Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Topical Curcumin as Chemoprotector Against Photoproducts Production: The Role of Cyclobutyl Pyrimidine Dimers, 8-Hydroxy2ʹDeoxyguanosine Expression and Epidermal Hyperplasia in Acute and Chronic UVB-Induced Mice
Authors Djawad K , Yusuf I, Miskad UA, Patellongi IJ, Massi MN
Received 1 June 2022
Accepted for publication 26 August 2022
Published 31 August 2022 Volume 2022:15 Pages 1787—1795
DOI https://doi.org/10.2147/CCID.S377055
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Khairuddin Djawad,1 Irawan Yusuf,2 Upik Anderiani Miskad,3 Ilhamjaya Jaya Patellongi,4 Muhammad Nasrum Massi5
1Department of Dermatology and Venereology, Hasanuddin University, Makassar, South Sulawesi, Indonesia; 2Department of Pathological Anatomy, Hasanuddin University, Makassar, South Sulawesi, Indonesia; 3Department of Physiology and Biostatics, Hasanuddin University, Makassar, South Sulawesi, Indonesia; 4Department of Physiology, Hasanuddin University, Makassar, South Sulawesi, Indonesia; 5Department of Medical Microbiology, Hasanuddin University, Makassar, South Sulawesi, Indonesia
Correspondence: Khairuddin Djawad, Email [email protected]
Background: Ultraviolet B (UVB) exposure leads to formation of photoproducts leading to cellular damage. Prevention using sunscreen can sometimes be inadequate and can be an economic burden. Recent studies have suggested the photoprotective effect of curcumin.
Objective: To examine the acute and chronic photoprotective effect of topical curcumin, using cyclobutyl pyrimidine dimers (CPD) and 8-hydroxy2ʹdeoxyguanosine (8-OHdG) expression as markers of DNA-induced damage, and epidermal hyperplasia on UVB-induced mice.
Methods: Three treatment groups were established. Group A (negative control) consisted of 5 mice, Group B and C were further divided into two categories to assess acute and chronic effects of topical curcumin and UVB radiation. Each consisted of six subgroups of five mice. Subgroup 1; UVB exposure only (positive control) subgroup 2; acetone and UVB exposure, subgroup 3– 6; topical curcumin application of 100nM, 1μM, 10μM, and 100μM concentrations, respectively. In Group C, there were two categories that received 3x/week UVB exposure for three weeks which effects were being observed at 24 hours and 10 days after the last exposure. The topical curcumin dose was 2mg/mL/cm2 applied 30 minutes prior to 343mJ/cm2/day UVB irradiation. Skin biopsy was done one hour after the last UVB exposure for immunohistochemical and histopathology examinations.
Results: Topical curcumin showed a limited yet robust protective effect against CPD and 8-OHdG expression in Group B, while in Group C all concentrations showed significant CPD and 8-OHdG inhibition after 10 days of UVB exposure. The 10μM and 100μM concentrations showed the best epidermal hyperplasia inhibition effect (p< 0.05). No significant differences were found in terms in efficacy either in single nor daily application.
Conclusion: Topical curcumin can prevent the formation of the photoproducts CPD and 8-OHdG and epidermal hyperplasia in both acute and chronic exposure in UVB-induced mice.
