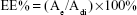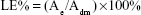Back to Journals » International Journal of Nanomedicine » Volume 12
Transferrin receptor-targeted pH-sensitive micellar system for diminution of drug resistance and targetable delivery in multidrug-resistant breast cancer
Authors Gao W, Ye G, Duan X, Yang X, Yang VC
Received 17 June 2016
Accepted for publication 16 November 2016
Published 7 February 2017 Volume 2017:12 Pages 1047—1064
DOI https://doi.org/10.2147/IJN.S115215
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Linlin Sun
Wei Gao,1 Guihua Ye,1 Xiaochuan Duan,1 Xiaoying Yang,1 Victor C Yang1,2
1Tianjin Key Laboratory on Technologies Enabling Development of Clinical Therapeutics and Diagnostics (Theranostics), School of Pharmacy, Tianjin Medical University, Tianjin, People’s Republic of China; 2Department of Pharmaceutical Sciences, College of Pharmacy, University of Michigan, Ann Arbor, MI, USA
Abstract: The emergence of drug resistance is partially associated with overproduction of transferrin receptor (TfR). To overcome multidrug resistance (MDR) and achieve tumor target delivery, we designed a novel biodegradable pH-sensitive micellar system modified with HAIYPRH, a TfR ligand (7pep). First, the polymers poly(l-histidine)-coupled polyethylene glycol-2000 (PHIS-PEG2000) and 7pep-modified 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-polyethylene glycol-2000 (7pep-DSPE-PEG2000) were synthesized, and the mixed micelles were prepared by blending of PHIS-PEG2000 and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-polyethylene glycol-2000 (DSPE-PEG2000) or 7pep-DSPE-PEG2000 (7-pep HD micelles). The micelles exhibited good size uniformity, high encapsulation efficiency, and a low critical micelle concentration. By changing the polymer ratio in the micellar formulation, the pH response range was specially tailored to pH ~6.0. When loaded with antitumor drug doxorubicin (DOX), the micelle showed an acid pH-triggering drug release profile. The cellular uptake and cytotoxicity study demonstrated that 7-pep HD micelles could significantly enhance the intracellular level and antitumor efficacy of DOX in multidrug-resistant cells (MCF-7/Adr), which attributed to the synergistic effect of poly(l-histidine)-triggered endolysosom escape and TfR-mediated endocytosis. Most importantly, the in vivo imaging study confirmed the targetability of 7-pep HD micelles to MDR tumor. These findings indicated that 7-pep HD micelles would be a promising drug delivery system in the treatment of drug-resistant tumors.
Keywords: poly(l-histidine), DSPE-PEG2000, HAIYPRH, MCF-7Adr
Introduction
Tumor multidrug resistance (MDR) and severe systematic side effects are two major impediments to successful chemotherapy.1 Overexpression of some adenosine triphosphate-binding cassette (ABC) transporters that induce tumor cell efflux toward various cytotoxicity agents is the major cause of MDR.2 Nonspecific systemic distributions of cytotoxic drugs induce damage to healthy tissues, which contributes to the side effect of chemotherapy. Developing special drug delivery systems based on nanotechnology has attracted great attention in the treatment of cancers during the last decades.3,4 Nanocarriers such as liposomes, micelles and nanoparticles can achieve tumor-targeted delivery due to the enhanced permeability and retention (EPR) effect, or tumor-specific recognition to antibodies/ligands anchored on the surface of nanocarriers,5 thereby reducing drug-induced systemic toxic effects.6 The EPR effect refers to the enhanced permeability of nanoparticles across the tumor vascular barrier through intercellular gaps and the retention in the tumor site owing to pressure created by poor lymphatic drainage.7 However, after internalization in tumor cells, nanoparticles are often trapped in endolysosomes and fail to deliver the payloads to their intracellular targets, thus significantly affecting the therapeutic efficacy of the drug.8 Recently, a number of pH-sensitive micelles in response to endolysosome pH (pHendo:6.0–5.0) have been developed for efficient intracellular delivery. These micellar systems, which combine the property of pH-triggered release and endolysosome escape, have been widely demonstrated to provide an efficient intracellular delivery and an enhancement of drug effectiveness.9–11 Most importantly, certain research groups have demonstrated that pHendo-triggered micellar system could circumvent the MDR effect in various tumor types.8,12–14
Poly(L-histidine) (PHIS) is a promising pH-sensitive biomaterial with good biocompatibility and biodegradability. It undergoes a rapid hydrophobic to hydrophilic transition at acidic pH via protonation of the unsaturated nitrogen atoms on the imidazole groups. Moreover, when PHIS becomes a strong polycation at acidic pH, it can facilitate endolysosome escape via the so-called “proton sponge” effect that ruptures the endosomal membrane.15 The first PHIS-based copolymer micelles (PHIS-coupled polyethylene glycol-2000 [PHIS-PEG2000]) were synthesized by Lee et al.16 However, the PHIS-PEG2000 micelle gradually dissembled below pH 7.4 and is thus not suitable to serve as a drug carrier. Therefore, optimizing the triggering pH to a more acid pH plays a key role to construct PHIS-based drug delivery system. Preparing mixed micelles with another amphiphilic compound has been proven as a feasible approach to manipulate pH response range.11,17 For instance, the triggering pH of PHIS-PEG2000 and poly(L-lactide)-PEG2000 (PLLA-PEG2000) mixed micelles could be tailored to 6.0 by optimizing the ratio of the two polymers. The fraction of hydrophobic polymer PLLA in the micellar core would stabilize the micelles from disassembly and switch the triggering pH to a more acid pH. Interestingly, this micelle system displayed a significant effect on circumventing MDR of breast and ovarian tumors in animal models.13,18 Wu et al designed a pH-sensitive mixed micelle based on 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-PEG2000 (DSPE-PEG2000) for cytosolic drug delivery, where DSPE-PEG2000 was an US Food and Drug Administration (FDA)-approved excipient being widely used in drug formulations.19,20 The mixed micelle exhibited a strong enhancement on tumor-specific internalization and anticancer efficacy in drug-sensitive tumors.20 Stimulated by these former research ideas, this study is aimed at utilizing and further modifying the PHIS-PEG2000/DSPE-PEG2000 micellar system to counter the MDR effect, an investigation that so far has not been attempted by other research groups.
