Back to Journals » Clinical Ophthalmology » Volume 14
To Peel or Not to Peel: Pars Plana Vitrectomy with Macular Membrane Peel in Eyes with Abnormalities of Vitreomacular Interface and Coexisting Dry Age-Related Macular Degeneration
Authors Furashova O , Engelmann K
Received 29 November 2019
Accepted for publication 3 January 2020
Published 11 February 2020 Volume 2020:14 Pages 389—396
DOI https://doi.org/10.2147/OPTH.S240480
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Olga Furashova, Katrin Engelmann
Department of Ophthalmology, Klinikum Chemnitz gGmbH, Chemnitz, Germany
Correspondence: Olga Furashova
Department of Ophthalmology, Klinikum Chemnitz gGmbH, Flemmingstrasse 2, Chemnitz 09116, Germany
Tel +49 371 333 33230
Fax +49 371 333 33223
Email [email protected]
Purpose: To evaluate the outcome of macular surgery with ILM- and epiretinal membrane peel associated with significant dry age-related macular degeneration (AMD) as defined by the Age-Related Eye Disease Study (AREDS).
Patients and Methods: Institutional. Retrospective case-control study. A total of 42 pseudophacic eyes of 39 patients (7 with full thickness macular hole and 35 with epiretinal membrane) with coexisting dry AMD underwent pars plana vitrectomy (PPV) with internal limiting membrane (ILM) and epiretinal membrane peel. Preoperative and postoperative data including best corrected visual acuity (BCVA), AMD grade according to AREDS, central retinal thickness (CRT), development of choroidal neovascularization (CNV), and central retinal atrophy have been evaluated. Twenty-eight fellow eyes with dry AMD of the included 39 patients served as a control group.
Results: A significant improvement in the visual acuity could be observed after surgery (initial BCVA 0.47± 0.31 logarithm of the minimal angle of resolution (logMAR) vs 0.33± 0.29logMAR 9 months postoperatively; p=0.006). CRT decreased significantly after surgery (p< 0.001). In the surgery group, there were 4 eyes (9.5%) with CNV and 1 eye (2.5%) with new central retinal atrophy development after surgery. All these eyes had preoperative AREDS 3 (4 eyes) or AREDS 4 (1 eye) AMD category. In the control group, there was 1 eye (4%) with CNV and 4 eyes (14%) with new central retinal atrophy development during the follow-up of 9 months. These eyes had initially AREDS 2 (1 eye), AREDS 3 (3 eyes) or AREDS 4 (1 eye) AMD category.
Conclusion: Eyes with dry AMD of AREDS 3 and AREDS 4 with coexisting VMI abnormalities improve significantly after PPV with membrane peel. While there is a higher risk of CNV development after surgery (9.5%) in these eyes, the vitrectomy does not seem to accelerate central retinal atrophy progression compared to the fellow eyes course.
Keywords: macular membrane peel, choroidal neovascularization, pars plana vitrectomy, age-related macular degeneration, ILM, epiretinal membrane peeling
Introduction
Abnormalities of the vitreomacular interface (VMI) such as epiretinal macular membranes (ERM) and full-thickness macular holes (FTMH) cause a significant reduction in central vision, especially reducing the reading visual acuity.1 PPV with membrane peel of ERM and inner limiting membrane (ILM) is now used worldwide to treat these abnormal conditions of the VMI with excellent results. The improvement of visual acuity can be achieved in up to 90% of the ERM cases and up to 98% in FTMH cases.2–5
Abnormalities of the VMI occur mostly in elderly people. The Blue Mountains Eye Study reported ERM prevalence to be up to 20% in eyes older than 70 years.6 Another common condition occurring in predominantly elderly people is age-related macular degeneration (AMD) . Non-exudative degenerative macular changes have been seen in at least 15% of adults of 43 to 86 years old in a big population study.7 Therefore, simultaneous presence of both abnormalities of the VMI and ERM or FTMH might not be uncommon. While the surgical outcome for ERM and FTMH is as good as 90% or even higher, the question arises about the best therapeutic strategy for the eyes having VMI abnormalities with co-existing AMD.
