Back to Journals » Cancer Management and Research » Volume 10
Transcatheter arterial chemoembolization monotherapy vs combined transcatheter arterial chemoembolization–percutaneous microwave coagulation therapy for massive hepatocellular carcinoma (≥10 cm)
Authors Wei Y , Dai F, Zhao T, Tao C, Wang L, Ye W , Zhao W
Received 26 April 2018
Accepted for publication 30 July 2018
Published 1 November 2018 Volume 2018:10 Pages 5273—5282
DOI https://doi.org/10.2147/CMAR.S172395
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kenan Onel
Video abstract presented by Yanyan Wei.
Views: 248
Yanyan Wei,* Feng Dai,* Tianhui Zhao, Chen Tao, Lili Wang, Wei Ye, Wei Zhao
Liver Disease Department, The Second Hospital of Nanjing, Medical School of Southeast University, Nanjing, China
*These authors contributed equally to this work
Background: The prognosis of massive hepatocellular carcinomas (MHCCs; ≥10 cm) remains worse.
Purpose: The aim of this study was to evaluate the clinical benefits of transcatheter arterial chemoembolization (TACE) or TACE combined with percutaneous microwave coagulation therapy (PMCT) and the long-term survival rate of MHCC patients treated with these techniques.
Patients and methods: A retrospective study was performed using data involving 102 MHCC patients admitted to the Second Hospital of Nanjing from September 2010 to August 2015. The median interval between treatments and overall survival (OS) was hierarchically analyzed using log-rank tests. Multivariate analysis was done using Cox regression model analysis.
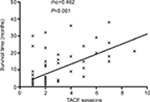
Results: The median survival time of MHCC patients was 3 months (range, 1–10 months) in the palliative group, 3 months (range, 1–39 months) in the TACE group, and 7.5 months (range, 3–30 months) in the TACE–PMCT group (P=0.038). The 6-, 12-, and 18-month OS rates for MHCC patients were 15%, 0%, and 0% in the palliative group, 30%, 25.63%, and 17.97% in the TACE group, and 50%, 41.67%, and 16.67% in the TACE–PMCT group, respectively (P=0.0467). In addition, TACE sessions had positive correlation with the survival time of MHCC patients (rho = 0.462, P<0.001). TACE treatment more than three times (HR =0.145, P<0.001) was an independent predictor of the survival of MHCC patients, which was identified by the Cox regression model analysis.
Conclusions: These results indicated that TACE–PMCT treatment in MHCC patients had advantages in prolonging OS and improving liver function. Multiple TACE treatments might be a suitable treatment for the MHCC patients.
Keywords: massive hepatocellular carcinoma, transcatheter arterial chemoembolization, TACE, percutaneous microwave coagulation therapy, PMCT
Introduction
Hepatocellular carcinoma (HCC) is one of the five most common causes of cancer-associated death worldwide. It is also an aggressive malignancy with poor prognosis.1 First of all, surgical resection is an effective treatment for a solitary lesion without vascular invasion and with sufficient liver function reserve in HCC patients.2 However, due to large tumor lesions, main blood vessels, including the portal vein, the hepatic artery and the vena cava are often infiltrated in patients with massive HCC (MHCC).3,4 In addition, most MHCC patients suffer from cirrhosis or liver dysfunction, which may also lead to difficulties in surgical intervention.5 Even if surgical intervention is performed, MHCC patients might still have poor prognosis.6–8 Second, due to large tumor lesions and poor radiation tolerance of normal liver tissue, the curative effect of radiotherapy is also limited in MHCC patients.9 Third, although capecitabine plus oxaliplatin regimen10 and gemcitabine plus oxaliplatin regimen11 could be safely administered with close monitoring and have moderate antitumor activity in patients with advanced HCC, they remain to be further investigated in MHCC patients.12 In summary, although the abovementioned treatment is limited in MHCC patients, it is necessary to further explore the appropriate regimen therapy to prolong the survival time of MHCC patients and improve their quality of life.
