Back to Journals » Cancer Management and Research » Volume 12
Thoracoscopic Surgery to Treat Lung Metastases from Refractory Choriocarcinoma
Authors Zhao L, Qin Y, Ma D, Li L, Han Z, Li S, Liu H
Received 25 February 2020
Accepted for publication 5 May 2020
Published 25 May 2020 Volume 2020:12 Pages 3851—3858
DOI https://doi.org/10.2147/CMAR.S251249
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Luo Zhao, Yingzhi Qin, Dongjie Ma, Li Li, Zhijun Han, Shanqing Li, Hongsheng Liu
Department of Thoracic Surgery, Peking Union Medical College Hospital, CAMS & PUMC, Beijing 100730, People’s Republic of China
Correspondence: Hongsheng Liu
Department of Thoracic Surgery, Peking Union Medical College Hospital, Beijing 100730, People’s Republic of China
Tel +86 18519667758
Fax +86 10 69152630
Email [email protected]
Purpose: To assess the use of video-assisted thoracoscopic surgery to treat lung metastases from refractory choriocarcinoma.
Patients and Methods: We reviewed patients diagnosed with refractory choriocarcinoma who underwent lung resection by video-assisted thoracoscopic surgery combined with chemotherapy between October 2013 and August 2019 at the Peking Union Medical College Hospital. The surgical records, pathologic findings and survival rates were analyzed.
Results: The study included 73 patients who underwent 78 thoracoscopic surgeries. Most patients underwent lobectomy (48.7%), and 17 patients (21.8%) underwent resection of more than one lobe. The median operation time and bleeding volume were 95 minutes and 50 mL, respectively. The median duration of chest tube use and hospital stay were 3 days and 4 days, respectively. Postoperative complications were documented in 6 patients (7.7%). The thoracic lymph nodes were harvested in 51 patients (65.4%), but none of these patients had positive nodes. A total of 69.2% of the patients had positive pathologic findings. The mean follow-up time was 30 months. During follow-up, 11 patients experienced disease relapse, and 2 of them died because of brain metastasis. The overall disease-free rate was 83.6%, and the survival rate was 97.0% after excluding those lost to follow-up. Patients with decreased postoperative β-hCG showed a higher disease-free rate during follow up (P< 0.05).
Conclusion: The minimally invasive video-assisted thoracoscopic approach is a valuable and safe treatment for refractory choriocarcinoma patients with lung metastases. Lymphadenectomy is not suggested for these patients. Patients with decreased postoperative β-hCG levels may achieve a much better prognostic result.
Keywords: video-assisted thoracoscopic surgery, lung metastases, choriocarcinoma
Introduction
Gestational trophoblastic tumors (GTNs) are recognized as a relatively rare disease that occurs in women of childbearing age. Since the 1950s, Song et al first introduced large dose of 5-Fluorouracil and other chemicals in the treatment of choriocarcinoma and achieved a breakthrough effect in Peking Union Medical College Hospital (PUMCH), and the mortality rate of the first treated patients has decreased to less than 15% from more than 90% in the past.1 At present, our hospital has been the largest medical center in the diagnosis and treatment of GTNs in the world. International Federation of gynecology and Obstetrics (FIGO) has proposed a staging system and prognostic scoring index for GTNs (Table 1). Choriocarcinoma, as one type of the GTNs, is usually secondary to hydatidiform mole, abortion or term delivery. It most commonly hematogenously metastasizes to the lungs, and most of these patients can be cured with chemotherapy.2 Nevertheless, 10% to 50% of these patients with lung metastasis may develop refractory choriocarcinoma after failing first-line chemotherapy. Some studies have supported the use of lobectomy or sub-lobar resections to cure a majority of these patients with refractory GTNs irrelevant with beta human chorionic gonadotropin (β-hCG) level.3–5 Compared to thoracotomy, video-assisted thoracoscopic surgery (VATS) is currently considered one of the standard treatments for various thoracic diseases. We herein report a retrospective study of 73 patients diagnosed with refractory choriocarcinoma and pulmonary metastases in the Peking Union Medical College Hospital and reviewed our experience with the VATS treatment of these patients.