The transferrin receptor (TfR) has been extensively explored as a drug delivery target for tumor cell recognition and internalization, due to its overexpression on malignant cell surface and capability for constitutive endocytosis via Tf–TfR pathway.21 Recently, it has been demonstrated that the emergence of drug resistance is partially associated with further overproduction of TfR, which therefore could render it a potential target for MDR tumors.22 Sheng et al23 have demonstrated that TfR ligand and doxorubicin (DOX) conjugate exhibited the ability to target drug-resistant tumors. Recently, HAIYPRH, a TfR ligand (7pep), has been screened through phage display.24 Compared to endogenous ligand Tf that has been used in many reported studies, such as Nogueira-Librelotto et al,25 7pep is structure-wise much simpler, is easy for synthesis, has low immunogenicity and lacks the ability to compete with Tf in the body for receptor binding. Du et al26 constructed TfR-specific nanocarriers by conjugating with functional 7pep to facilitate oral drug delivery via enhancement of the interaction between the nanocarrier and gastrointestinal epithelial cells that overexpress TfR. In another study, Gao et al27 reported that the 7pep-modified lipid/cholesterol-grafted poly(amidoamine) polymer hybrid nanoparticles exhibited the greatest inhibition on tumor growth via TfR-mediated targeted delivery.
In this study, we attempted the preparation of a mixed micelle based on PHIS-PEG2000 and DSPE-PEG2000 with an appropriate pH response range for overriding the MDR effect and modified the micelle with 7pep to further increase the anti-MDR effect in achieving targeted delivery in MDR breast tumors. The polymers PHIS-PEG2000 and 7pep-modified DSPE-PEG2000 (7pep-DSPE-PEG2000) were first synthesized. The mixed micelles were prepared by blending of PHIS-PEG2000 and DSPE-PEG2000 hybrid micelles (HD micelles) or 7pep-DSPE-PEG2000 (7-pep HD micelles). DOX, a first-line antitumor drug, was then loaded into the micelles.28 The characteristics of the mixed micelles were thoroughly evaluated, including particle size, zeta potential, drug loading efficiency (LE%), encapsulation efficiency (EE%), critical micelle concentration (CMC), pH sensitivity and drug release. The effect of HD micelles on increasing the intracellular drug accumulation on MCF-7/Adr cells was evaluated in consideration of different formulations, dose and incubation time, as well as 7pep modification. The antitumor efficacy of 7-pep HD micelles, HD micelles and free DOX on MCF-7/Adr cells was compared using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay. Finally, the target delivery of 7-pep HD micelles were evaluated in vivo on nude mice bearing MCF-7/Adr xenograft.
Materials and methods
Materials
PHIS (3.0 kDa) was purchased from GL Biochem Co., Ltd (Shanghai, China). 7pep was purchased from ChinaPeptides Co., Ltd (Shanghai, China). DSPE-PEG2000 and DSPE-PEG2000-succinimidyl ester (NHS) were purchased from the Advanced Vehicle Technology Pharmaceutical L.T.D. Co. (Shanghai, China). Methoxy PEG-2000 succinimidyl carboxymethyl ester (mPEG2000-NHS) was purchased from JenKem Technology Co., Ltd (Beijing, China). Doxorubicin hydrochloride (DOX·HCl) was provided by Melone Pharmaceutical Co., Ltd (Dalian, China). 3-Amino-6-chloro-N-(diaminomethylene)-5-(ethyl(isopropyl)amino)pyrazine-2-carboxamide (EIPA) and cytochalasin D (CytD) were purchased from ApexBio (Beijing, China). Dynasore was provided by Selleck (Houston, TX, USA). Chlorpromazine (CPZ) and filipin were obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Methyl-β-cyclodextrin (MβCD) was purchased from Yuanye Biotechnology (Shanghai, China). 1,1-Dioctadecyl-3,3,3,3-tetramethylindotricarbocyanine iodide (DiR) was purchased from Molecular Probes Inc. (Eugene, OR, USA). Triethylamine, dimethyl sulfoxide (DMSO), dimethyl sulfoxide-d6 (DMSO-d6), α-cyano-4-hydroxycinnamic acid (CHCA) and chloroform were purchased from Sigma-Aldrich Co. Ltd (San Francisco, CA, USA). All other reagents used were of analytical grade.
The cell lines MCF-7/Adr (adriamycin-resistant human mammary adenocarcinoma) were purchased from the Institute of Basic Medical Science at the Chinese Academy of Medical Science (Beijing, China). Cells were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium supplemented with 10% (v/v) fetal bovine serum (FBS) and antibiotics (penicillin 100 U/mL and streptomycin 100 mg/mL). RPMI-1640 and FBS were provided by Thermo Fisher Scientific Inc. (Waltham, MA, USA). Antibiotics were purchased from M&C Gene Technology Ltd (Beijing, China).
Healthy female nu/nu nude mice, with a weight of 18–20 g and ~6 weeks old, were supplied by Peking University Health Science Center. The experimental procedures were carried out in accordance with the US National Institutes of Health guidelines. All animal experimentation procedures were conducted at Peking University and approved by the Institutional Animal Care and Use Committee of Peking University.
Methods
Synthesis of PHIS-PEG2000 and 7pep-DSPE-PEG2000
The synthesis of PHIS-PEG2000 was according to previous reports.20 PHIS3000 (30 mg) was dissolved in 2 mL of DMSO and stirred for 12 hours at 30°C. An excess amount of mPEG2000-NHS (1.2:1, mol/mol) was added dropwise to the above solution. The reaction was carried out under the nitrogen gas for 72 hours at 30°C. After the reaction, the solution was dialyzed with cellulose ester membranes (molecular weight 3,500 Da; Spectrum Medical Industries, Rancho Dominguez, CA, USA) against deionized water for 24 hours to remove the unreacted mPEG-NHS and PHIS. The solution was lyophilized. Then, the product was dissolved in DMSO-d6 and tested using a proton nuclear magnetic resonance (1H-NMR) spectroscopy (Bruker Advance III 400 MHz; Bruker, Fallanden, Switzerland). To measure the average molecular weight of the product, the product solution was mixed with CHCA and tested by an Axima-CFR plus (Kratos Analytical Ltd, Manchester, UK) mass spectrometer equipped with a nitrogen laser (337.1 nm).