Previous studies have demonstrated that interventional treatments such as transcatheter arterial chemoembolization (TACE) monotherapy or combined therapies could improve unresectable HCC patient prognosis.13–16 In addition, TACE is recommended as the standard of care for unresectable HCC at Barcelona Clinic Liver Cancer (BCLC) stage A–B.17,18 Percutaneous microwave coagulation therapy (PMCT) is a minimally invasive technique. PMCT produces high temperature by electrodes inserted into tumor tissue, which can lead to rapid coagulation and necrosis of tumor tissue, so as to achieve the goal of eliminating tumor.5,20 This method gradually became one of the most important treatments for HCC.21,22 Importantly, TACE could reduce the cooling effect of hepatic blood flow on microwave thermal coagulation by blocking the tumor vascular bed.23 Therefore, TACE is expected to play a vital role in promoting tumor damage and improving the ability of PMCT to kill the tumor tissue in situ. To sum up, in theory, the therapeutic effect of TACE combined with PMCT on MHCC is better than that of TACE alone. However, in clinical practice, TACE combined with PMCT could prolong survival time and improve prognosis of MHCC patients. To the best of our knowledge, the benefits of TACE combined with PMCT for MHCC patients have not been well explored. In addition, the effect of TACE sessions on the prognosis of MHCC was unclear. Therefore, by comparing the efficacy and safety of TACE–PMCT treatment with TACE monotherapy in MHCC patients, the therapeutic regimen and TACE sessions suitable for MHCC patients will be elucidated in the present study. Importantly, treatment programs for 102 MHCC patients were determined through the BCLC proposal and patients’ informed consent.24,25 Therefore, to reach the purpose of the abovementioned study, our study attempted to explore the predictive factors for MHCC patients through comprehensive retrospective analysis of medical history, imaging features, and laboratory results.
Patients and methods
Patient data
Patients
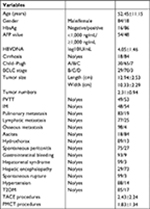
According to the inclusion and exclusion criteria of this study, 102 patients were enrolled including 84 males (82.4%) and 18 females (17.6%), aged 24–78 years, with a mean age of 52.45±11.15 years.
The inclusion criteria for the study population were as follows: 1) patients who were diagnosed with HCC according to the standards for the diagnosis and treatment of primary liver cancer established by the Ministry of Health of the People’s Republic of China by contrast imaging (computed tomography [CT] or magnetic resonance imaging [MRI] or contrast-enhanced ultrasonography [CEUS], and hepatic angiography) with positive tumor markers in combination with liver cirrhosis or tissue biopsy;26 2) patients who had MHCC with the largest diameter tumor of at least 10 cm (mHCC); 3) patients who had no history of hepatic encephalopathy above third degree or ascites refractory to diuretics; 4) patients who had controlled diabetes mellitus and hypertension using medication; and 5) patients who had no multiple organ failure and no severe underlying diseases.
Patients were excluded from this study if they: 1) received other treatments in another hospital; 2) had missing data; 3) were not tracked adequately; 4) underwent liver transplantation and received previous hepatic interventional or surgical treatments; 5) had diffuse-type HCC; 6) previously received any other local invasive therapies, such as radio frequency ablation (RFA), 125I seed implantation, or percutaneous ethanol injection (PEI); and 7) had another type of malignant tumor.
Collection of data and primary end point assessment
Clinical data were collected from each patient at the time of MHCC diagnosis including gender, age, other chronic diseases (eg, hypertension and type 2 diabetes mellitus [T2DM]), Child–Pugh grade, and BCLC stage. Imaging features were also collected including tumor size, tumor number, cirrhosis, portal vein tumor thrombus (PVTT), intrahepatic metastasis (IM), and extrahepatic metastasis (EM). All laboratory indicators were collected in the week before surgery. Laboratory results determined the alpha-fetoprotein (AFP), hepatitis B surface antigen (HbsAg), and hepatitis C virus antibody (HCV-Ab) levels. The main end point was survival time, which was defined as the duration from the time of primary treatment for MHCC to death or to August 2016, whichever was earlier. The secondary end point was outcomes during follow-up, including survival and death.
Treatments and follow-up
Eighty-one patients were initially treated with TACE. Hepatic artery angiography was performed using the Seldinger technique. TACE was performed using the following procedures. After using 5-French catheter selection to perform arteriography of the superior mesenteric, celiac, and common hepatic arteries, the hepatic artery was catheterized with a coaxial microcatheter. After the microcatheter was positioned into or as close as possible to the tumor-feeding branch, an emulsion of doxorubicin hydrochloride (Adriamycin) and iodized oil (lipiodol; Guerbet, Aulnay-sous-Bois, France) was slowly infused through the catheter. Oily TACE was performed as selectively as possible, and a microcatheter was routinely used. The doses of iodized oil and doxorubicin were determined according to the tumor size and tumor vascularity. The maximum doses of iodized oil and doxorubicin for a single session of TACE were 25 mL and 40 mg, respectively. Infusion of the lipiodol mixture was followed by particulate embolization with 300–500 µm-diameter Embosphere Microspheres (Merit Medical Systems, Inc., Rockland, MA, USA).