Keywords: curcumin, cyclobutyl pyrimidine dimers, 8-hydroxy2ʹdeoxyguanosine, epidermal hyperplasia, ultraviolet B, UVB, CPD
Introduction
Prolonged exposure to sunlight causes acute and chronic consequences, such as sunburn, epidermal hyperplasia, photoaging, and skin cancer. The ultraviolet (UV) spectrum in sunlight can be distinguished to three groups based on its wavelengths: UVC (<290nm), UVB (290–320nm), and UVA (320–400nm). The UVC spectrum is mostly absorbed by the ozone layer, leaving UVB and UVA to reach the skin, of which UVB causes more serious acute and chronic effects to the skin. UVB is absorbed by chromophores in the dermis such as deoxyribonucleic acid (DNA) and urocanic acid in the form of photon energy which leads to various biological effects.1 UV is absorbed by various molecules such as thymine, and cytosine to form photoproducts such as cyclobutyl pyrimidine dimers (CPD) and 6–4 photoproducts which are formed immediately after UVB absorption by nucleotide. CPD is the main photoproduct which is formed within the pyrimidine bases in the DNA chain and is considered to play an important role in mammalian cells mutations.2
Besides promoting CPD formation, nucleotides are highly susceptible to free radical injury. UV radiation induces the production of reactive oxygen species (ROS) such as superoxide anion, hydrogen peroxide and the hydroxyl radical.3 Oxidation of nucleotide bases promotes transversion of guanine to thymine and is a characterized mutation caused by ROS where guanine at the 8th position is oxidized to produce 8-hydroxy2ʹdeoxyguanosine (8-OHdG).4 These molecular and biochemical processes will result in clinical and histopathology changes of the skin manifesting in epidermal hyperplasia, sunburn, photoaging and skin cancer.5 One study concludes that after 2.5kJ/m2 UVB exposure, epidermal hyperplasia that leads to epidermal thickening begins within a few hours and reaches its peak after 48–72 hours.6 Other studies have shown epidermal hyperplasia to appear at a maximum rate after 24 hours of chronic exposure of 4.8kJ/m2 UVB twice a week for three weeks.7 Epidermal hyperplasia can act as protective response towards UVB exposure as well as an indicator to assess the photoprotective capability of certain chemical compounds when applied to the skin prior to UVB radiation.8
The acute and chronic effects of UV radiation can be prevented through sun exposure avoidance. However, this approach is often not practical. Chemical sunscreens have been shown to be effective in preventing acute and chronic effects, but they are not easily available in rural communities and are expensive for low-income population. Hence, other protection modalities need to be developed. Curcuma longa is a widely known natural ingredient used for various purposes, including as a natural sunblock when used in powder form. Its active compound, curcumin, contains various polyphenols known for its antioxidant properties preventing the production of ROS after UV radiation.9
However, the protective effect of curcumin against the acute and chronic effects of UV exposure in the inhibition of photoproducts formation, has not been well explained. Various studies carried out have been only assessing the effect of curcumin based on a single curcumin application before UVB exposure. Topical application of 10µM prior to UVB exposure inhibited the formation of malondialdehyde (MDA) in UV-induced mice. Assessment of the protective effect of a chemical can be assessed based on the application of the material before UV exposure. However, the best protective concentration of curcumin is not yet known. In addition, the effects regarding frequency of daily application prior to UVB exposure is also yet to be explored. Furthermore, the protective effect of curcumin against chronic UVB exposure has also yet to be studied as well as the extent of this protective effect is not clearly known.
This study aims to explore the photoprotective of acute and chronic effect of various doses of curcumin on UVB-induced mice. Acute effects were observed after single UVB exposure, while chronic effects were observed after three-time weekly UVB exposure three weeks. In addition, the study also assessed the effects of long-term effects of UVB radiation through assessment of epidermal thickness and determine the best concentration through photoproduct expression of CPD, 8-OHdG expression, and epidermal hyperplasia examination.
Materials and Methods
Study Design
The study is a pure experimental study with a post-test design comparison with a control group conducted at the Animal Laboratory, Faculty of Medicine, Hasanuddin University, Makassar, South Sulawesi, Indonesia. The histopathological samples were prepared and analyzed in the Pathology Laboratory of Wahidin Sudirohusodo Hospital, Makassar, South Sulawesi, Indonesia. The immunohistochemical staining was carried out at the Health Biotechnology Laboratory, Research Center, Hasanuddin University, Makassar, South Sulawesi, Indonesia.
Subjects
Subjects used in our study were healthy albino mice aged 7 weeks old that weighed 20–30 grams and bred by Maros Center for Research and Development Center, Makassar, South Sulawesi, Indonesia. Mice were chosen, randomly grouped, and placed inside cages for one week under standard room temperature (28 ± 2°C), humidity 50 ± 10% with 12-hour light and 12-hour dark cycle.
Chemicals
Synthetic topical curcumin was acquired and prepared by the Pharmacognosy Phytochemical Laboratory, Faculty of Pharmacy, Hasanuddin University), Makassar, South Sulawesi, Indonesia. Four concentrations, 100nM, 1µM, 10µM and 100µM concentrations were prepared in 100mL acetone. Method of synthesis was proprietary. The UVB light source was ten FS40T12/UVB sun lamps (200–340 nm, peak emission 314 nm) calibrated using FLUX radiometer.