7pep-DSPE-PEG2000 was synthesized by conjugating 7pep to DSPE-PEG2000-NHS.26 Briefly, the 7pep and DSPE-PEG2000-NHS at a molar ratio of 1:2 were dissolved in anhydrous dimethylformamide. The trimethylamine was added to the mixture to adjust the pH to 8.0–9.0. The reaction was carried out under the nitrogen gas for 72 hours at 30°C. After the reaction, the solution was dialyzed with cellulose ester membranes (molecular weight 2,000 Da; Spectrum Medical Industries) against deionized water for 24 hours to remove the unreacted 7pep. The solution was lyophilized. The product was confirmed by an Axima-CFR plus mass spectrometer, following the same method as described in the previous paragraph. The final product was composed of 7pep-DSPE-PEG2000 and DSPE-PEG2000-NHS. The amount of 7pep-DSPE-PEG2000 in the final product was determined by element analysis method using vario EL III Element Analyzer (Elementar, Langenselbold, Germany).
Preparation of the HD micelles
The mixed micelles were prepared using the self-emulsion/solvent evaporation method.29 To prepare the HD micelles, PHIS-PEG2000 and DSPE-PEG2000 with different molecular ratios were dispersed in Na2B4O7 (10 mM) solution and stirred for 12 hours at room temperature. Chloroform (chloroform:Na2B4O7 solution: 1:10 [v/v]) was then added dropwise to the polymer solution and sonicated in the ice water bath for 10 minutes to form the emulsion. The emulsion was stirred for another 12 hours in fume cupboard at room temperature and further dried under high vacuum for 1 hour to remove chloroform. The PHIS-PEG2000 and 7pep-DSPE-PEG2000 hybrid micelles (7-pep HD micelles) were prepared following a similar process to that of HD micelles except for the addition of 7pep-DSPE-PEG2000 in the Na2B4O7 solution (10 mM).
To obtain the drug-loaded mixed micelles, DOX·HCl was first reacted with triethylamine in chloroform for 3 hours to obtain DOX base and then added dropwise to the polymer solution. The rest preparation process was the same as described earlier.
For in vivo fluorescent imaging, the near-infrared fluorescent probe DiR was loaded into the HD micelles and 7-pep HD micelles. The preparation process was the same as that of empty micelles except for the addition of DiR in the chloroform.
Characterization of various drug-loaded micelles
Particle size and zeta potential of the micelles
The particle size and zeta potential of the micelles were determined by dynamic light scattering (DLS) analysis using a Malvern Zetasizer Nano ZS (Malvern Instruments Ltd, Malvern, UK). The morphology of drug-loaded micelles was observed by a transmission electron microscope (TEM) (HT7700; Hitachi, Ltd, Tokyo, Japan).
EE% and LE%
The concentration of DOX was quantified by a ultraviolet (UV)-visible spectrophotometer (Hitachi, Ltd) at 485 nm. The EE% and LE% of the micelles were calculated as follows:
|
|
Ae represents the weight of DOX in micelles after dialysis, Adi is the weight of drug input and Adm is the weight of DOX-loaded micelles.
CMC
The CMC of the micelles was determined by the standard pyrene method.20 First, the micellar solutions at varied concentrations ranging from 0.5 to 100 μg/mL were mixed with pyrene. The mixed solutions were sonicated for 30 minutes and placed at room temperature for 1 hour. The fluorescence of pyrene was determined using a RF-5301 PC fluorescence spectrometer (Shimadzu, Kyoto, Japan). The excitation wavelength is 339 nm. The CMC plot was obtained by the intensity ratio of the first and the third energy bands (I373/I383) in the emission spectra profile against the micelle concentration, and the CMC value was obtained from the crossover point in this plot.
The pH sensitivity
The dissociation process of the micelles with pH can be tracked by measuring the fluorescence of pyrene that was loaded in the micelles and the zeta potential changes.16,20 For the fluorescence method, the micellar solutions were mixed with pyrene and sonicated for 30 minutes and then placed at room temperature for 1 hour. HCl solution was added dropwise to adjust the pH of the micellar solutions. The fluorescence emission spectrum of the pyrene was measured every 0.5–1 pH change. The excitation wavelength is 339 nm. The fluorescence change with pH was estimated by the intensity ratio of the first and the third peaks (I373/I383) in the spectra profile. For the zeta potential method, the micelles were dispersed in 10 mM Na2B4O7 solution (pH=7.5, adjusted by HCl solution). HCl solution was then added dropwise to adjust the pH of the micellar solutions. The zeta potential of the micelle was measured every 0.5–1 pH changes.
In vitro DOX release at different pH value
The in vitro DOX release from the micelles was determined using a dialysis method. The dialysis was conducted in 30 mL phosphate-buffered saline (PBS) (pH 7.4 or 5.0) under continuous shaking at a speed of 200 times per minute for 48 hours at 37°C. Typically, 1 mL micellar solution and 1 mL media were added in a dialysis bag (molecular weight 3500 Da). At each time point (0.5, 1, 2, 4, 8, 12 and 24 hours), 1 mL media were withdrawn from the beaker and replaced by an equal volume of the fresh media. The concentration of DOX in the media was determined by an ultra performance liquid chromatography system (Acquity; Waters, Milford, MA, USA) with an UV detector at 233 nm, an octadecylsilyl column (Agilent Zorbax SB-C18, 4.6×250 mm, 5 μm) and a flow rate of 0.2 mL/min. The mobile phase consisted of water (10 mM ammonium acetate adjusted to pH 3.5 by 0.1 M HCl), methanol and acetonitrile (40:5:50, v/v).
Flow cytometry analysis
The flow cytometry analysis was used to determine the intracellular concentration of DOX after incubation with different DOX formulations. First, MCF-7/Adr cells were seeded into 12-well plates and incubated until reaching 70%–80% confluence. Various DOX formulations were added and incubated with cells for 3 hours at 37°C. Then, the cells were trypsinized, rinsed with cold PBS (pH=7.4) and suspended in 400 μL of PBS. The DOX fluorescence intensity was determined using a FacScan flow cytometer (FacsVerse™; BD Biosciences, San Jose, CA, USA). The number of cells collected was 10,000. A gate is set for each sample with a majority of the cells, only excluding small fragments of cells.