PMCT was initiated 1–3 weeks after TACE. PMCT was performed using the KY-2000 microwave therapy instrument (HengDa Electronic Co., Ltd., Xuzhou, China). The PMCT procedure was performed by an experienced hepatobiliary surgeon after local anesthesia using 2% lidocaine. The entire procedure was guided and constantly monitored using real-time ultrasound (MyLabTwice; Esaote Co., Ltd., Genoa, Italy). After anesthesia was achieved, a 15 cm 16 G electrode needle was inserted into the center of the nodule, and coagulation therapy was performed at 2,450 MHz with 60–80 W output for 8–20 minutes per ablation. The ablation was performed repeatedly until the tumors attained complete necrosis as monitored by real-time ultrasound, and the hyperechoic area overlapped the area of the tumor with a surrounding ≥1 cm safety margin. To relieve pain in the patients, dolantin (100 mg) was injected intramuscularly 5 minutes before the PMCT.
After treatment with TACE and PMCT, liver protection, anti-inflammatory, and sedation therapies were prescribed. A follow-up study by repeat CT (plain and enhanced) and serum AFP-level measurement was conducted once every 1–2 months.
Statistical analyses
The Kruskal–Wallis test was performed to analyze continuous variables, and the results were expressed as mean ± SD for normal distribution and M (Q1–Q3) for skewed distribution. For categorical variables, the chi-squared test and Fisher’s exact test were utilized. Survival curves were estimated using the Kaplan–Meier method and compared using the log-rank test. Univariate analysis was performed by the Cox regression model. Multivariate analysis was carried out using the Cox proportional hazard model to generate adjusted HRs and 95% CIs. Any value of P<0.05 was considered statistically significant. All statistical analyses were performed using the SPSS 22.0 software (IBM Corporation, Armonk, NY, USA).
Ethical approval
All procedures in the current study were in accordance with the ethical standards of the institutional research committee and with the Declaration of Helsinki. The study was approved by the ethics committee of the Second Hospital of Nanjing, and the written informed consent for participation was obtained. This study had no influence on the subsequent management of patients.
Results
General condition
Eighty-one patients received 197 TACE treatments (mean, 2.4; range, 1–14) and 22 PMCT sessions (mean, 1.8; range, 1–5). In the TACE group, 69 patients received 166 TACE treatments (average, 2.4; range, 1–14). In the TACE–PMCT group, 12 patients received 31 TACE treatments (mean, 2.5; range, 1–7) and 22 PMCT sessions (mean, 1.8; range, 1–5). The median follow-up time was 41 months (range, 6–96 months).
The sample was predominantly males (82.4%), full grown adults with a long period of hepatitis B virus (HBV) infection (84.3%). Most patients (60.47%) received antiviral therapy. In the present study, the majority of the patients had cirrhosis (82.4%) and 48 patients (47.1%) had AFP levels more than 1,000 ng/mL. Most patients (63.7%) had Child–Pugh B functional status, whereas 30 patients (29.4%) and seven patients (6.9%) had Child–Pugh A and C functional status, respectively. Most patients (68.6%) had tumors classified as BCLC stage C, while 29 patients (28.4%) and three patients (2.9%) had tumors classified as BCLC stage B and D, respectively. The mean number of tumors was 2.31±0.94 (range 1–3), the mean tumor length was 12.94±2.53 cm (range 10–23.2 cm), and the mean tumor width was 10.33±2.29 cm (range 3.5–19.1 cm). In these 102 patients with MHCC, the detailed proportion of all patients with complications or other chronic diseases is summarized in Table 1.