 |
Table 1 FIGO Staging System for GTN |
Patients and Methods
Study Design and Population
We reviewed 73 patients from the PUMCH who were diagnosed with refractory choriocarcinoma with lung metastasis during first-line chemotherapy and underwent VATS lobectomy or sub-lobar resections between October 2013 and August 2019. Refractory choriocarcinoma was defined as a plateau (variaty<10%) or increase (elevated>10%) in β-hCG level after at least two consecutive cycles of chemotherapy. The normal range of β-hCG is no more than 5 mIU/mL. The clinical information from the patients’ hospital records was retrospectively reviewed. The independent medical ethical committee of the Peking Union medical College approved this retrospective study (IRB number S-K1051) and all the patients signed extensive informed consents.
Multidrug salvage chemotherapy regimens were used for all patients after the diagnosis of refractory choriocarcinoma. The operations were all performed during chemotherapy to reduce the possibility of trophoblast cell metastasis. All available preoperative and postoperative data were collected. Before surgical resection, computed tomography (CT) was performed to assess the location and number of lung metastases. For peripheral nodules, wedge resection was the main method. For central nodules or multiple nodules in one lobe, lobectomy or segmental resection was performed. For multiple nodules in different lobes, more than one lobe resection was performed. Adjuvant chemotherapy was suggested one month after surgery. After surgery, all patients were followed up with serum β-hCG levels and chest CT monitoring after treatment. Relapse was defined as elevated serum β-hCG levels (>10%), radiological occurrence of new lung nodules or death from the disease during follow up.
We analyzed all surgical specimens postoperatively. Positive pathologic findings were defined as the presence of trophoblastic cells in the specimen, whereas the presence of no trophoblastic cells with hemorrhagic tissue, necrosis, or fibrogenesis was defined as negative.
The patient characteristics were summarized using medians or means. Continuous data were compared using one-way Student’s t-tests. The chi-square test was used to analyze the correlations among categorical variables. Differences were considered statistically significant at P<0.05. Survival analysis was performed with the Kaplan-Meier method. The Statistical Package for Social Sciences program was used for all statistical analyses.
Results
Patient Characteristics
A total of 73 patients with refractory choriocarcinoma who underwent VATS were enrolled in this study. The patient characteristics are summarized in Table 2. The median age was 31 years (range 21 to 53). All the patients were treated with multiple chemotherapy cycles and regimens preoperatively. The median preoperative chemotherapy cycles and regimens were 13 (range 1–43) and 3 (range 1–7), respectively. The median International Federation of Gynecology and Obstetrics (FIGO) score was 10 (range 3 to 18). Sixty-four patients were in stage 3 (FIGO staging), and nine patients were in stage 4.
 |
Table 2 Patient Characteristics |
Surgical Management and Pathologic Findings (Tables 3 and 4)
A total of 73 patients underwent 78 thoracoscopic surgeries, and 5 of them underwent surgery twice with a mean interval of 12 months between the first and second surgery. Twenty-four patients had abnormal serum β-hCG levels before surgery. The most frequently performed procedure was lobectomy (48.7%). Seventeen patients (21.8%) underwent resection of more than one lobe. Seven patients (9.0%) underwent synchronous bilateral VATS. Sixteen patients (18.8%) underwent single-port VATS and did not receive a chest tube after surgery. A total of 106 lesions were resected in the 78 patients during surgery. A single metastasis was found in 57 patients (73%). The mean size of the largest lesion was 1.8 cm (range, 0.5–10). We found severe thoracic adhesions in 6 patients (7.7%), 5 of whom had a history of previous ipsilateral thoracic surgery or radiotherapy. There was 1 VATS conversion caused by severe thoracic cavity adhesions. The median operation time was 95 minutes (range, 25 to 285 minutes). The median intraoperative bleeding volume was 50 mL (range, 5–600). Three patients received blood transfusions, mostly because of preoperative anemia caused by chemotherapy. The median duration of chest tube use and hospital stay were 3 days (range, 1–12 days) and 4 days (range, 1–14 days), respectively. Postoperative complications were documented in 6 patients (7.7%). The most common complications were pneumothorax (3.8%) and pneumonia (2.6%). In patients who received wedge resection, the operation time (55 min versus 116.4min), bleeding (17.2mL versus 112.5mL), duration of chest tube stay (1.1 days versus 3.3 days) were significantly less than patients who received lobectomy (P<0.001). The postoperative complication rate was significantly higher in patients with severe thoracic adhesions than in the other patients (33.3% versus 5.6%, P<0.05). Twenty-four patients had abnormal serum β-hCG levels, and 96% (23 of 24) had reduced β-hCG levels (>10%) after surgery, which was significantly higher than that of patients who had normal serum β-hCG levels before surgery (95.8% versus 55.6%, P<0.001). The thoracic lymph nodes were harvested in 65.4% (51 of 78) of the patients, and a mean of 9 nodes were harvested; none of these patients had positive nodes. Of all the patients, 69.2% (54 of 78) had positive pathologic findings. The positive rate was higher in patients with abnormal β-hCG levels than in patients with normal β-hCG (75.0% versus 66.7%, P=0.462). In stage 4 patients, the positive rate of the pathologic findings was 55.6%.