Laser confocal microscopy analysis
Confocal microscopy analysis was also used to observe the intracellular concentration of DOX after incubation with different DOX formulations. MCF-7/Adr cells were seeded into glass bottom dishes for 24 hours until cell adhesion. The cells were incubated with DOX and DOX-loaded micelles for 4 hours and then washed with PBS. Living cells were directly observed using a laser scanning confocal microscope (FV1000; Olympus, Tokyo, Japan). DOX was excited at 480 nm and detected at 555–590 nm.
Immunofluorescence for TfRs
The TfRs expression on MCF-7/Adr cells was confirmed using immunofluorescence. First, the cells were fixed with 4% (v/v) formaldehyde for 10 minutes at 3°C and permeabilized using PBS containing 0.1% Triton (PBS-Triton). Then, the cells were incubated with blocking buffer (5% bovine serum albumin [BSA], 0.3 M glycine, 0.1% Tween dissolved in PBS) for 1 hour, followed by incubation with anti-TfR antibody (ab84036; 1:1,000 dilution; Abcam, Cambridge, MA, USA) in PBS overnight at 4°C. The cells were washed and treated with the secondary antibody fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit immunoglobulin G (SA134; 1:1,000 dilution; Solarbio, Beijing, China) for 1 hour at 37°C. Cell nucleus was stained with Hoechst 33258 (4 μg/mL) for 15 minutes at room temperature. The negative control was incubated with 1% BSA instead of anti-TfR receptor antibody to rule out the possibility of nonspecific binding. Finally, the cells were observed using a laser scanning confocal microscope (FV1000, Olympus).
Investigation of endocytosis mechanism
Endocytosis mechanism studies were performed to clarify the endocytosis pathway of 7-pep HD micelles/DOX and HD micelles/DOX.30 First, MCF-7/Adr cells were seeded into 12-well plates and incubated until reaching 70%–80% confluence. Then, the cells were preincubated with different endocytosis inhibitors including 100 μM EIPA, 50 μM CPZ, 80 μg/mL dynasore, 5 μM MβCD, 2.5 μg/mL filipin and 0.5 μM CytD, for 1 hour. 7-pep HD micelles/DOX and HD micelles/DOX were added into the above medium and incubated for another 1 hour. Then, the cells were trypsinized, rinsed with cold PBS (pH=7.4) and suspended in 400 μL of PBS. The DOX fluorescence intensity was determined using a FACScan flow cytometer (FacsVerse, BD Biosciences). The number of cells collected was 10,000.
In vitro cytotoxicity assays
The in vitro cytotoxicity of various DOX formulations was evaluated by the MTT assay. Typically, cells were seeded into 96-well plates (5,000 cells per well) and incubated for 24 hours. Serial concentrations of DOX formulations diluted with RPMI-1640 were added in the wells and incubated for 48 hours at 37°C. The cells were washed twice with PBS and incubated with the complete growth medium diluted in MTT for 4 hours. The MTT medium was then removed. A 200 μL of DMSO was added to each well under slow shaking for 10 minutes. The absorbance intensity at 490 nm was recorded using a Multiskan™ GO microplate reader from Thermo Fisher Scientific, Waltham, MA, USA, and the cytotoxicity was expressed as the percentage of cell viability compared to that of the control group.
In vivo imaging
The biodistributions of HD micelles and 7-pep HD micelles were determined using an In Vivo Image System (FX Pro; Kodak, Rochester, NY, USA). To establish the animal model, MCF-7/Adr cells were subcutaneously inoculated in the right flanks of female nude mice (~6 weeks old). After the tumor volume reached ~150 mm3, the mice were injected via the tail vein with DiR-loaded micelles (DiR) (500 ng/mL), 0.2 mL for each mouse. At each time point (1, 2, 4, 8, 12 and 24 hours), the mice were inhalation anesthetized with isoflurane and photographed for both fluorescence and X-ray imaging pictures. The parameters of the fluorescence camera are fixed during the test (emission filter: 790 nm; exposure time: 30 s; f-stop is 3.5; field of view [FOV]: 145 mm; focal plane: 10 mm). At the end of the test, mice were sacrificed, and the main organs and tumors were collected for ex vivo fluorescence imaging.
Statistical analysis
All experiments were performed more than three times, and quantitative data were expressed as mean ± standard deviation (SD) unless otherwise specified. Two-tailed Student’s t-test analysis was used to evaluate the differences between groups. A P-value <0.05 or <0.01 was considered statistically significant or highly significant.
Results and discussion
Synthesis of PHIS-PEG2000 and 7pep-DSPE-PEG2000
The synthesis scheme of PHIS-PEG2000 and 7pep-DSPE-PEG2000 is illustrated in Figure 1A. The PHIS-PEG2000 was synthesized via a reaction between the amine group of the PHIS3000 and the NHS group of the mPEG2000-NHS. Successful synthesis of PHIS-PEG2000 was confirmed by a mass spectrometer (Figure 1B) and 1H-NMR (Figure S1). As seen in the mass spectrum, the peak of ~5,000 indicated the molecular weight of PHIS-PEG2000. In the 1H-NMR spectrum of PHIS-PEG2000, the signal at 3.50 ppm was characteristic of OCH2CH2 in the PEG2000 units, and the peak at 7.40–8.40 ppm was for the protons on the imidazole ring in the PHIS.
The 7pep was conjugated to NHS-DSPE-PEG2000 via a reaction between the amine group of the 7pep and the NHS group of the DSPE-PEG2000-NHS (Figure 1A). After the reaction, the remaining 7pep was removed by dialysis. The final product was a mixture of DSPE-PEG2000-NHS and 7pep-DSPE-PEG2000. As seen in the mass spectrum (Figure 1C), the peak of ~3,800 indicated the molecular weight of 7pep-DSPE-PEG2000, while the peak (~2,900) indicated the molecular weight of DSPE-PEG2000. According to the element analysis, the amount of 7pep-DSPE-PEG2000 in the final product was 60.3%.
