified mainstay of therapy for CSCR. For this study we propose to test the effectiveness of topical NSAIDs in the treatment of CSCR as a safe alternative to current treatment options.
Methods
The study received IRB approval by the University Health System (San Antonio, Texas; UHS) and Medical Center Ophthalmology Associates (San Antonio, Texas; MCOA). Patient consent was not required given the retrospective nature of the study and the information collected had no effect on patients’ treatment or plan of care. All stages of the study were conducted in accordance with the principles set forth by the Declaration of Helsinki.
This IRB-approved retrospective review was conducted on patients that were diagnosed with a new case of CSCR. Inclusion criteria included: 1) presence of subretinal fluid under the fovea as seen on OCT; 2) no prior treatment; and 3) diagnosis of acute CSCR as first presentation to the eye clinic with visual symptoms of less than 3 months duration. Exclusion criteria included: 1) the presence of choroidal neovascular membranes or ocular disorders other than CSCR that could affect the macula or the posterior retina; 2) initiation of a treatment other than topical NSAIDs; and 3) a follow-up period of more than 7 weeks since the initial visit. Patients were divided into control (observation only) and treatment groups, who were treated 4-times a day with topical NSAIDs eye drops. Topical formulations included Nevanac (nepafenac ophthalmic, 0.1%), Ilevro (nepafenac ophthalmic, 0.3%), and Xibrom (bromfenac ophthalmic, 0.09%). Data were collected retrospectively from 2009 to 2018 from a total of 6 medical centers, 2 of which were affiliated with UHS and the other 4 were affiliated with MCOA . Investigators at MCOA treated their CSCR patients with topical NSAIDs, while patients at UHS centers were observed. The primary outcomes include changes in the visual acuity score,10 central macular thickness (CMT), and volume of subretinal fluid before and after therapy or observation. CMT values were obtained from Cirrus or Heidelberg OCT machines in-built software that were available at MCOA and UHS, respectively. This comparison became possible with an earlier study that has demonstrated central thickness difference of only 2.78 μm between these two machines, the value for which was statistically non-significant.11 Volume analysis of the OCT scans were performed using the ImageJ software manual method, where areas of subretinal fluid or the maximal height of subretinal fluid was manually drawn and the number of pixels analyzed. For all comparisons, the investigators were blinded to the treatment or control labels. The data were graphed using Microsoft Excel and was analyzed for their statistical significance using the Excel Student’s t-test.
Results
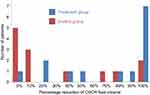
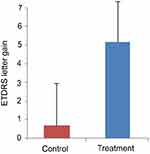
Clinical charts from 27 patients/eyes with CSCR were reviewed, with 14 eyes being treated with topical NSAIDs and 13 eyes observed. The mean age was 49.4 and 44.4 years for the control and treatment groups, respectively (p=0.14). OCT was obtained at the initial presentation and in the follow-up period, which was on average between 4 and 5 weeks. There was no statistical significance between CMT and visual acuity of treatment and control groups at the initial presentation (Figure 1). At the follow-up visit, patients in the control group were skewed toward having no improvement in the subretinal fluid volume, versus patients in the treatment group, the majority of whom had up to full resolution of the subretinal fluid (Figure 2). CMT reductions in the control and treatment groups were 43.5 µm and 196.2 µm, respectively (p<0.006). Likewise, percentage reductions of subretinal fluid volume were 11.1% and 64.3% in the control and treatment groups, respectively (p<0.02) (Figure 3). There was no statistical significance (p-value=0.067) in visual acuity letter gain for the control (0.69 letters) and treatment (5.2 letters) groups (Figure 4). Details regarding patient’s age, sex, initial date of follow up, and CMT values at initial and follow-up visits are illustrated in Table 1.