 |
Table 3 Surgical and Pathologic Characteristics of Patients Undergoing Lung Resection for Choriocarcinoma Metastases in PUMCH from 2013 to 2019 |
 |
Table 4 The Comparison of Clinical Characters and Pathologic Results of Refractory Choriocarcinoma Patients (N=78) |
Follow-Up
The mean follow-up time was 30 months (3–73 months). The lost follow-up rate was 8.2% (6/73). During follow-up, 11 patients experienced disease relapse with elevated β-hCG levels, and 2 of them died because of brain metastasis. The patients who were lost to follow-up were excluded from the survival analysis. The overall disease-free rate was 83.6%, and the survival rate was 97.0%. Patients with decreased postoperative β-hCG showed a higher disease-free rate during follow up (P<0.05). The Kaplan-Meier analysis results were shown in Figure 1. Patients with preoperative abnormal β-hCG, single lesion, and larger lesion size also showed a higher disease-free rate during follow up, however, no significance were found. (Figure 2)
 |
Figure 1 The disease-free analysis of patients (N=67). (A) The overall disease-free rate was 83.6%. (B) Patients with decreased postoperative β-hCG showed a higher disease-free rate. |
 |
Figure 2 Subgroup disease-free analysis were showed by preoperative β-hCG level (A), lesion numbers (B), lesion size (C) and FIGO stage (D). |
Discussion
Chemotherapy for GTNs is one of the most remarkable successes in the treatment of human malignant tumors.6,7 When first diagnosed as GTNs, patients are classified according to the FIGO score and different chemotherapy regimens are selected. Most patients can be cured by chemotherapy alone. However, some patients have refractory choriocarcinoma with lung metastases after failing first-line chemotherapy. Surgical excisions, including hysterectomy, pulmonary resections and other procedures, may be invaluable in the treatment of highly selected patients with persistent drug-resistant diseases.8,9 Combined salvage chemotherapy with surgical excision of persistent lesions will cure most refractory choriocarcinoma patients. Refractory choriocarcinoma associated with lung metastases may be caused by ineffective drugs reaching the lung lesions due to central necrosis or fibrin-encapsulated tumors or the presence of one or more drug-resistant cell clones in the lung lesions after chemotherapy. Some retrospective studies have shown that pulmonary surgery is effective for refractory choriocarcinoma patients with lung metastases, although the series was relatively small.10,11 This is the largest retrospective study to evaluate the benefit of surgery in treating refractory choriocarcinoma. In our study, the positive pathology rate was 69.2% in all patients. Furthermore, 95.8% of the patients with abnormal β-hCG levels had reduced β-hCG levels after surgery, which is a significantly higher proportion than that of patients with normal β-hCG levels. These results suggest that surgical treatment is more effective for patients with drug-resistant pulmonary lesions and abnormal β-hCG levels.