Characterization of the mixed micelles
The pH-sensitive HD micelles were successfully prepared in this study. After optimizing the formulations, the HD micelles (DSPE-PEG2000:PHIS-PEG2000=35:65, w/w) were chosen for good size distributions and proper pH sensitivity. As shown in Table 1 and Figure 2A, the average hydrodynamic diameter of the HD micelles was 98.743±5.584 nm with a polydispersity index (PDI) of 0.228. When loaded with antitumor drug DOX, the size increased to 119.100±11.913. The morphology of HD micelles/DOX micelle was observed by TEM. As seen in Figure 2B, the micelle particles were spherical in shape with the diameter within 100 nm, smaller than that measured in DLS (120 nm). It should be noted that TEM analyzes dry sample, whereas DLS analyzes particles in solution under Brownian motion. The mean hydrodynamic diameter by DLS includes several solvent layers, and thus made the particle size larger than that by TEM.31
Generally, the hydrodynamic diameter of individual core–shell-type micelle is a few tens of nanometers. The size of HD micelles in our study is much larger than that, which is probably due to the secondary aggregates of smaller individual micelles.20 In addition, HD micelles carry negative charges that stabilized them from aggregation. The drug EE% and LE% of HD micelles/DOX were 91.277%±2.777% and 2.385%±1.421%, respectively (Table 1). Modification with 7pep did not change the size and pH sensitivity of the HD micelles (Figure S2). DiR-loaded HD micelle was prepared by the same process as HD micelles, and the size and zeta potential were similar to those of HD micelles (data not shown). Furthermore, the CMC of the HD micelle was 5.25 μg/mL (Figure 2D), lower than that of PHIS-PEG2000 micelle (8.32 μg/mL) (Figure 2C), indicating that blending the hydrophobic polymer in the core of PHIS micelle makes it more stable upon dilution in the bloodstream or other biological fluids following dosing.32
The pH sensitivity of the mixed micelles
The pH sensitivity is the key feature of the micelles. To alleviate MDR, the micellar system should be stable at physiological pH and tumor microenvironment (pH: 7.4–6.5), but rapidly dissociate in endosomes (pH: 6.5–5.5) after endocytosis to facilitate drug endosomal escape. In this study, we tailored the stimulating pH by varying the two polymer ratios in the micellar formulations and evaluated it with zeta potential change. Micelle dissociation was attributed to the protonation of the imidazole ring, which can be reflected by the zeta potential reversal from negative to positive. As shown in Figure 3A, the potential of the mixed micelle (with 65% PHIS-PEG2000 in weight) is constant at pH 8–6.5 and exhibited a sharp increase at pH 6.3–5.8 from approximately −4 mV (pH=7.4) to 4 mV (pH=4), indicating a quick dissociation of the micelle system below pH 6.3. On the contrary, the potential of mixed micelles with 50% and 80% PHIS displayed a gradual increase below pH 8.0, proving their instability at pH 8–6.5.
Moreover, the dissociation of polymeric micelles can be monitored by the micropolarity change of the pyrene encapsulated in the micelles, which is reflected by the intensity ratio of the first and the third peaks (I373/I383) in the emission spectra profile.16 As shown in Figure 3B, the HD micelle (with 65% PHIS-PEG2000 in weight) was stable at pH 7.5–6.5 but underwent a sharp dissociation at pH 6.5–4.5, which is constant with the results from the abovementioned zeta potential tests. Alternatively, Figure 3C displayed that the PHIS-PEG2000 micelle without DSPE-PEG2000 underwent a gradual dissociation at pH 7.2, which was not a desirable pH response range. The pH response tests here demonstrated that blending DSPE-PEG2000 to PHIS-PEG2000 micellar formulations could manipulate the triggering pH to a more acid pH. By changing the two polymer ratios in the micellar formulations, the micelle (DSPE-PEG2000:PHIS-PEG2000=35:65, w/w) with a proper stimulating pH (~6.5) for intracellular drug delivery and reversing MDR was obtained and used in the following tests.
In vitro drug release test
The in vitro drug release of DOX-loaded HD micelles mixed micelles (HD micelles/DOX) was tested at pH 5.5 and 7.4. As shown in Figure 3D, <6% of DOX was released from the HD mixed micelles at pH 7.4 over 24 hours. The drug release at pH 5.5 was much faster than that at pH 7.4, and the cumulative DOX release is >30% within the first 4 hours. The result demonstrated that DOX release from their loaded HD mixed micelles was pH-dependent because of disassembly of the micelles at an acid pH. As noted, the micelle barely released the drug at physiological pH, which might allow the loaded drug to maintain in the carriers until reaching the action site. After the micelle was endocytosed by tumor cells, it would exhibit a burst release at pH 5.5–6.0 (in endosomes) and 4.5–5.5 (in lysosomes). Together with the proton sponge effect of protonated PHIS, the micellar system offers the potential to increase drug accumulation and efficacy in MDR tumors as we tested later.
Cellular uptake of the DOX-loaded HD micelles
To elucidate the ability of HD micelles/DOX in reversing drug resistance, we performed the cellular uptake studies on MCF-7/Adr cells (Figure 4A) using flow cytometry. Compared with free DOX, all the DOX-loaded micelles significantly increased the cellular uptake of DOX, in particular with the case of HD65 (containing 65% PHIS in weight), which shows an increment of approximately 2.3-fold over free DOX after 6 hours of incubation. The lesser increment displayed by HD50 and HD80 might be due to the early drug release before endocytosis. The confocal experiments were further performed to assess the cellular uptake and distributions of free DOX and HD65/DOX. Figure 4B shows the images of MCF-7/Adr cells after incubation with 1 μg/mL free DOX or HD micelles/DOX for 6 hours at 37°C. The red fluorescence signal in the picture represents DOX. Compared to the free DOX, the HD micelles/DOX group showed significantly higher intracellular fluorescence level, confirming the results obtained from the abovementioned flow cytometry analysis. The large enhancement of intracellular DOX might attribute to the different intracellular trafficking between DOX-loaded micelle and free DOX. The free DOX passively diffused through cell membranes, and thus, large portion of DOX will be effluxed directly by the membrane-bound transporter P-gp. On the other hand, the DOX-loaded micelles internalized via endocytosis, thereby bypassing the action of P-gp, and thus accumulated high drug levels in cells. The pH-sensitive HD micelles then facilitated the drug endosomal escape, leading to a rapid distribution of DOX throughout the cells, and delivered a large fraction of the drug to their intracellular target before the P-gp efflux could act.13 In other words, the high drug level in the cytoplasm may exceed the efflux ability of P-gp, thus inducing more drug to remain in the cytoplasm to maintain a therapeutic concentration. A drug efflux test was conducted (Figure S3). As expected, the HD micelles did not eliminate the drug efflux compared to the free DOX. Without a continuous cellular uptake, the intracellular DOX level dropped, and the efflux effect became dominant.