General characteristics of subjects in the three groups
According to the BCLC proposal and the patients’ informed consent, a total of 102 patients were included in this study. Twenty-one patients were treated with palliative treatment. Sixty-nine patients were treated with TACE alone, and 12 patients were treated with TACE–PMCT. The demographic and clinicopathological characteristics of the three groups are summarized in Table 2. The mean survival time of MHCC patients in the TACE–PMCT group was significantly longer than the other two groups, but the incidence of hydrothorax in TACE–PMCT was higher than the other two groups (P=0.038, P=0.022). Other laboratory and imaging parameters were not significantly different among the three groups including AFP levels, tumor length, tumor width, cirrhosis, PVTT, IM, EM, and complications except for hydrothorax (all of them, P>0.05). Gender, Child–Pugh grade, BCLC stage, antiviral therapy timing, hypertension, and T2DM were also not significantly different among the three groups (all of them, P>0.05).
Relationship between survival time and local invasive treatment strategies
There were no fatal treatment-related complications for MHCC patients. This fact indicates that the local invasive treatments for MHCC patients receiving TACE or PMCT were safe in the short term. The median survival time was 3 months (range, 1–10 months) in the palliative group, 3 months (range, 1–39 months) in the TACE group, and 7.5 months (range, 3–30 months) in the TACE–PMCT group. The results showed that there were significant differences in the survival time of MHCC patients among the three groups (P=0.038). In addition, Figure 1 shows that the 6-, 12-, and 18-month survival rates for MHCC patients were 15%, 0%, and 0% in the palliative group, 30%, 25.63%, and 17.97% in the TACE group, and 50%, 41.67%, and 16.67% in the TACE–PMCT group, respectively. These results indicate significant differences among the three groups in OS rate.
In addition, there were 53 patients with PVTT. Of these patients, eleven patients received palliative treatment, 36 patients received TACE treatment, and six patients received TACE–PMCT treatment. The median survival time of these patients with PVTT was 2 months (range, 2–5 months) in the palliative group, 3 months (range, 2–13.5 months) in the TACE group, 6 months (range, 3–21 months) in the TACE–PMCT group. Although this result did not reach statistical significance (P=0.180), a trend was suggested that MHCC patients with PVTT receiving TACE–PMCT treatment had a longer survival time. Therefore, TACE–PMCT treatment was effective and safe for MHCC patients, even for those patients with PVTT.
Clinical features of MHCC patients based on the number of TACE treatments
According to previous studies, TACE with lipiodol and gelatin sponge is highly effective for MHCC.23,27 However, it has not been confirmed whether the TACE treatments could impact on the survival time and OS rate in MHCC patients. In this study, the proportion of patients receiving TACE treatment was the highest, including the TACE group and TACE–PMCT group. Figure 2 shows that the number of TACE treatments was positively correlated with the survival time of MHCC patients in the TACE group and TACE–PMCT group. Next, TACE sessions were divided into two groups: TACE treatments more than three times group (n=66) and TACE treatments less than or equal to three times group (n=15). Then, we further studied the factors affecting the prognosis of MHCC.
The data showed that the median survival time of MHCC patients was 3 months (range, 1–32 months) in the TACE treatments less than or equal to three times group and 20 months (range, 6–39) in the TACE treatments more than three times group. Therefore, patients in the TACE treatments more than three times group had significantly longer survival time than those in the TACE treatments less than or equal to three times group. Likewise, the data showed that HBV-DNA levels in the TACE treatments more than three times group were higher than those in the TACE treatments less than or equal to three times group (P=0.04). However, other parameters including HBsAg, AFP levels, Child–Pugh grade, BCLC stage, IM, EM, complications, and comorbidities had no significant differences between the two groups. Moreover, there were also no significant differences in patients’ age, gender, tumor numbers and size, and cirrhosis between the two groups. The detailed comparison of demographic and clinical characteristics of patients based on TACE sessions is summarized in Table 3.
To sum up, the survival time of MHCC patients in the TACE treatments more than three times group was longer than those in the TACE treatments less than or equal to three times group. In addition, HBV-DNA levels of MHCC patients in the TACE treatments more than three times group were higher than those in the TACE treatments less than or equal to three times group. Therefore, we further studied the risk factors associated with the survival of MHCC.
Risk factors associated with the survival of MHCC by subgroup analyses
The Kaplan–Meier survival analyses were used to analyze the cumulative survival rate among the subgroups. Figure 1 shows that the 6-, 12-, and 18-month cumulative survival rates for MHCC patients treated with the TACE–PMCT were higher than those treated with TACE and palliative treatment. In addition, Figure 3 shows that the 6-, 12-, and 18-month cumulative survival rates for MHCC patients who received TACE treatments more than three times were higher than those who received TACE treatments less than or equal to three times (93.33%, 79.44%, 63.56% vs 19.54%, 12.45%, 6.23%, log-rank P<0.001).