Study Protocol
The mice were grouped into three groups: group A, group B, and group C. Group A acted as negative control with a total of five mice, while Group B and C were divided to assess the acute and chronic effects of topical curcumin and UVB radiation (Figure 1). Group B was further divided into two categories, the first was to assess the one-time application of topical curcumin applied 20 minutes prior to UVB radiation, and the second was to assess the effects daily application of topical curcumin for seven days prior to single UVB exposure. To assess each of the concentrations, the second category was further divided to six subgroups each containing five mice. Subgroup one acted as positive control which only received UVB exposure, subgroup two received topical acetone for 20 minutes followed with UVB exposure, and subgroup 3–6 received four 100nM, 1µm, 10µM, and 100µM concentration, respectively, for 20 minutes followed by UVB exposure. Skin biopsy was taken one hour after UVB radiation for immunohistochemical staining to assess the levels of CPD and 8-OHdG.
Similarly, group C was designated to assess the chronic effects of UVB radiation and was divided into two categories to assess the effects of UVB radiation in two different time periods (24 hours and 10 days). Each category was divided to six subgroups each consisting of five mice, and all received thrice weekly exposure of UVB radiation for three consecutive weeks. Histopathological and immunohistochemistry (IHC) examination were performed after 24 hours and 10 days of last UVB exposure. Dosage of all topical application was 2 mg/mL/cm2 applied 20 minutes prior to UVB exposure. Dose of UVB exposure was set to 343mJ/cm2/day.
Immunohistochemistry
IHC was used to assess CPD and 8-OHdG expression and performed according to the streptavidin-biotin-peroxidase method labeled with streptavidin-biotin (Dako, Carpinteria, USA). The protocol used in this study followed a previous similar study.10 Before staining, each slide was deparaffinized with xylene for 15 minutes and dehydrated with 100%, 90%, 80%, 70%, and 60% alcohol, each for 10 minutes. The preparation was then washed with dH20 for two cycles, each for five minutes, and incubated with PBS solution for 5 minutes. Furthermore, samples were incubated using 0.3% hydrogen peroxide for 15 minutes.
Samples were staining using a primary anti-CPD antibody (KAM`IYA, United States) and anti-8-OHdG antibody (GENESIS Bio-med, Spain) and incubated overnight at 4°C, followed by incubation with secondary antibody and streptavidin for 30 minutes each. The preparation was then further stained using 3.3 diamino benzidine tetrahydrochloride for 10 minutes until a brown pigment was visible for microscopic examination. Lastly, staining using hematoxylin was then performed to better visualize the cell nucleus for 30 seconds and then washed using water for 5 minutes. A second dehydration process using 70%, 80%, 90%, and 96% for 2 minutes each was then performed and submerged in xylene for 5 minutes and malinol before preparations were covered using deck glass.
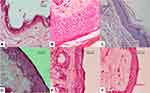
The result of the immunohistochemical examination was classified based on a study by Chen et al11 using the number of the stained cells and color intensity using the following classification: 0; no expression or <10% of the epidermal cells showing weak intensity, 1; <50% epidermal cells showing weak intensity or <10% showing strong intensity, 2; >50% epidermal cells showing weak intensity or >10% showing strong intensity. Scoring was determined by multiplying the percentage of cells that yielded positive signal with the intensity of the positive signal. Four categories were established with negative, mild (0–2), moderate (3–4), and strong (5–6) (Figure 1A–C).
Epidermal Thickness Assessment
Histopathological preparations were obtained from the back of mice through biopsy and fixed with formalin buffer. Specimens were placed on a flat surface and divided into two parts. Slides of 4µM thickness were then prepared from the center of the specimen and stained with hematoxylin-eosin (HE). Examination was performed using microscope equipped with a digital camera (Olympus DP12). Evaluation of epidermal hyperplasia were made based on epidermal thickness in the interfollicular region of the basal layer to the granular layer, as measured by CorelDraw® version 13.
Statistical Examination
Data were analyzed using SPSS version 22 (SPSS Inc. Chicago, IL, USA). The CPD and 8-OHdG expression analysis were done using Mann–Whitney and Kruskal Wallis tests. ANOVA test was followed by a post-hoc to assess the decrease in epidermal thickness. A p-value <0.05 was considered statistically significant.