Additionally, the cellular uptake with different drug concentrations and incubation times was evaluated. As shown in Figure 4C, the MCF-7/Adr cells were treated with 1 μg/mL free DOX or HD micelles/DOX. The intracellular drug level in the HD micelles/DOX group increased rapidly from 2 to 6 hours, whereas it showed little increment in the DOX group. It was probably due to rapid balancing between the uptake and efflux in the free DOX group. In the HD micelles/DOX group, however, more drug-loaded micelles internalized via endocytosis as time was prolonged. Alternatively, we also examined the cellular uptake of free DOX and HD micelles/DOX at different drug concentrations (Figure 4D). In each concentration, the cellular uptake of HD micelles/DOX was higher than that of DOX. The intracellular drug level in the HD micelles/DOX group displayed a concentration-dependent manner, of which the drug uptake was enhanced by 1.4-fold and 3.3-fold when the concentration was increased from 1 to 2 and 10 μg/mL, respectively. On the other hand, the cellular uptake of free DOX did not change significantly with concentration, especially at low concentrations (1–2 μg/mL), which might be due to the strong efflux action by P-gp. The results suggested that the higher intracellular level of HD micelles/DOX could be obtained with longer incubation times or higher concentrations. PEGylated nanoparticles usually possessed a long in vivo circulation property and gradually accumulated at tumor site. As a result, the time- and concentration-dependent property of the micelle could be an advantage in drug delivery.
Cellular uptake of 7pep-targeted micelles
To realize tumor-targeted delivery and further enhance the anti-drug-resistant effect, a TfR ligand 7pep was modified on the surface of HD micelles. It has been widely proved that TfRs are overexpressed on various types of malignant cells, including the MCF-7/Adr cells used in this study.21,23 Here, we investigated the TfR expression on MCF-7/Adr cells by immunofluorescence (as shown in Figure 5A). The large amount of FITC-labeled secondary antibody (green) on MCF-7/Adr cells confirmed the TfR expression. The negative control did not show any green fluorescence signal.
To evaluate the effect of 7pep modification on cellular uptake of the micelles, flow cytometry analysis analysis and confocal experiments were performed. First, the internalization of 7-pep HD micelles with different modification rates (10%, 30%, 50% and 60%) was examined using flow cytometry. As shown in Figure 5B, the 7-pep HD micelles (60%) exhibited the highest intracellular DOX level among the four tested formulations ranging from 10% to 60% of modification of the HD micelles polymer. Compared to HD micelles, 7-pep HD micelles (60%) significantly enhanced the cellular uptake (1.7-fold). This is consistent with the data of the confocal experiments. As shown in Figure 5C, the red signal representing DOX was increased in the 7-pep HD micelles group than the HD micelles group. Since 7-pep HD micelles possessed similar composition and characteristics to HD micelles except 7pep modification, their difference here obviously resulted from 7pep. To further investigate the role of 7pep modification on cellular uptake, the receptor competitive experiment was performed using flow cytometry. An excess of 7pep (10 μg/mL) was preincubated with cells to saturate the TfR receptors before applying DOX-loaded HD micelles and 7-pep HD micelles. As shown in Figure 6A, with the addition of 7pep, the intracellular level of DOX in the 7-pep HD micelles group was reduced 33%, whereas that in the HD micelles group was not affected. The results indicated that free 7pep and 7-pep HD micelles competed with the same receptor (TfR); thus, 7pep could block the cellular uptake of 7-pep HD micelles.
In total, these results revealed that the 7-pep HD micelles could markedly enhance the DOX accumulation in drug-resistant MCF-7/Adr cells, which was attributed to the specific binding of 7pep with TfRs on the surface of the cells.
Endocytic mechanisms of the micelles
To clarify the endocytic pathway of the targeting and nontargeting micelles, the MCF-7/Adr cells were treated with 7-pep HD micelles/DOX or HD micelles/DOX in the presence of different endocytosis inhibitors, including MβCD and filipin (inhibitors of caveolar-mediated endocytosis); CPZ (inhibitors of clathrin-mediated endocytosis); dynasore (inhibitors of both clathrin- and caveolar-mediated endocytosis); EIPA (inhibitors of macropinocytosis) and CytD (inhibitors of actin polymerization, thus affecting several endocytic mechanisms).33 As shown in Figure 6B, the internalization of 7-pep HD micelles/DOX and HD micelles/DOX was inhibited by CPZ, MβCD, filipin, dynasore and CytD, indicating that both clathrin- and caveolar-mediated pathways were involved in the endocytosis. Macropinocytosis might not participate in the endocytosis, as EIPA did not result in a drop in the intracellular DOX level of neither micelle systems. Importantly, the CPZ inhibition rate increased from 18% (HD micelles/DOX) to 28% (7-pep HD micelles/DOX), indicating that 7pep modification enhanced the clathrin-mediated endocytosis, which is the main pathway of TfR-mediated endocytosis.34 The results here are consistent with the previous report by Du et al, in which 7pep-modified PEG2000-block-poly (ε-caprolactone) (7pep-PEG2000-PCL) micelles were fabricated, and the endocytic mechanism in a human colon carcinoma cell line (Caco-2) was demonstrated as including both unspecific caveolar-mediated pathway and TfR-dependent clathrin-mediated pathway.26 Similar results were also published in their later study, where 7pep-modified DSPE-PEG2000 micelles did not change the endocytic pathways of DSPE-PEG2000 micelles in human breast adenocarcinoma MCF-7 cells but enhanced the clathrin-mediated endocytosis.34 Together with the results in cellular uptake and the receptor competitive experiments, we can conclude that the increased cellular uptake by 7pep modification was due to a TfR-mediated mechanism. In addition, the results also proved that 7-pep HD micelles or HD micelles internalized via endocytosis. It has been widely demonstrated that after endocytosis, nanoparticles are often trapped in endolysosomes and fail to deliver the payloads to their intracellular targets.8 Du et al26 have proved in their study that 7pep-modified PEG2000-PCL micelles were colocalized with lysosomes after endocytosis. As a result, the pH-sensitive 7-pep HD micelles micelles might increase the antitumor efficacy of DOX via PHIS-induced endolysosome escape.