 |
Table 1 Clinical characteristics of patients who were either treated or observed for acute central serous chorioretinopathy |
Discussion
Although the natural history of CSCR has a favorable prognosis, potential anatomical and functional loss complications observed with chronic or recurrent acute cases further highlight the need for a safe and early effective treatment. There are different therapeutic approaches that are used for CSCR and yet, the potential use of NSAIDs in the treatment of CSCR has been largely neglected. NSAID eye drops are the mainstay of therapy for post-operative cystoid macular edema (CME) but there are only a few non-randomized studies that show the effectiveness of this medication in the treatment of CSCR.12,13
Post-operative CME is largely attributed to increased production of prostaglandins and thus, NSAIDs are routinely used in post-operative patients to inhibit cyclooxygenase (COX-1 and COX-2) enzymes with subsequent reduced production of prostaglandins and their downstream inflammatory effects.14 There are case reports related to the development of CSCR following the use of latanoprost eye drops, suggesting that prostaglandin may also play a role in the development of CSCR.15,16 In one study, CSCR patients that were treated with topical nepafenac showed a rapid improvement in best corrected visual acuity (BCVA) and CMT compared to the observation group.12 Another study also shows that 107 patients that were treated with oral aspirin (100 mg daily for the first month, then 100 mg every other day for the next 5 months) demonstrated faster visual recovery with reduced rates of CSCR recurrence compared to the control group. This latter study suggested that the beneficial effects of oral aspirin may be attributed to increased plasminogen activator inhibitor 1 (PAI-1) in CSCR patients.17 Finally, NSAIDs like indomethacin are shown to reduce serum and urinary concentration of aldosterone,18,19 suggesting that topical NSAIDs may also cover CSCR-protecting mechanisms previously observed with systemic spironolactone and eplerenone therapy.20,21 Therefore, considering NSAIDs' protective effects against CSCR, perhaps through reduced production of prostaglandins, reduced secretion of aldosterone, and/or their anticoagulation effects, topical NSAIDs appear as an effective and safe treatment option for this form of retinopathy.
Our results support the fact that patients treated with topical NSAIDs had a significantly faster resolution of subretinal fluid. The limitations of our study, however, include a relatively small sample size (n=27). Indeed, while there was a large difference in visual acuity gain between the treatment and control groups, the difference is not statistically significant owing to a small sample size. Furthermore, considering that patients with CSCR are often type A personality, it is possible that the placebo effects of starting topical eye drops may also contribute to faster resolution of subretinal fluid. Therefore, future studies are directed into repeating these findings with a larger sample size in addition to applying artificial tears as a placebo in the control group. Moreover, patients need longer follow-up periods to determine whether topical NSAIDs may be also beneficial in reducing the recurrence rate of CSCR. Finally, the amount of drug penetration into ocular tissues can vary for different formulations22,23 and thus, it would be worthwhile to compare the effectiveness of different NSAIDs in the treatment of CSCR.
Conclusion
The results of our study demonstrate that topical bromfenac and nepafenac are effective means of mediating a quick resolution of subretinal fluid in patients with acute-onset CSCR.
Disclosure
Dr Roberto Diaz-Rohena reports personal fees from Allergan, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Nicholson BP, Atchison E, Idris AA, Bakri SJ. Central serous chorioretinopathy and glucocorticoids: an update on evidence for association. Surv Ophthalmol. 2018;63(1):1–8. doi:10.1016/j.survophthal.2017.06.008
2. Liew G, Quin G, Gillies M, Fraser-Bell S. Central serous chorioretinopathy: a review of epidemiology and pathophysiology. Clin Exp Ophthalmol. 2013;41(2):201–214. doi:10.1111/j.1442-9071.2012.02848.x
3. Okushiba U, Takeda M. Study of choroidal vascular lesions in central serous chorioretinopathy using indocyanine green angiography. Nihon Ganka Gakkai Zasshi. 1997;101(1):74–82.
4. Liegl R, Ulbig MW. Central serous chorioretinopathy. Ophthalmologica. 2014;232(2):65–76. doi:10.1159/000360014
5. Caccavale A, Romanazzi F, Imparato M, Negri A, Morano A, Ferentini F. Central serous chorioretinopathy: a pathogenetic model. Clin Ophthalmol. 2011;5:239–243. doi:10.2147/OPTH.S17182
6. Salehi M, Wenick AS, Law HA, Evans JR, Gehlbach P. Interventions for central serous chorioretinopathy: a network meta-analysis. Cochrane Database Syst Rev. 2015;22(12):CD011841.