Results of univariate survival analysis demonstrated that TACE treatments more than three times were a prognostic predictor. TACE treatments more than three times were associated with better OS rate. Other prognostic variables are presented in Table 3. Of these, non-spontaneous peritonitis, non-pulmonary metastasis, non-hepatic encephalopathy, TACE–PMCT vs palliative treatment, and TACE vs palliative treatment were related to better OS rate.
To eliminate the confounding factors, Cox proportional hazard model was used to evaluate the risk factors for the survival of MHCC patients. Variables included in the analysis were local invasive treatment, the number of TACE treatments, spontaneous peritonitis, pulmonary metastasis, and hepatic encephalopathy. Table 4 summarizes that TACE treatments more than three times were independent protective factors for the survival of MHCC patients, namely palliative treatment and TACE treatments less than or equal to three times were risk factors for the survival of MHCC patients.
Discussion
HCC is a frequent cancer that is among the leading causes of cancer deaths worldwide.28 Most cases were detected until tumor progressed to large mass or to multiple lesions, at which point surgery was no longer a suitable treatment option.29 MHCCs were defined as those with a maximum tumor diameter of ≥10 cm.3,30 For patients with large, unresectable tumor lesions, TACE was recommended as the suitable therapeutic option.12 However, most of the prognosis was unsatisfactory.9,12,13,19 The mechanism of PMCT for HCC is based on the heating effect of microwaves and the sensitivity of the tumor to the heating action,31 but the “heat sinking” effect limited the range of PMCT.23 TACE could alleviate the adverse cooling effect induced by abundant blood flow on microwave thermal coagulation by blocking the tumor vascular bed.20,23 Seki et al32 reported that the combination of TACE and PMCT might result in higher rates of necrosis and longer survival times compared to treatment with TACE or PMCT alone and improve the prognosis of HCC patients. Up to date, there are limited studies on the efficacy and safety of MHCC patients treated with TACE plus PMCT. In the present study, we mainly compared the effects of TACE–PMCT treatment with TACE treatment on the prognosis of MHCC patients.
It is well known that HCC possesses an abundant blood supply, especially in MHCC patients. TACE can obstruct the artery supply of the tumor through the hepatic artery. The iodine oil can fill up the portal vein surrounding the tumor and decrease the blood flow volume of the portal vein.15–18 PMCT produces high temperature by electrodes inserted into tumor tissue, which can lead to rapid coagulation and necrosis of tumor tissue, so as to achieve the goal of eliminating tumor.5,20 In addition, TACE can lead to ischemia and inflammatory edema of the tumor tissue, which could accelerate tumor necrosis and enhance the coagulation effect of microwaves.16 This provides a theoretical basis for the combination of TACE and PMCT treatment for MHCC patients. In the present study, it was found that there were no fatal treatment-related complications for patients. It is indicated that MHCC patients who received local invasive treatments were safe in the short term. In addition, survival time of MHCC patients treated with TACE–PMCT was significantly longer than those treated with TACE and palliative. Importantly, the 6-, 12-, and 18-month OS rates for MHCC patients in the TACE–PMCT group were also higher than those in the TACE group and palliative group. Therefore, TACE–PMCT treatment was a safe and preferable treatment. MHCC patients receiving TACE–PMCT treatment could significantly prolong the survival time and improve the OS rates.
According to some studies, the complete necrosis rate of TACE only reaches 10–20% for HCC patients.32 TACE can reduce tumor volume, induce tumor necrosis, and prevent tumor dissemination. However, a large area of chemoembolization may cause liver function impairment and even liver function failure.12,32 Therefore, multiple TACE procedures might be appropriate. By analyzing the survival time and the OS rate, it was found that MHCC patients who received TACE treatments more than three times had longer survival time and better OS rate than those who received TACE treatments less than or equal to three times. Herein, the larger tumor size could be controlled by repeated TACE treatment, and liver function could keep stable. Likewise, IMs or multicenter lesions could be controlled more easily by repeated PMCT in the absence of tumor-feeding vessels caused by the repeated TACE treatment. Therefore, liver dysfunction was decreased by the combination of TACE and PMCT treatment or repeated TACE treatment, making this therapy preferable to radical treatments.