Result
CPD and 8-OHdG Expression Following Acute and Chronic UVB Exposure
The CPD and 8-OHdG expression in group B were examined after single and daily topical curcumin application for seven days followed with UVB radiation. We found that topical curcumin reduced CPD expression where all concentrations displayed moderate and high expression of CPD (40% and 60% respectively) compared to the positive control and acetone only group which showed 100% IHC expression. Conversely, we found negative and mild-moderate 8-OHdG expression especially in groups receiving concentration of 1μM and 10μM. A reduced protective effect was shown by the 100nM and 100μM group. The total number of applications of topical curcumin, one time and seven times, did not significantly influence protective effects against CPD and 8-OHdG expression (p <0.05) (Figure 2).
 |
Figure 2 Effects of curcumin application against single UVB-induced CPD and 8-OHdG expression. |
Subsequently, we then examined the CPD and 8-OHdG expressions in group C which received chronic UVB exposure three times weekly for three consecutive weeks. The 100μM curcumin group exhibited the best protective effect towards CPD levels, with all samples resulted in negative/mild IHC expression 24 hours after the last UVB exposure (p<0.05). Meanwhile, mild/negative CPD expression 10 days after the last UVB exposure was shown by 1µM, 10µM, and 100µM. As for 8-OHdG levels, all concentrations resulted in mild/negative IHC expression 24 hours and 10 days after UVB exposure (p <0.05). Similar to group B, no significant differences in CPD and 8-OHdG levels were found between single and daily application. (p<0.05) (Figure 3).
 |
Figure 3 Effects of curcumin application against chronic UVB-induced CPD and 8-OHdG expression. |
UVB-Induced Epidermal Hyperplasia
Epidermal hyperplasia was assessed through histopathology in mice without UVB exposure (Figure 4A) and only UVB exposure (Figure 4B), followed with topical curcumin application before chronic UVB exposure within 24 hours and 10 days after the last UVB exposure. All concentrations of 100nM, 1µM, 10µM, and 100µM topical curcumin applied prior to UVB exposure exhibited less visible epidermal hyperplasia (Figure 4B–F). Furthermore, we found that the 10µM and 100µM concentrations provided the best results in prevention of epidermal hyperplasia.
Discussion
UVB absorption by chromophores causes inflammation, epidermal hyperplasia, and changes in gene expression. It is associated with proliferation and differentiation, cytokine production, and growth factor.10 Chronic UV exposures may lead to defects in the recovery process of DNA damage and are associated with oncogenesis and/or tumor suppressor genes mutation that causes the initiation of cutaneous cancer.12
CPD is a UV hotspot mutagen10 and one of the main photoproduct that is directly formed after UVB absorption by nucleotide. While 8-OHdG is formed indirectly from oxidative stress. Both compounds play a significant role in the carcinogenesis process.2 Thus, they can be used to assess the protective effect of curcumin against photodamage. In addition, acetone used as solvent in this study has not shown to influence the efficacy of curcumin and has long been used to evaluate the efficacy of curcumin.13 A direct protective effect from DNA damage occurs through inhibition of UV exposure to the skin or absorption of photon energy by agents acting as sunscreens.14 In our study, we used synthetic topical curcumin creams in four concentrations with acetone acting as solvent. Previous studies such as by Lüeret al have seek to explore the anti-inflammatory capability of synthetic curcumin and have found that it can reduce the number of expression of cytokines and chemokines such as IL-6 and IL-8.15 To prove the efficacy of topical curcumin we also prepared one group that was only applied with acetone as a comparison. Dosage of 2mg/mL/cm2 was determined as it is the standard dosage of sunscreen application.16 Furthermore, our study found that in single UVB exposure, the protective effect of curcumin was shown to have minimal effect in various groups and with the best effect exhibited at 10µM concentration. No significant differences were found in either single or daily application of curcumin. Whereas in the group that received chronic UVB exposures, curcumin showed a protective effect against CPD expression with the 100μM concentration showing the most significant protective effect in both 24 hours and 10 days. These results are consistent with previous studies that reported the increased protective effects of curcumin against CPD expression with increased curcumin concentrations.17