Before surgery, we need to perform an accurate preoperative imaging evaluation of these patients. CT is the most recommended examination. For a single nodule, we need to choose an appropriate surgical method, such as wedge resection, lobectomy or segmentectomy, which mostly depends on the size and location of the nodule. For patients with multiple or bilateral nodules, we can choose synchronous or staged resection, which should be individualized according to the general condition of patients, number of lobes to be resected, pulmonary function, etc. In our study, almost 50% of patients received lobectomy, mainly because the nodules were central or multiple on a single lobe, which could not be resected partially. Regarding incision selection, a single-port VATS is preferred in patients undergoing a simple wedge resection with no thoracic drainage tube placement, which may reduce postoperative pain and the length of hospital stay and improve patient satisfaction. In our study, wedge resection patients had significantly shorter hospital stays and less bleeding than lobectomy patients. As all the patients have suffered hardships after so many chemotherapy cycles before the operation, they are highly concerned about the incision length, incision numbers, pain and hospitalization time of the surgical treatment. Therefore, in refractory choriocarcinoma patients with lung metastases, the surgical approaches need to be carefully designed to maximize the benefits with minimal trauma and pulmonary function loss. Furthermore, the VATS technology gives us a chance to perform synchronous bilateral thoracotomy with a shorter operation time, less trauma and shorter hospital stay than the open route.
Several studies have examined the outcomes of pulmonary surgery in refractory choriocarcinoma patients with lung metastasis. However, most studies contained a relatively small case number.5,12 In our study, the overall disease-free rate and survival rate were 83.6% and 97.0%, respectively, which is much higher than those in previous studies. Patients with decreased postoperative β-hCG showed a higher disease-free rate during follow up (P<0.05). We can conclude that decreased postoperative β-hCG will be a predictor in better prognosis after surgery. Meanwhile, patients with preoperative abnormal β-hCG, single lesion, larger lesion size (>2cm) were prone to show a much higher disease-free rate, indicating an important role in selected patients. However, no significance was found between these groups due to small case numbers. For patients with distal metastases that were not lung metastases (FIGO stage 4), such as brain or renal metastases, if drug-resistant pulmonary lesions were found in the course of treatment, we also performed lesion resection. The postoperative pathological findings showed that the positive rate is comparable, and the treatment effect is better after the operation. We also found that all stage 4 patients were alive, and normal β-hCG levels were detected during follow-up. Therefore, surgical treatment may be helpful in highly selected stage 4 refractory choriocarcinoma patients. However, we still need more powerful evidence in the future.
We can conclude several experiences according to our study. 1. Patients without a past history of ipsilateral lung surgery, pleurisy, or tuberculosis may have a low incidence of pleural adhesion. In comparison, for patients with a history of ipsilateral lung surgery, the incidence of pleural adhesion, VATS conversion and postoperative complications were much higher. 2. Most patients have undergone several cycles of chemotherapy and usually have anemia and leukopenia during the perioperative period, thus requiring appropriate blood transfusions and therapy with leukocyte-increasing agents according to the routine blood tests after surgery. 3. As the small number of these patients, we explosively carried out systematic mediastinal lymphadenectomy to study whether there was lymph node metastasis in the early lobectomy cases. According to our study, 65.4% of the patients underwent lymphadenectomy, and 466 lymph nodes were examined. However, there were no lymph node metastases in any of the samples. We suggest that no lymphadenectomy is required for refractory choriocarcinoma patients with lung metastases. The pathophysiological reason is that choriocarcinoma mainly depends on hematogenous metastases rather than lymph node metastases, so theoretically, there are no lymph node metastases. 4. During the differential diagnosis, we need to be vigilant about the possibility of choriocarcinoma combined with lung cancer. The typical characteristics of lung metastases from choriocarcinoma are as follows: solid and yellow with necrosis in the middle of the nodule (Figure 3). In our study, a patient was found to have lung metastases in both the right lower lobe and middle lobe nodules, in which the right middle lobe nodules indicated positive lung metastases of choriocarcinoma, while the right lower lobe pathologically showed minimally invasive adenocarcinoma. Finally, a right lower lobectomy was carried out to achieve radical resection at the same time. Therefore, if atypical signs of choriocarcinoma are present and the nodules are resected intraoperatively, we recommend performing intraoperative pathology to avoid misdiagnosing other lung diseases, such as lung cancer.