In 2008, Mojana et al described 5 patients with exudative AMD, who did not respond well to intravitreal injections of vascular endothelial growth factor (VEGF) inhibitors because of the co-existing central vitreomacular traction.8 Berinstein et al reported reduced macular hole closure rate in eyes with significant macular drusen.5 There have also been some case reports on CNV development after successful macular hole surgery in eyes with pre-existing aging changes.9–11
It is therefore obvious that the surgical outcome of PPV with membrane peel in eyes with coexisting degenerative macular changes should not be expected to be as good as in eyes without these. Obeid et al demonstrate anatomic and functional improvement in eyes with ERM and coexisting dry AMD after PPV, although 2 of the 25 analyzed eyes progressed to neovascular AMD within 2 years after surgery.12
The purpose of the current study is to assess the surgical outcome of PPV with macular membrane peel in eyes with VMI abnormalities and coexisting dry AMD, especially to estimate the rate of CNV and central retinal atrophy development after surgery and to compare these results with the fellow control eyes.
Materials and Methods
The present study was approved by the Institutional Review Board of Saxony (Dresden, Germany) under the number EK-BR-108/19-1 and adhered to the tenets of the Declaration of Helsinki. Informed patients’ consent was waived because of the retrospective anonymous design and because no study-related investigations were necessary. The investigation has been registered on ClinicalTrials.Gov (ClinicalTrials.gov Identifier NCT03927430).
Patient Selection
The patient database in the Chemnitz Hospital was reviewed for billing codes of epiretinal membrane and macular hole according to the International Classification of Diseases, 10th Revision between January 2016 and December 2018. Patients included in this study met the following criteria: 1) diagnosed with ERM or FTMH and co-existing dry AMD; 2) undergone PPV with ILM and epiretinal membrane peel; 3) spectral-domain optical coherence tomography (SD-OCT) at the initial visit and at last follow-up visit with image quality score >30; 4) no evidence of CNV on initial fluorescein angiography (FA). The exclusion criteria were: 1) history of other macular disease, severe non-proliferative or proliferative diabetic retinopathy, other retinal vascular diseases, glaucoma, myopic retinopathy, or other diseases interfering with OCT images in any one of the eyes; 2) active CNV or history of CNV.
Ophthalmic Examination
All patients underwent a complete ophthalmic examination of both eyes including BCVA, applanation tonometry, slit-lamp biomicroscopy, indirect binocular ophthalmoscopy, SD-OCT imaging, FA at least at initial visit. Additional FA on follow-up visits was performed if necessary. SD-OCT examination was performed using Spectralis OCT (Heidelberg Engineering Inc., Heidelberg, Germany). The macula was scanned with an acquisition speed of 40,000 A-scans per second using the “fast macular volume” protocol, consisting of a 25-line horizontal raster scan covering 20° × 20° centered on the fovea with the standard nine frames. The eye tracking system (ART Module, Heidelberg Engineering Inc.) was used to minimize motion artifacts.
AMD Assessment
The diameter of the biggest drusen in the scanned macular area was measured using the built-in distance measuring tool of the OCT software. All measurements were done by one examiner (OF). The drusen were then classified according to the Age-Related Eye Disease Study (AREDS). Central retinal atrophy was defined as complete retinal pigment epithelium and outer retinal atrophy in the scanned macular area and was measured using the built-in distance measuring tool of the OCT software. CNV presence was defined as active leakage on FA with corresponding typical exudative changes on SD-OCT.
Surgical Procedure
All subjects underwent a standard three-port 23-gauge PPV. All epiretinal membranes as well as the ILM were removed using Brilliant Blue for staining. Twenty-six eyes (62%) received intraoperative autologous platelet concentrate in cases with existing full-thickness macular hole, lamellar macular hole and macular pseudohole. Twenty-six cases (62%) were finished with air or C2F6-tamponade in case of existing or threatening macular holes.
Statistical Analysis
IBM SPSS Statistics, version 23.0.0.0 for Windows (IBM, Armonk, NY, USA) was used to perform the analysis. Visual acuity measurements were converted from decimal numbers to logMAR for all analyses. Data for continuous variables are expressed as mean ± standard deviation. Differences between the baseline and the final data were calculated using paired t-test or the Wilcoxon signed rank test. Frequency of conversion to neovascular AMD and categorical characteristic comparisons were done using a chi-square analysis. For representing statistical significance, p<0.01 was chosen.
Results
In total, 42 pseudophacic eyes from 39 patients were included in this study. The demographic and clinical characteristics of the study participants are summarized in Table 1. Only 28 fellow eyes with dry AMD could serve as controls, 14 had to be excluded from the analysis (3 patients had bilateral macular surgery and both eyes were included in the case series, 3 because of poor visual acuity due to other ocular diseases, 5 eyes were diagnosed with wet AMD prior to study beginning).