The difference in curcumin protective effect between single and chronic UVB groups may be due to the increased rate of repaired cells in the chronic UVB group. In these groups, subjects were exposed to UVB three times weekly for three weeks and examined 24 hours after the last exposure which allowed more CPD to be repaired than subjects in the group that only received single UVB exposure. Some investigators observed dimer reparation occurred 9 hours after UVB radiation.18 In our study, CPD expression was still visible in both UVB control group at 1 hour, 24 hours and 10 days after exposure, with expression at 10 days may be a sign of permanent DNA damage.17 These photolesions can inhibit DNA replication and transcription and therefore generate permanent DNA damage, which subsequently lead to cell death, photoaging and photocarcinogenesis.19 With the impairment of the cellular repairment mechanism, ROS and other free radicals can cause permanent DNA damage and is one of the initial and typical stages of UV-induced carcinogenesis.10
The application of curcumin prior to UVB exposure prevents CPD expression and cell damage as well as play a role in accelerating CPD repair by influencing cellular processes such as cycle cell, differentiation and DNA repairment through the mutS homolog 2 (MSH2) enzyme.10,20 Furthermore, an in vitro study on squamous cell carcinoma cell lines showed that curcumin decreases UV-induced apoptosis and inflammation through inhibition of nuclear factor-κB (NF-κB).20,21
In contrast with CPD, the effect of curcumin against 8-OHdG, one of the key markers for cellular oxidative stress, following single UVB exposure showed strong protective effect. The best protective effect was shown by 10μM concentration. In multiple exposures, 8-OHdG expression was not seen in all samples at 24 hours and 10 days. These results suggested that curcumin possesses protective effect towards ROS formation after chronic UVB exposure Interestingly, higher curcumin concentrations increased the protective effect except the 100μM. This phenomenon also occurred in a previous study.9,22 The decline in protective effect observed on the 100µM might be explained by the pro-oxidant and antioxidant effects of curcumin, with the earlier effect being observed at higher concentrations.23
The antioxidant effect of curcumin is mediated through several mechanism including scavenge different forms of free radicals, such as ROS and reactive nitrogen species (RNS), induction of the antioxidant enzymes, superoxide dismutase (SOD), catalase, and glutathione peroxidase (GSH), and inhibition of ROS-generating enzymes such as lipoxygenase/cyclooxygenase and xanthine hydrogenase/oxidase which can be revealed by assessing 8-OHdG expression.24 This was supported by previous studies where Curcuma prevented UV-induced apoptosis and eliminated oxidative stress through lipid peroxidase inhibition using linoleic acid, a polyunsaturated fatty acid that is oxidized and forms fatty acid radicals.25 A decline in the lipid peroxidase level is achieved through maintaining the activity of various antioxidant enzymes that play a central role in regulating lipid peroxidases, such as SOD, catalase, and GSH. The protective effect of curcumin is also clear in human cells by protecting against radiation-induced DNA damage. This is mainly attributed to the antioxidant effect and scavenger against free hydroxyl radicals.26 In addition, curcumin is a lipophilic compound, which makes it an efficient scavenger of peroxyl radicals. Phenolic OH in curcumin play a major role in that activity; therefore, it is also considered as a chain-breaking antioxidant.27
The protective effect towards CPD and 8-OhDG production is in line with the protective effect against epidermal hyperplasia. As epidermal hyperplasia is a well-known normal response towards UV radiation, its decrease resulting from topical curcumin application can therefore be associated with its protective effect against UB radiation. The 10µM concentration was shown to be the most effective concentration to inhibit epidermal hyperplasia. Similar with the photoproducts analysis described previously, a decreased effect was also seen on the 100µM concentration, which was consistent with other studies.28 However, these differences are not statistically significant. This might be attributed by the suppression of C-jun/Activator protein 1 (AP-1) activation,25 and fibroblast growth factor receptor 2 (FGFR2) expression,29 or through the induction of the p53-p21/CIP1 cascade. The antiproliferative effect of curcumin is mediated by modulation in the cell cycle through CDK inhibitor expression which inhibits CKD-cyclin kinase complex activity. In addition, curcumin is also known to inhibit UVB-induced cytotoxicity.30
From these results, we therefore determined that 10µM, and 100µM topical curcumin provided the best overall photoprotective effect by presenting the lowest level of CPD and 8-OHdG after UVB radiation in both acute and chronic models.