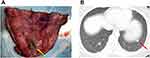 |
Figure 3 Typical lung metastases from choriocarcinoma. (A) Solid and yellow with necrosis in the middle of the nodule (yellow arrow). (B) CT image (red arrow). |
There are still several limitations in our study. First, this is a retrospective study, and without comparing patients who did not receive surgical treatment, it is difficult to evaluate the exact benefits of surgery in refractory choriocarcinoma patients. Compared with previous studies, the positive rate of pathologic findings, the disease-free survival rate and the overall survival rate in our study are significantly improved, however, patient selection bias may exist. In addition, the positive rate of pathologic findings still needs improvement, but it is difficult to distinguish whether the living trophoblasts exist from CT imaging preoperatively. Further randomized controlled trials are needed to elucidate these questions.
Conclusion
This retrospective study showed that pulmonary surgery is valuable and safe in the treatment of refractory choriocarcinoma patients with lung metastases. Patients with abnormal preoperative β-hCG levels may achieve a much better prognostic result after surgery than those with normal preoperative β-hCG levels. VATS is recommended as a minimally invasive approach to achieve enhanced recovery after surgery. We can also perform synchronous bilateral thoracotomy. A preoperative evaluation is very important for individualized therapy. We suggest that lymphadenectomy is not necessary in these patients. The differential diagnoses should exclude primary lung cancer combined with choriocarcinoma metastases.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Song HZ, Wu PC, Wang YE, Yang XY, Dong SY. Pregnancy outcomes after successful chemotherapy for choriocarcinoma and invasive mole: long-term follow-up. Am J Obstet Gynecol. 1988;158(3):538–545. doi:10.1016/0002-9378(88)90021-X
2. El-Helw LM, Hancock BW. Treatment of metastatic gestational trophoblastic neoplasia. Lancet Oncol. 2007;8:715–724. doi:10.1016/S1470-2045(07)70239-5
3. Cao Y, Xiang Y, Feng F, Wan X, Yang X. Surgical resection in the management of pulmonary metastatic disease of gestational trophoblastic neoplasia. Int J Gynecol Cancer. 2009;19(4):798–801. doi:10.1111/IGC.0b013e3181a3d014
4. Feng F, Xiang Y. Surgical management of chemotherapy-resistant gestational trophoblastic neoplasia. Expert Rev Anticancer Ther. 2010;10(1):71–80. doi:10.1586/era.09.169
5. Feng F, Hu H, Wu L, et al. Thoracotomy in refractory gestational trophoblastic neoplasia with lung metastasis after normalization of serum beta human chorionic gonadotropin (beta-hCG) with salvage chemotherapy. Onco Targets Ther. 2014;7:171–176. doi:10.2147/OTT.S56361
6. Lurain JR, Singh DK, Schink JC. Primary treatment of metastatic high-risk gestational trophoblastic neoplasia with EMA-CO chemotherapy. J Reprod Med. 2006;51:767–772.
7. Chapman-Davis E, Hoekstra AV, Rademaker AW, et al. Treatment of nonmetastatic and metastatic low-risk gestational trophoblastic neoplasia: factors associated with resistance to single-agent methotrexate chemotherapy. Gynecol Oncol. 2012;125:572–575. doi:10.1016/j.ygyno.2012.03.039
8. Hanna RK, Soper JT. The role of surgery and radiation therapy in the management of gestational trophoblastic disease. Oncologist. 2010;15:593–600. doi:10.1634/theoncologist.2010-0065
9. Soper JT. Role of surgery and radiation therapy in the management of gestational trophoblastic disease. Best Pract Res Clin Obstet Gynaecol. 2003;17:943–957. doi:10.1016/S1521-6934(03)00091-9
10. Fleming EL, Garrett L, Growdon WB, et al. The changing role of thoracotomy in gestational trophoblastic neoplasia at the New England Trophoblastic Disease Center. J Reprod Med. 2008;53:493–498.
11. Powles T, Savage PM, Stebbing J, et al. A comparison of patients with relapsed and chemo-refractory gestational trophoblastic neoplasia. Br J Cancer. 2007;96(5):732–737. doi:10.1038/sj.bjc.6603608
12. Yang J, Xiang Y, Wan X, Yang X. The prognosis of gestational trophoblastic neoplasia patient with residual lung tumor after completing treatment. Gynecol Oncol. 2006;103:479–482. doi:10.1016/j.ygyno.2006.03.015
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