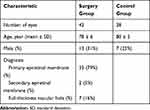 |
Table 1 General Characteristics of Included Patients |
Table 2 summarizes the preoperative and postoperative data on anatomical and functional outcome during the 9 months of the mean follow-up time. Overall, a statistically significant BCVA improvement could be seen after surgery with mean logMAR decrease of 0.14 (Figure 1). In the subgroup analysis of eyes with intact ellipsoid zone (EZ) preoperatively, there was a mean BCVA improvement of −0.16 logMAR at the final visit (0.28 logMAR at baseline vs 0.12 logMAR at the final visit; p=0.002). In eyes with disrupted EZ BCVA was significantly worse at baseline, but increased from 0.57 logMAR to 0.44 logMAR at final visit (p=0.047).
 |
Table 2 Preoperative and Postoperative Data on Anatomical and Functional Outcome |
CRT decreased significantly after surgery, while it remained stable in the control group (Table 2).
We could see 4 cases (9.5%) of CNV development in the surgery group, 3 of them with AREDS 3 and 1 with AREDS 4 (geographic atrophy) initially. All CNV cases developed in eyes with initial epiretinal membrane without macular hole and were observed during the first 2 months after surgery. Three of these 4 eyes received intraoperative autologous platelet concentrate. In the control group, there was 1 case (4%) of CNV development during the whole follow-up time.
New central retinal atrophy developed only in 1 eye (2%) in the surgery group. In the control group, there were 4 cases (14%) of central retinal atrophy: 3 eyes developed new central retinal atrophy, while 1 eye with initial AREDS 4 (because of already diagnosed central retinal atrophy) showed progression of central retinal atrophy over the study course.
The mean time from cataract surgery to PPV was 24 months in the whole study group and 48 months in the eyes with post-surgical CNV development.
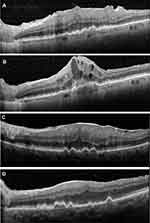
Figure 2 illustrates the different postoperative course after PPV of two eyes with epiretinal membrane and initial AREDS 3 drusen. Figure 3 shows the change in AREDS categories of dry AMD after PPV.
Discussion
The present study demonstrates the anatomical and functional improvement after PPV with membrane peel in eyes with VMI abnormalities, especially ERM or FTMH, and coexisting age-related degenerative macular changes during a mean follow-up time of 9 months.
There is a low number of studies evaluating the visual outcome after macular membrane surgery in eyes with coexisting dry AMD. Obeid et al observed a mean BCVA increase of 0.19 logMAR in eyes with dry AMD undergoing PPV for ERM after 2.6 years of follow-up.12 Wilde et al demonstrated less favorable visual outcomes of ERM surgery in eyes with subretinal drusenoid deposits.13 In another study by Lee et al, significant anatomical and visual improvement after PPV for ERM could be demonstrated, but final visual outcome of eyes with simultaneous non-exudative AMD (BCVA improvement of 0.19 logMAR over 1 year period) was worse than that of eyes with idiopathic ERM without degenerative macular changes (0.28 logMAR BCVA improvement).14
In our study, the mean BCVA improvement after PPV with macular membrane peel was 0.14 logMAR after a mean follow-up of 9 months. Eyes with initially intact EZ showed even better visual recovery of 0.16 logMAR, while BCVA change in eyes with disrupted EZ at baseline was 0.12 logMAR. This is consistent with other studies showing that EZ integrity is associated with better functional outcomes.9,10,15 Still, even in eyes with disrupted EZ, there was a significant improvement of BCVA postoperatively.
Obeid et al reported a CNV incidence of 16% in the vitrectomized eyes during the minimum follow-up time of 2 years compared to 4% in the control group.12 In contrast, Roller et al suggested that vitrectomy might reduce the risk of CNV and geographic atrophy development in eyes with coexisting AREDS 3 drusen.16 In his pilot study, the vitrectomy was associated with reduced progression to CNV or geographic atrophy compared to fellow eyes over the course of 5.5 years. Possible explanations for this discrepancy in study results may be related to different AMD disease stages and different follow-up time periods. Furthermore, it underlines the complicated and not always easy predictable course of the disease in these patients.