Serum AFP levels were an important tumor biomarker of HCC. A few decades ago, Nomura et al33 and Tangkijvanich et al34 suggested that serum AFP levels could be used as an indicator to assess the clinical features and prognosis of HCC. Then, Choi et al35 further demonstrated that serum AFP levels prior to RFA were important predictors of long-term outcomes in HCC. Afterward, Carr et al36 reported that elevated AFP levels are associated with worse survival of patients with large tumors. More recent studies by Blank et al37 and Terentiev and Moldogazieva38 reported that preoperative serum AFP was an independent predictive factor among HBV–HCC patients following surgical resection. However, in our study, higher preoperative AFP level was not identified as an independent risk factor for the survival of MHCC patients. The reason for the different results may be due to different treatment modalities and the setting of AFP subgroup boundaries. In our study, serum AFP levels more than 1,000 ng/mL accounted for over a half of total MHCC patients. In addition, it is well known that the survival time of MHCC patients was significantly shorter than HCC patients with small or large tumor lesions. Therefore, the massive volume of tumor lesions accounts for crucial role in the prognosis of MHCC patients.
In general, MHCC patients treated with PMCT had mild adverse reactions. During and after treatment, local pain was common and tolerable. Subcapsular hematoma, internal bleeding, biliary duct damage, and so on were infrequent. However, hydrothorax and peritonitis were common complications caused by PMCT.20 In this study, the higher incidence of hydrothorax in the TACE–PMCT group was found compared to other two groups, but hydrothorax can be resolved by applying albumin and diuretic. In addition, pulmonary metastasis, peritonitis, and hepatic encephalopathy were associated with the prognosis of MHCC patients by univariate analysis. However, it was confirmed that the tumor characteristics, complications, IM, EM, and comorbidities were not associated with the prognosis of MHCC patients by multivariate analysis in this study. These results were different to other previous studies on other types of HCC.5,39–43 These results have not been reported in MHCC patients. The main reason might be associated with the features of MHCC including larger tumor volume, higher incidence of PVTT, and metastases.3,30
Limitations
Although some valuable and important conclusions have been obtained, there are still some limitations in this study. The first limitation of the present study was its retrospective nature. The data were collected retrospectively from the patient’s information system. However, there was one among-group difference in baseline characteristics, including the presence of hydrothorax. Moreover, due to this retrospective nature, a mild complication, for example, pain and low-grade fever, that did not need further treatment or was successfully treated with painkillers or physical hypothermia, may not have been documented in the patient’s information system and was therefore not traceable in a retrospective manner. The second limitation was that the selection of treatment modality inevitably depended not only on medical but also on nonmedical and/or economic considerations. However, no significant differences were found in the baseline characteristics among the three groups. Moreover, the patients in the three groups were treated strictly according to the study protocol. The third limitation was that the inclusion period of this study had a wide range from 2010 to 2015. In this period, diagnostic and treatment protocols might have been changed due to new insights and developments. However, subsequent treatments of the three groups were performed according to the same multidisciplinary treatment protocol by the same team.
Conclusion
TACE following PMCT is a safe and efficient treatment strategy in MHCC patients. In addition, multiple TACE treatments should be considered as an alternative treatment therapies for the unresectable MHCC. However, the results of this retrospective study need to amplify the sample to identify the benefits from TACE–PMCT treatments and be validated by prospective clinical trial.
Acknowledgments
This study was partially supported by grants from the Science and Technology Commission of Nanjing (no. 201605033 to Wei Ye), the Project of Six Talent Peaks of Jiangsu Province (no. WSN-177 to Wei Ye), the Project of Jiangsu Provincial Medical Youth Talent (Wei Ye), Nanjing Medical Science and Technology Development Foundation (no. YKK-17173 to Wei Ye), Medical Science and Technology Development Foundation, and Nanjing Department of Health (no. YKK-16192 to Lili Wang).