In vitro cytotoxicity assay
Further, we evaluated the antitumor efficacy of DOX-loaded HD micelles and 7-pep HD micelles against MCF-7/Adr tumors using the MTT assays (Figure 6C). As expected, free DOX exhibited very low cytotoxicity, even at a high concentration of 40 μg/mL, with over half amount of the cells being shown viable (40 μg/mL). Both 7-pep HD micelles and HD micelles showed a significantly enhanced cytotoxicity over free DOX, indicating the ability to alleviate the MDR effect. Compared to unmodified HD micelles, 7-pep HD micelles showed a much higher inhibition rate, especially in the low concentration range (0.1 and 0.5 μg/mL), whereas both free DOX and HD micelles/DOX did not yield obvious effects. The remarkable cytotoxicity increment of 7-pep HD micelles might attribute to a synergistic effect of Tf-mediated endocytosis and PHIS-induced endosome escape.
In vivo imaging
To investigate the tumor-targeting effect of the HD micelles and 7-pep HD micelles, in vivo imaging was performed on the female BALB/c nude mice bearing MCF-7/Adr tumors. The near-infrared fluorescent probe DiR was loaded into the HD micelles and 7-pep HD micelles. As shown in Figure 7A, significantly intensified fluorescence was observed in the tumor site of the mice receiving both HD micelles and 7-pep HD micelles, and tumor accumulation of DiR was gradually increased over the time up to 24 hours, which might be attributed to the prolonged circulation time and the EPR effect by the PEGylated micelle surface. Compared to the unmodified HD micelles, the 7-pep HD micelles exhibited much earlier tumor distribution and higher levels of fluorescence intensity at the tumor site at all time points examined. In the ex vivo imaging after 24 hours (Figure 7B), the tumor fluorescence level of 7-pep HD micelles was 2.1-fold higher than HD micelles. These results demonstrated that the 7pep modification enhanced the target delivery of HD micelles to drug-resistant tumors. However, despite an increment in tumor sites, ligand-modified nanoparticles are unavoidably distributed in other organs, especially in liver and spleen.7 The red signal on the back of the mice is mainly the fluorescence in liver, as it can be seen from the ex vivo imaging. The targetability of the nanocarrier is a very important element for tumor therapy, especially for drug-resistant tumor, because an enhanced drug dose is always required, and thus, a targeted delivery appears to be able to alleviate the systematic side effect.
To sum up, as illustrated in Figure 8, the pH-sensitive 7-pep HD micelles induced higher intracellular DOX level and diminution in drug resistance, which might be attributed to the synergistic effect of PHIS-triggered endolysosome escape and TfR-mediated endocytosis. Moreover, the 7pep modification enhanced the target delivery of HD micelles to drug-resistant tumors in vivo, probably due to the TfR-mediated specific binding and internalization on MCF-7/Adr tumors.
Conclusion
In this study, we prepared 7pep-modified PHIS-PEG2000 and DSPE-PEG2000 mixed micelles loaded with DOX for the treatment of MDR breast tumor. The mixed micelles were ~100 nm with good size uniformity and negative zeta potential. The pH response range was specially tailored to the endosome/lysosome pH (~6.5), and the micelles exhibited a large increment of intracellular drug level and antitumor efficacy in MCF-7/Adr cells. By further modifying the HD mixed micelles with 7pep, the cellular accumulation of DOX was further enhanced via TfR-mediated endocytosis of these micelles. Most importantly, a targeted delivery of the 7-pep HD micelles to MDR tumors was achieved in vivo, due to the overexpression of TfR in MDR tumors. Overall, this study provided a biodegradable micellar system incorporated with the properties of anti-MDR and targetable delivery, which might offer great potential to be used as a drug carrier in the treatment of MDR tumors.
Acknowledgment
This study was supported by the National Science Foundation of China Grant 81402857 and by the National Science Foundation of Tianjin City (Grant No 15JCZDJC36300). This project is partially supported by the National Science Foundation of China A3 program (Grant 813611403), of which Dr Wei Gao is a participating faculty member.
Disclosure
The authors report no conflicts of interest in this work.