Conclusion
The use of topical curcumin especially the 10µM dan 100µM concentrations showed photoprotective effect towards acute and chronic UVB exposure on mice by inhibiting CPD and 8-OHdG production for up to ten days after UVB exposure. There were no differences in photoprotective effect in either single or daily application of topical curcumin.
Data Sharing Statement
All data used during the current study available from the corresponding author on reasonable request.
Ethics Approval
Ethical approval was obtained from the Animal Research Committee at Hasanuddin University (No: UH07100058), Indonesia following the Institutional Animal Care and Use Committee (IACUC) Protocol requirements.
Acknowledgment
The authors would like to thank Jonathan Kurnia Wijaya, MD, Pg.Dip (Derm) for layout and editing of the manuscript, as well as to all the laboratory staff and assistants for their technical assistance.
Author Contributions
All authors made significant contributions to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study received support from the Department of Dermatology and Venereology, Faculty of Medicine, Hasanuddin University.
Disclosure
The authors declared no conflict of interest.
References
1. Rünger TM. Cutaneous Photobiology. In: Kang S, Amagai M, Bruckner AL, Enk AH, Morgolis DJ, McMichael AJ, editors. Fitzpatrick’s Dermatology.
2. Streilein JW. Suceptibility: posible relationship to photoaging and photocarcinogenesis. In: Gilchrest B, editor. Photodamage. Oxford: Wiley-Blackwell; 1995:68–79.
3. Soehnge H, Ouhtit A, Ananthaswamy ON. Mechanisms of induction of skin cancer by UV radiation. Front Biosci. 1997;2:d538–51. doi:10.2741/A211
4. D’Orazio J, Jarrett S, Amaro-Ortiz A, Scott T. UV radiation and the skin. Int J Mol Sci. 2013;14(6):12222–12248. doi:10.3390/ijms140612222
5. Nishisgori C. Current concept of photocarcinogenesis. Photochem Photobiol Sci. 2015;14(9):1713–1721. doi:10.1039/C5PP00185D
6. Ouhtit A, Muller HK, Davis DW, Ullrich SE, McConkey D, Ananthaswamy HN. Temporal events in skin injury and the early adaptive responses in ultraviolet-irradiated mouse skin. Am J Pathol. 2000;156(1):201–207. doi:10.1016/S0002-9440(10)64720-7
7. Wolf P, Donawho CK, Kripke ML. Effect of sunscreens on UV radiation-induced enhancement of melanoma growth in mice. JNCI. 1994;86(2):99–105. doi:10.1093/jnci/86.2.99
8. Scott TL, Christian PA, Kesler MV, et al. Pigment-independent cAMP-mediated epidermal thickening protects against cutaneous UV injury by keratinocyte proliferation. Exp Dermatol. 2012;21(10):771–777. doi:10.1111/exd.12012
9. Ali RE, Rattan SI. Curcumin’s biphasic hormetic response on proteasome activity and heat-shock protein synthesis in human keratinocytes. Ann N Y Acad Sci. 2006;1067:394–399. doi:10.1196/annals.1354.056
10. Miskad UA, Semba S, Kato H, et al. High PRL-3 expression in human gastric cancer is a marker of metastasis and grades of malignancies: an in situ hybridization study. Virchows Archiv. 2007;450(3):303–310. doi:10.1007/s00428-006-0361-8
11. Chan WY, Cheung KK, Schorge JO, et al. Bcl-2 and p53 protein expression, apoptosis, and p53 mutation in human epithelial ovarian cancers. Am J Pathol. 2000;156(2):409–417. doi:10.1016/S0002-9440(10)64744-X
12. Tsai KD, Lin JC, Yang S, et al. Curcumin protects against UVB-induced skin cancers in SKH-1 hairless mouse: analysis of early molecular markers in carcinogenesis. Evid Based Complementary Altern Med. 2012;2012:1–11. doi:10.1155/2012/593952
13. Huang MT, Ma W, Yen P, et al. Inhibitory effects of topical application of low doses of curcumin on 12-O-tetradecanoylphorbol-13-acetate-induced tumor promotion and oxidized DNA bases in mouse epidermis. Carcinogenesis. 1997;18(1):83–88. doi:10.1093/carcin/18.1.83
14. Majidinia M, Bishayee A, Yousefi B. Polyphenols: major regulators of key components of DNA damage response in cancer. DNA Repair. 2019;82:102679. doi:10.1016/j.dnarep.2019.102679
15. Lüer SC, Goette J, Troller R, Aebi C. Synthetic versus natural curcumin: bioequivalence in an in vitro oral mucositis model. BMC Complement Altern Med. 2014;14(1):53. doi:10.1186/1472-6882-14-53
16. Taylor S, Diffey B. Simple dosage guide for suncreans will help users. BMJ. 2002;324(7352):1526. doi:10.1136/bmj.324.7352.1526/a
17. Mitchell DL, Greinert R, De Gruijl FR, et al. Effects of chronic low-dose ultraviolet B radiation on DNA damage and repair in mouse skin. Cancer Res. 1999;59(12):2875–2884.