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Wang X, Wang Z, Wu L. Combined measurements of tumor number and size helps estimate the outcome of resection of Barcelona clinic liver cancer stage B hepatocellular carcinoma. BMC Surg. 2016;16:22. | ||
Aitken KL, Hawkins MA. The role of radiotherapy and chemoradiation in the management of primary liver tumours. Clin Oncol. 2014;26(9):569–580. | ||
Carr BI, Guerra V. Features of massive hepatocellular carcinomas. Eur J Gastroenterol Hepatol. 2014;26(1):101–108. | ||
Vauthey JN, Lauwers GY, Esnaola NF, et al. Simplified staging for hepatocellular carcinoma. J Clin Oncol. 2002;20(6):1527–1536. | ||
Klein J, Dawson LA. Hepatocellular carcinoma radiation therapy: review of evidence and future opportunities. Int J Radiat Oncol Biol Phys. 2013;87(1):22–32. | ||
Carr BI, Guerra V. Features of massive hepatocellular carcinomas. Eur J Gastroenterol Hepatol. 2014;26(1):101–108. | ||
Chang YJ, Chung KP, Chang YJ, Chen LJ. Long-term survival of patients undergoing liver resection for very large hepatocellular carcinomas. Br J Surg. 2016;103(11):1513–1520. | ||
Giuliante F, de Rose AM, Guerra V, Ardito F, Nuzzo G, Carr BI. Clinical characteristics and survival of European patients with resectable large hepatocellular carcinomas. J Gastrointest Cancer. 2013;44(3):329–335. | ||
Zeng ZC, Tang ZY, Fan J, et al. A comparison of chemoembolization combination with and without radiotherapy for unresectable hepatocellular carcinoma. Cancer J. 2004;10(5):307–316. | ||
Boige V, Raoul JL, Pignon JP, et al. Multicentre phase II trial of capecitabine plus oxaliplatin (XELOX) in patients with advanced hepatocellular carcinoma: FFCD 03-03 trial. Br J Cancer. 2007;97(7):862–867. | ||
Zhu AX, Blaszkowsky LS, Ryan DP, et al. Phase II study of gemcitabine and oxaliplatin in combination with bevacizumab in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24(12):1898–1903. | ||
Lencioni R. Chemoembolization in patients with hepatocellular carcinoma. Liver Cancer. 2012;1(1):41–50. | ||
Murata S, Mine T, Sugihara F, et al. Interventional treatment for unresectable hepatocellular carcinoma. World J Gastroenterol. 2014;20(37):13453–13465. | ||
Ray CE, Brown AC, Green TJ, et al. Survival outcomes in patients with advanced hepatocellular carcinoma treated with drug-eluting bead chemoembolization. Am J Roentgenol. 2015;204(2):440–447. | ||
de Stefano G, Iodice V, Signoriello G, Scognamiglio U, Farella N. P1325: Efficacy and safety of combined sequential treatment with RFA and sorafenib in patients with HCC in intermediate stage ineligible for TACE: A prospective randomized open study. J Hepatol. 2015;62:S852–S852. | ||
Veltri A, Moretto P, Doriguzzi A, Pagano E, Carrara G, Gandini G. Radiofrequency thermal ablation (RFA) after transarterial chemoembolization (TACE) as a combined therapy for unresectable non-early hepatocellular carcinoma (HCC). Eur Radiol. 2006;16(3):661–669. | ||
Kadalayil L, Benini R, Pallan L, et al. A simple prognostic scoring system for patients receiving transarterial embolisation for hepatocellular cancer. Ann Oncol. 2013;24(10):2565–2570. | ||
Cillo U, Vitale A, Grigoletto F, et al. Prospective validation of the Barcelona Clinic Liver Cancer staging system. J Hepatol. 2006;44(4):723–731. | ||
Liapi E, Geschwind JF. Chemoembolization for primary and metastatic liver cancer. Cancer J. 2010;16(2):156–162. | ||
Sun H, Ni J, Jiang X, et al. The effect of lipiodol deposition in HCC after TACE on the necrosis range of PMCT. Onco Targets Ther. 2017;10:3835–3842. | ||
Seki T, Wakabayashi M, Nakagawa T, et al. Ultrasonically guided percutaneous microwave coagulation therapy for small hepatocellular carcinoma. Cancer. 1994;74(3):817–825. | ||
Yin XY, Xie XY, Lu MD, et al. Percutaneous thermal ablation of medium and large hepatocellular carcinoma: long-term outcome and prognostic factors. Cancer. 2009;115(9):1914–1923. | ||