In our cohort, the rate of CNV development seems to be higher in the PPV group compared to the control group. On the other hand, central retinal atrophy developed more frequently in fellow eyes. These findings are consistent with the study of Roller et al,16 demonstrating less atrophy in vitrectomized eyes. One possible explanation might be the released vitreomacular adhesion and traction in eyes after PPV. There have been several studies postulating that posterior vitreous detachment might play a protective role against AMD progression.17–19 Other factors contributing to reduced progression of AMD after vitrectomy have been described in detail by the study group of Roller et al and include possible removal of inflammatory mediators during vitrectomy as well as increased oxygen tension in the vitreous after surgery.20,21
Despite all this possible positive influence of PPV, the rate of CNV development in the present study was as high as 9.5% compared to 4% in the control group. Given that all the CNV cases were seen during 2 months after surgery, we can assume that there is a possible correlation between the surgery and the CNV development afterward. It is conceivable that further CNV cases might have developed later, as the patients have been observed only for 9 months, but the correlation with the surgery in these potential cases is less likely because of the longer time period after the vitrectomy.
There was no documented intraoperative damage to outer retinal layers or bruch membrane, that might have been resulting in CNV development. The underlying pathogenesis of CNV development remains unknown. Mechanical trauma during membrane peel might alone induce CNV development due to tractional forces on the central retina. Furthermore, intense light exposure during PPV and higher inflammation level after intraocular surgery might also negatively influence the course of the underlying AMD disease. Oh et al suggested that dye toxicity can be a possible explanation for CNV development,9 but in our cases without macular hole there was no direct way for the dye to pass to the retinal pigment epithelium and neurosensory retina. All 4 postoperative CNV cases showed a major occult portion: 2 cases with a pure occult CNV and 2 cases with a minimally classic CNV according to fluorescein angiography criteria. This is consistent with other studies, showing that the vast majority of CNV secondary to AMD is occult.22,23
Lamin et al showed recently that increased drusen volume was associated with higher risk of occult CNV development compared to classic CNV type.24 In our study, 3 of 4 CNV cases showed AREDS 3 drusen category and 1 case even AREDS 4 preoperatively.
As cataract surgery is a known risk factor for CNV development in eyes with pre-existing degenerative macular changes, we analyzed the mean time from the cataract surgery to PPV in the surgery group. The cataract surgery was done in all 4 eyes with CNV development on average 48 months prior to vitrectomy, while in the whole study population the mean time from cataract surgery to vitrectomy was 24 months. Therefore, cataract surgery alone cannot explain CNV development in these eyes either.
One major limitation of the study is the absence of OCT-angiography at baseline. This might have helped to detect quiescent CNV prior to surgery, a new distinct entity of CNV, which can be easily overseen on conventional SD-OCT and fluorescein angiography.
Using fellow eyes as a control group helped us to avoid genetic and environmental differences between the groups, like the study group of Mason et al had done. On the other hand, the control group was smaller than the study group, because some eyes had to be excluded from analysis for several reasons. Therefore, another limitation is related to an imperfect case-control study design with not all eyes in the study group being “paired matched.”
Conclusion
In summary, we report the outcomes of surgical repair of VMI abnormalities, predominantly epiretinal membranes, in eyes with co-existing dry AMD. Our results demonstrate a statistically significant improvement in visual acuity as well as reduction in central retinal thickness during the first 9 months after surgery. While the development of central retinal atrophy was not accelerated in vitrectomized eyes compared to controls, the rate of CNV seems to be higher after PPV. Better protective strategies are needed to improve the surgical outcome and preserve better visual acuity by inhibition of CNV development in these eyes. Further studies should assess whether perioperative anti-VEGF treatment might help to reduce the rate of CNV development.
Data Sharing Statement
The authors report that no further data besides what is included in the manuscript will be shared.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ting FS, Kwok AK. Treatment of epiretinal membrane: an update. Hong Kong Med J. 2005;11(6):496–502. Epub 2005/ 12/13.
2. de Bustros S, Thompson JT, Michels RG, et al. Vitrectomy for idiopathic epiretinal membranes causing macular pucker. Br J Ophthalmol. 1988;72(9):692–695. doi:10.1136/bjo.72.9.692
3. Shimada H, Nakashizuka H, Hattori T, et al. Double staining with brilliant blue G and double peeling for epiretinal membranes. Ophthalmology. 2009;116(7):1370–1376. doi:10.1016/j.ophtha.2009.01.024
4. Chang WC, Lin C, Lee CH, et al. Vitrectomy with or without internal limiting membrane peeling for idiopathic epiretinal membrane: a meta-analysis. PLoS One. 2017;12(6):e0179105. doi:10.1371/journal.pone.0179105
5. Berinstein DM, Hassan TS, Williams GA, et al. Surgical repair of full-thickness idiopathic macular holes associated with significant macular drusen. Ophthalmology. 2000;107(12):2233–2239. doi:10.1016/S0161-6420(00)00417-6
6. Mitchell P, Smith W, Chey T, et al. Prevalence and associations of epiretinal membranes. The blue mountains eye study, Australia. Ophthalmology. 1997;104(6):1033–1040. doi:10.1016/S0161-6420(97)30190-0
7. Klein R, Klein BEK, Linton KL, et al. Prevalence of age-related maculopathy. The beaver dam eye study. Ophthalmology. 1992;99:933–943.