7. Iacono P, Battaglia Parodi M, Falcomatà B, Bandello F. Central serous chorioretinopathy treatments: a mini review. Ophthalmic Res. 2015;55(2):76–83. doi:10.1159/000441502
8. Abouammoh MA. Advances in the treatment of central serous chorioretinopathy. Saudi J Ophthalmol. 2015;29(4):278–286. doi:10.1016/j.sjopt.2015.01.007
9. Schwartz R, Habot-Wilner Z, Martinez MR, et al. Eplerenone for chronic central serous chorioretinopathy-a randomized controlled prospective study. Acta Ophthalmol. 2017;95(7):e610–e618. doi:10.1111/aos.13273
10. Gregori NZ, Feuer W, Rosenfeld PJ. Novel method for analyzing snellen visual acuity measurements. Retina. 2010;30(7):1046–1050. doi:10.1097/IAE.0b013e3181d87e04
11. Sander B, Al-Abiji HA, Kofod M, Jørgensen TM. Do different spectral domain OCT hardwares measure the same? Comparison of retinal thickness using third-party software. Graefes Arch Clin Exp Ophthalmol. 2015;253(11):1915–1921. doi:10.1007/s00417-015-3075-2
12. Alkin Z, Osmanbasoglu OA, Ozkaya A, Karatas G, Yazici AT. Topical nepafenac in treatment of acute central serous chorioretinopathy. Med Hypothesis Discov Innov Ophthalmol. 2013;2(4):96–101.
13. Khan NA, Atiqa K, Sobia K, Aisha K, Ahmed AK. Topical Bromfenac in the Treatment of Central Serous Chorioretinopathy. Adv Ophthalmol Vis Syst. 2017;7(7):00248.
14. McCafferty S, Harris A, Kew C, et al. Pseudophakic cystoid macular edema prevention and risk factors; prospective study with adjunctive once daily topical nepafenac 0.3% versus placebo. BMC Ophthalmol. 2017;17(1):16. doi:10.1186/s12886-017-0405-7
15. Artunay O, Senel A, Sengul A, Rasier R, Bahcecioglu H. Central serous chorioretinopathy associated with topical latanoprost therapy. Ocul Immunol Inflamm. 2011;19(6):453–455. doi:10.3109/09273948.2011.619680
16. Ayhan Tuzcu E, Keskin U, Coskun M, Ilhan O, Daglıoglu M, Oksuz H. Bilateral serous detachment associated with latanoprost/timolol fixed combination use: a report of one phakic case. Case Rep Ophthalmol Med. 2012;2012:305379.
17. Caccavale A, Imparato M, Romanazzi F, Negri A, Porta A, Ferentini F. A new strategy of treatment with low-dosage acetyl salicylic acid in patients affected by central serous chorioretinopathy. Med Hypotheses. 2009;73(3):435–437. doi:10.1016/j.mehy.2009.03.036
18. Kutyrina IM, Androsova SO, Warshavskii VA, Tareyeva IE. Effects of indomethacin on the renal function and renin-aldosterone system in chronic glomerulonephritis. Nephron. 1982;32(3):244–248. doi:10.1159/000182853
19. Pedrinelli R, Arzilli F, Cavasinni L, Poli L, Sassano P, Salvetti A. The effect of indomethacin on plasma renin activity and urinary aldosterone of patients with essential hypertension. J Endocrinol Invest. 1978;1(4):315–320. doi:10.1007/BF03350976
20. Cakir B, Fischer F, Ehlken C, et al. Clinical experience with eplerenone to treat chronic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2016;254(11):2151–2157. doi:10.1007/s00417-016-3373-3
21. Gong Q, Sun XH, Yuan ST, Liu QH. The relation of the serum aldosterone level and central serous chorioretinopathy - a pilot study. Eur Rev Med Pharmacol Sci. 2017;21(3):446–453.
22. Bucolo C, Marrazzo G, Platania CB, Romano GL, Drago F, Salomone S. Effects of topical indomethacin, bromfenac and nepafenac on lipopolysaccharide-induced ocular inflammation. J Pharm Pharmacol. 2014;66(7):954–960. doi:10.1111/jphp.12224
23. Bucolo C, Drago F, Salomone S. Ocular drug delivery: a clue from nanotechnology. Front Pharmacol. 2012;3:188. doi:10.3389/fphar.2012.00188
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.