Xu LF, Sun HL, Chen YT, et al. Large primary hepatocellular carcinoma: transarterial chemoembolization monotherapy versus combined transarterial chemoembolization-percutaneous microwave coagulation therapy. J Gastroenterol Hepatol. 2013;28(3):456–463. | ||
Hernández-Guerra M, Hernández-Camba A, Turnes J, et al. Application of the Barcelona Clinic Liver Cancer therapeutic strategy and impact on survival. United European Gastroenterol J. 2015;3(3):284–293. | ||
Prajapati HJ, Kim HS. Treatment algorithm based on the multivariate survival analyses in patients with advanced hepatocellular carcinoma treated with trans-arterial chemoembolization. PLoS One. 2017;12(2):e0170750. | ||
Ministry of Health of the People’s Republic of C. [Updated standards for the diagnosis and treatment of primary liver cancer]. Zhonghua Gan Zang Bing Za Zhi. 2012;20:419–426. Chinese | ||
Xie LL, Sun CJ, Li XD, Wang YH, Wang CE. Arterial embolization of massive hepatocellular carcinoma with lipiodol and gelatin sponge. Indian J Cancer. 2015;51(Suppl 2):49–51. | ||
Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. | ||
Fattovich G, Giustina G, Degos F, et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112(2):463–472. | ||
Carr BI, Guerra V, Pancoska P. Thrombocytopenia in relation to tumor size in patients with hepatocellular carcinoma. Oncology. 2012;83(6):339–345. | ||
Seki T, Wakabayashi M, Nakagawa T, et al. Ultrasonically guided percutaneous microwave coagulation therapy for small hepatocellular carcinoma. Cancer. 1994;74(3):817–825. | ||
Seki T, Tamai T, Nakagawa T, et al. Combination therapy with transcatheter arterial chemoembolization and percutaneous microwave coagulation therapy for hepatocellular carcinoma. Cancer. 2000;89(6):1245–1251. | ||
Nomura F, Ohnishi K, Tanabe Y. Clinical features and prognosis of hepatocellular carcinoma with reference to serum alpha-fetoprotein levels. Analysis of 606 patients. Cancer. 1989;64(8):1700–1707. | ||
Tangkijvanich P, Anukulkarnkusol N, Suwangool P, et al. Clinical characteristics and prognosis of hepatocellular carcinoma: analysis based on serum alpha-fetoprotein levels. J Clin Gastroenterol. 2000;31(4):302–308. | ||
Choi D, Lim HK, Rhim H, et al. Percutaneous radiofrequency ablation for recurrent hepatocellular carcinoma after hepatectomy: long-term results and prognostic factors. Ann Surg Oncol. 2007;14(8):2319–2329. | ||
Carr BI, Guerra V, Giannini EG, et al. Low alpha-fetoprotein HCC and the role of GGTP. Int J Biol Markers. 2014;29(4):395–402. | ||
Blank S, Wang Q, Fiel MI, et al. Assessing prognostic significance of preoperative alpha-fetoprotein in hepatitis B-associated hepatocellular carcinoma: normal is not the new normal. Ann Surg Oncol. 2014;21(3):986–994. | ||
Terentiev AA, Moldogazieva NT. Alpha-fetoprotein: a renaissance. Tumour Biol. 2013;34(4):2075–2091. | ||
Bholee AK, Peng K, Zhou Z, et al. Radiofrequency ablation combined with transarterial chemoembolization versus hepatectomy for patients with hepatocellular carcinoma within Milan criteria: a retrospective case-control study. Clin Transl Oncol. 2017;19(7):844–852. | ||
Choi YH, Chung JW, Son KR, et al. Novel intraarterial therapy for liver cancer using ethylbromopyruvate dissolved in an iodized oil. Acad Radiol. 2011;18(4):471–478. | ||
Paul SB, Gamanagatti SR, Gupta AK, et al. Tumor size determines the outcome of trans-arterial chemoembolization (TACE) for unresectable hepatocellular carcinoma (HCC): ongoing work at a tertiary care centre in India. J Gastroen Hepatol. 2010;25:A100–A100. | ||
Nhu Q, Knowles H, Pockros P, Frenette C. An Unexpected Pulmonary Complication Following TACE With Low-Dose Doxorubicin-Eluting Beads and Small-Volume Lipiodol for a Small HCC. Am J Gastroenterol. 2015;110:S345–S346. | ||
Goel A, Fidelman N, Yao F, Roberts J, Terrault N. Pre-Transplant Transarterial Chemoembolization (TACE) for Hepatocellular Carcinoma (HCC) and Risk of Hepatic Artery Complications (HA-C) in Liver Transplant (LT) Recipients. Liver Transplant. 2012;18:S256–S256. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.