8. Mojana F, Cheng L, Bartsch D-UG, et al. The role of abnormal vitreomacular adhesion in age-related macular degeneration: spectral optical coherence tomography and surgical results. Am J Ophthalmol. 2008;146:218–227. doi:10.1016/j.ajo.2008.04.027
9. Oh HN, Lee JE, Kim HW, Yang JW, Yun IH. Occult choroidal neovascularization after successful macular hole surgery treated with ranibizumab. Clin Ophthalmol. 2012;6:1287–1291.
10. Natarajan S, Mehta HB, Mahapatra SK, Sharma S. A rare case of choroidal neovascularization following macular hole surgery. Graefes Arch Clin Exp Ophthalmol. 2006;244(2):271–273. doi:10.1007/s00417-005-0004-9
11. Tabandeh H, Smiddy WE. Choroidal neovascularization following macular hole surgery. Retina. 1999;19(5):414–417. doi:10.1097/00006982-199919050-00010
12. Obeid A, Ali FS, Deaner JD, et al. Outcomes of pars plana vitrectomy for epiretinal membrane in eyes with coexisting dry age-related macular degeneration. Ophthalmol Retina. 2018;2(8):765–770. doi:10.1016/j.oret.2018.01.004
13. Wilde C, Awad M, Dua H, et al. Epiretinal membrane surgery outcomes in eyes with subretinal drusenoid deposits: a case control study. Ophthalmol Retina. 2018;2(12):1218–1226. doi:10.1016/j.oret.2018.06.009
14. Lee EK, Lee S-Y, Yu HG. Epiretinal membrane in nonexudative age-related macular degeneration: anatomical features, visual outcomes and prognostic factors. Retina. 2016;36:1557–1565. doi:10.1097/IAE.0000000000000953
15. Banker AS, Freeman WR, Kim JV, et al. Vision-threatening complications of surgery for full-thickness macular holes. Vitrectomy for macular hole study group. Ophthalmology. 1997;104(9):1442–1452. doi:10.1016/S0161-6420(97)30118-3
16. Roller AB, Mahajan VB, Boldt HC, et al. Effects of vitrectomy on age-related macular degeneration. Ophthalmology. 2010;117:1381–1386. doi:10.1016/j.ophtha.2009.11.007
17. Krebs I, Brannath W, Glittenberg C, et al. Posterior vitreomacular adhesion: a potential risk factor for exudative age-related macular degeneration? Am J Ophthalmol. 2007;144:741–746. doi:10.1016/j.ajo.2007.07.024
18. Schulze S, Hoerle S, Mennel S, Kroll P. Vitreomacular traction and exudative age-related macular degeneration. Acta Ophthalmol. 2008;86:470–481. doi:10.1111/j.1755-3768.2008.01210.x
19. Robison CD, Krebs I, Binder S, et al. Vitreomacular adhesion in active and end-stage age-related macular degeneration. Am J Ophthalmol. 2009;148:79–82. doi:10.1016/j.ajo.2009.01.014
20. Barbazetto IA, Liang J, Chang S, et al. Oxygen tension in the rabbit lens and vitreous before and after vitrectomy. Exp Eye Res. 2004;78:917–924. doi:10.1016/j.exer.2004.01.003
21. Becker M, Davis J. Vitrectomy in the treatment of uveitis. Am J Ophthalmol. 2005;140:1096–1105. doi:10.1016/j.ajo.2005.07.017
22. Freund KB, Yanuzzi LA, Sorenson JA. Age-related macular degeneration and choroidal neovascularization. Am J Ophthalmol. 1993;115(6):786–791. doi:10.1016/S0002-9394(14)73649-9
23. Olsen TW, Feng X, Kasper TJ, et al. Fluorescein angiographic lesion type frequency in neovascular age-related macular degeneration. Ophthalmology. 2004;111(2):250–255. doi:10.1016/j.ophtha.2003.05.030
24. Lamin A, Dubis AM, Sivaprasad S. Changes in macular drusen parameters preceding the development of neovascular age-related macular degeneration. Eye. 2019;33:910–916. doi:10.1038/s41433-019-0338-x
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.