References
Kathawala RJ, Gupta P, Ashby CR Jr, Chen ZS. The modulation of ABC transporter-mediated multidrug resistance in cancer: a review of the past decade. Drug Resist Updat. 2015;18:1–17. | ||
Montanari F, Ecker GF. Prediction of drug-ABC-transporter interaction – recent advances and future challenges. Adv Drug Deliv Rev. 2015;86:17–26. | ||
Yan Y, Bjornmalm M, Caruso F. Particle carriers for combating multidrug-resistant cancer. ACS Nano. 2013;7(11):9512–9517. | ||
Dawar S, Singh N, Kanwar RK, et al. Multifunctional and multitargeted nanoparticles for drug delivery to overcome barriers of drug resistance in human cancers. Drug Discov Today. 2013;18(23–24):1292–1300. | ||
Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Deliv Rev. 2014;66:2–25. | ||
Fernandez-Fernandez A, Manchanda R, McGoron AJ. Theranostic applications of nanomaterials in cancer: drug delivery, image-guided therapy, and multifunctional platforms. Appl Biochem Biotechnol. 2011;165(7–8):1628–1651. | ||
Wilhelm S, Tavares AJ, Dai Q, et al. Analysis of nanoparticle delivery to tumours. Nat Rev Mater. 2016;1(5):16014. | ||
Yin Q, Shen J, Zhang Z, Yu H, Li Y. Reversal of multidrug resistance by stimuli-responsive drug delivery systems for therapy of tumor. Adv Drug Deliv Rev. 2013;65(13–14):1699–1715. | ||
Zhang X, Chen D, Ba S, et al. Poly(l-histidine) based triblock copolymers: pH induced reassembly of copolymer micelles and mechanism underlying endolysosomal escape for intracellular delivery. Biomacromolecules. 2014;15(11):4032–4045. | ||
Qiu L, Li Z, Qiao M, et al. Self-assembled pH-responsive hyaluronic acid-g-poly((L)-histidine) copolymer micelles for targeted intracellular delivery of doxorubicin. Acta Biomater. 2014;10(5):2024–2035. | ||
Li X, Yang X, Lin Z, et al. A folate modified pH sensitive targeted polymeric micelle alleviated systemic toxicity of doxorubicin (DOX) in multi-drug resistant tumor bearing mice. Eur J Pharm Sci. 2015;76:95–101. | ||
Lee ES, Na K, Bae YH. Doxorubicin loaded pH-sensitive polymeric micelles for reversal of resistant MCF-7 tumor. J Control Release. 2005;103(2):405–418. | ||
Mohajer G, Lee ES, Bae YH. Enhanced intercellular retention activity of novel pH-sensitive polymeric micelles in wild and multidrug resistant MCF-7 cells. Pharm Res. 2007;24(9):1618–1627. | ||
Chen S, Yang K, Tuguntaev RG, et al. Targeting tumor microenvironment with PEG-based amphiphilic nanoparticles to overcome chemoresistance. Nanomedicine. 2016;12(2):269–286. | ||
Johnson RP, Jeong YI, John JV, et al. Dual stimuli-responsive poly(N-isopropylacrylamide)-b-poly(L-histidine) chimeric materials for the controlled delivery of doxorubicin into liver carcinoma. Biomacromolecules. 2013;14(5):1434–1443. | ||
Lee ES, Shin HJ, Na K, Bae YH. Poly(L-histidine)-PEG block copolymer micelles and pH-induced destabilization. J Control Release. 2003;90(3):363–374. | ||
Zhao Y, Zhou Y, Wang D, et al. pH-responsive polymeric micelles based on poly(2-ethyl-2-oxazoline)-poly(D,L-lactide) for tumor-targeting and controlled delivery of doxorubicin and P-glycoprotein inhibitor. Acta Biomater. 2015;17:182–192. | ||
Yin H, Lee ES, Kim D, Lee KH, Oh KT, Bae YH. Physicochemical characteristics of pH-sensitive poly(L-histidine)-b-poly(ethylene glycol)/poly(L-lactide)-b-poly(ethylene glycol) mixed micelles. J Control Release. 2008;126(2):130–138. | ||
Fang X, Yang T, Wang L, et al. Nano-cage-mediated refolding of insulin by PEG-PE micelle. Biomaterials. 2016;77:139–148. | ||
Wu H, Zhu L, Torchilin VP. pH-sensitive poly(histidine)-PEG/DSPE-PEG co-polymer micelles for cytosolic drug delivery. Biomaterials. 2013;34(4):1213–1222. | ||
Dufes C, Al Robaian M, Somani S. Transferrin and the transferrin receptor for the targeted delivery of therapeutic agents to the brain and cancer cells. Ther Deliv. 2013;4(5):629–640. | ||
Xu F, Yang T, Fang D, Xu Q, Chen Y. An investigation of heat shock protein 27 and P-glycoprotein mediated multi-drug resistance in breast cancer using liquid chromatography-tandem mass spectrometry-based targeted proteomics. J Proteomics. 2014;108:188–197. | ||
Sheng Y, Xu J, You Y, Xu F, Chen Y. Acid-sensitive peptide-conjugated doxorubicin mediates the lysosomal pathway of apoptosis and reverses drug resistance in breast cancer. Mol Pharm. 2015;12(7):2217–2228. | ||
Brammer LA, Bolduc B, Kass JL, Felice KM, Noren CJ, Hall MF. A target-unrelated peptide in an M13 phage display library traced to an advantageous mutation in the gene II ribosome-binding site. Anal Biochem. 2008;373(1):88–98. | ||
Nogueira-Librelotto DR, Codevilla CF, Farooqi A, Rolim CM. Transferrin-conjugated nanocarriers as active-targeted drug delivery platforms for cancer therapy. Curr Pharm Des. Epub 2016 Oct 26. | ||
Du W, Fan Y, Zheng N, et al. Transferrin receptor specific nanocarriers conjugated with functional 7peptide for oral drug delivery. Biomaterials. 2013;34(3):794–806. | ||
Gao LY, Liu XY, Chen CJ, et al. Core-shell type lipid/rPAA-Chol polymer hybrid nanoparticles for in vivo siRNA delivery. Biomaterials. 2014;35(6):2066–2078. | ||
Arola OJ, Saraste A, Pulkki K, Kallajoki M, Parvinen M, Voipio-Pulkki LM. Acute doxorubicin cardiotoxicity involves cardiomyocyte apoptosis. Cancer Res. 2000;60(7):1789–1792. | ||
Mandal B, Bhattacharjee H, Mittal N, et al. Core-shell-type lipid-polymer hybrid nanoparticles as a drug delivery platform. Nanomedicine. 2013;9(4):474–491. | ||
Cai D, Gao W, He B, et al. Hydrophobic penetrating peptide PFVYLI-modified stealth liposomes for doxorubicin delivery in breast cancer therapy. Biomaterials. 2014;35(7):2283–2294. | ||
Adelantado C, Rodriguez-Farinas N, Rodriguez Martin-Doimeadios RC, Zougagh M, Rios A. Analysis of silica nanoparticles by capillary electrophoresis coupled to an evaporative light scattering detector. Anal Chim Acta. 2016;923:82–88. | ||
Trivedi R, Kompella UB. Nanomicellar formulations for sustained drug delivery: strategies and underlying principles. Nanomedicine (Lond). 2010;5(3):485–505. | ||
Bae YM, Park YI, Nam SH, et al. Endocytosis, intracellular transport, and exocytosis of lanthanide-doped upconverting nanoparticles in single living cells. Biomaterials. 2012;33(35):9080–9086. | ||
Du W, Fan Y, He B, et al. Bionano interactions of mcf-7 breast tumor cells with a transferrin receptor targeted nanoparticle. Mol Pharm. 2015;12(5):1467–1476. |
Supplementary materials
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.