18. Sutherland BM, Harber LC, Kochevar IE. Pyrimidine dimer formation and repair in human skin. Cancer Res. 1980;40(9):3181–3185.
19. Park JM, Kang TH. Transcriptional and posttranslational regulation of nucleotide excision repair: the guardian of the genome against ultraviolet radiation. Int J Mol Sci. 2016;17(11):1840. doi:10.3390/ijms17111840
20. Zhou H-Y, Sun -Y-Y, Chang P, Huang H-C. Curcumin inhibits cell damage and apoptosis caused by thapsigargin-induced endoplasmic reticulum stress involving the recovery of mitochondrial function mediated by mitofusin-2. Neurotox Res. 2022;40(2):449–460. doi:10.1007/s12640-022-00481-y
21. Hassan F-U, Rehman MS-U, Khan MS, et al. Curcumin as an alternative epigenetic modulator: mechanism of action and potential effects. Front Genet. 2019;10:514. doi:10.3389/fgene.2019.00514
22. Huei Chen H, Tong Rong J, Sheau Farn Y. Inhibitory effect of curcumin, an anti-inflammatory agent, on vascular smooth muscle cell proliferation. Eur J Pharmacol. 1992;221(2–3):381–384. doi:10.1016/0014-2999(92)90727-L
23. Sharma K, Agrawal S, Gupta M. Development and validation of UV spectrophotometric method for the estimation of curcumin in bulk drug and pharmaceutical dosage forms. Int J Drug Dev Res. 2012;4(2):375–380.
24. Cao J, Jia L, Zhou H-M, Liu Y, Zhong L-F. Mitochondrial and nuclear DNA damage induced by curcumin in human hepatoma G2 cells. Toxicol Sci. 2006;91(2):476–483. doi:10.1093/toxsci/kfj153
25. Lin JK, Lin-Shiau SY. Mechanisms of cancer chemoprevention by curcumin. Proc Natl Sci Counc. 2001;25(2):59–66.
26. Okada K, Wangpoengtrakul C, Tanaka T, Toyokuni S, Uchida K, Osawa T. Curcumin and especially tetrahydrocurcumin ameliorate oxidative stress-induced renal injury in mice. J Nutr. 2001;131(8):2090–2095. doi:10.1093/jn/131.8.2090
27. Priyadarsini KI, Maity DK, Naik GH, et al. Role of phenolic O-H and methylene hydrogen on the free radical reactions and antioxidant activity of curcumin. Free Radic Biol Med. 2003;35(5):475–484. doi:10.1016/S0891-5849(03)00325-3
28. Y-p L, Chang RL, Lou Y-R, et al. Effect of curcumin on 12-O-tetradecanoylphorbol-13-acetate-and ultraviolet B light-induced expression of c-Jun and c-Fos in JB6 cells and in mouse epidermis. Carcinogenesis. 1994;15(10):2363–2370. doi:10.1093/carcin/15.10.2363
29. Khandelwal AR, Rong X, Moore-Medlin T, et al. Photopreventive effect and mechanism of AZD4547 and curcumin C3 complex on UVB-induced epidermal hyperplasia. Cancer Prev Res. 2016;9(4):296–304. doi:10.1158/1940-6207.CAPR-15-0366
30. Yehuda Greenwald M B, Frušić-Zlotkin M, Soroka Y, et al. Curcumin protects skin against UVB-induced cytotoxicity via the keap1-Nrf2 pathway: the use of a microemulsion delivery system. Oxid Med Cell Longev. 2017;2017:5205471. doi:10.1155/2017/5205471
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.