Back to Journals » International Journal of Nanomedicine » Volume 14
Therapeutic Potential Of Foeniculum vulgare Mill. Derived Selenium Nanoparticles In Arthritic Balb/c Mice
Authors Arif A, Bhatti A , John P
Received 9 August 2019
Accepted for publication 9 October 2019
Published 31 October 2019 Volume 2019:14 Pages 8561—8572
DOI https://doi.org/10.2147/IJN.S226674
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Ammara Arif, Attya Bhatti, Peter John
Atta-Ur-Rahman School of Applied Biosciences, National University of Sciences and Technology, Islamabad, Pakistan
Correspondence: Attya Bhatti
Atta-ur-Rahman School of Applied Biosciences, National University of Sciences and Technology, H-12, Islamabad, Pakistan
Tel +92 51 90856128
Fax +92 51 90856102
Email [email protected]
Purpose: Rheumatoid arthritis is an inflammatory autoimmune multifactorial disorder that primarily affects the joints. Currently available treatment options, although effective, still present some side effects. This study proposes an alternative treatment option for rheumatoid arthritis through elucidation of therapeutic potential of Foeniculum vulgare Mill.-derived selenium nanoparticles in arthritic Balb/c mice.
Methods: Synthesis and characterization of selenium nanoparticles were followed by their toxicity analysis on healthy mice. Subsequently, anti-arthritic efficacy of two doses (5 mg/kg and 10 mg/kg) of synthesized selenium nanoparticles was checked on arthritic mice using multiple parameters.
Results: Selenium nanoparticles in 10 mg/kg dose turned out to be more effective in treatment of rheumatoid arthritis as evident by significant reduction in paw volume and normal clinical chemistry parameters of treated arthritic mice. This dose also showed significant antioxidant activity in 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay.
Conclusion: Foeniculum vulgare Mill.-derived selenium nanoparticles retain significant anti-arthritic and antioxidant potential and consequently can further be explored as an alternative treatment option for rheumatoid arthritis.
Keywords: rheumatoid arthritis, nanotherapy, selenium deficiency, plant seed extract, Balb/c mice
Introduction
Rheumatoid arthritis (RA) is a polygenic, autoimmune, inflammatory disease that mainly affects the joints.1,2 It is prevalent in 0.5% to 1% population of the world.3 Characteristics of the disease include inflammation of joints, persistent synovitis and bone erosion primarily influencing peripheral joints.4 It usually exhibits a symmetric distribution. If not treated timely, consequences may include joint deformity, disability and even death in certain cases. The organs effected as a result of extra-articular manifestations of RA include heart, skin, lungs and eyes.5 Early and accurate diagnosis and timely initiation of treatment may help curb the disease to a significant extent. Current treatment options include non-steroidal anti-inflammatory drugs (NSAIDs), disease-modifying anti-rheumatic drugs (DMARDs), biologics and combination therapy.6 Methotrexate (MTX) is usually the drug of choice preferred by rheumatologists because of its potent efficacy. But, it comes with its own side effects which include gastrointestinal disorders, infections, pneumonitis, hepatic dysregulation and nephrotoxicity.7 Tocilizumab, leflunomide, rituximab and tofacitinib are other major highly targeted drugs that have revolutionized the management of rheumatoid arthritis. Still, a persistent concern for clinicians is the elevated risk of infections that is associated with these treatment options.8 In this state of events, it is essential that scientists explore an alternative therapeutic avenue for the control of this disease. One of such options is nanotherapy. Nanoparticles not only have increased bioavailability but also show less toxicity. They have the advantage of controlled release at the target site.9
One other vital observation made by scientists studying rheumatoid arthritis was that selenium concentration is decreased in the synovial fluid, plasma, leukocytes and erythrocytes of patients suffering from rheumatoid arthritis.10–12 Selenium serves as the catalytic center of enzyme glutathione peroxidase which plays a key role in body’s redox regulation and consequently controls inflammatory pathways.13 Selenium nanoparticles (SeNP), thus, present an attractive solution to this problem as the traditional supplements usually have increased toxicity and low absorption. SeNP have also exhibited antioxidant, anti-inflammatory and immunomodulatory activities in previous studies.14,15
When it comes to the synthesis of nanoparticles, there are multiple approaches involved which include physical, chemical and biological methods. Physical and chemical synthesis techniques have certain drawbacks which include enormous energy consumption, high costs, utilization of toxic solvents and formation of hazardous by-products. In contrast, the biggest advantage of biological synthetic method is the environment-friendly nature of the procedure.16 Furthermore, among the biological methods, plant-mediated synthetic approaches are cost-effective, low maintenance and nontoxic.17 Foeniculum vulgare Mill. is a medicinal plant that has been used to treat multiple ailments for decades and is reported to display anti-inflammatory, antioxidant, antimicrobial and hepatoprotective activities.18 It is now being used by nano-scientists in the synthesis of nanoparticles. Previously, gold and silver nanoparticles have been made using this plant.19,20
This study, thus, aims to combine the antioxidant and anti-inflammatory therapeutic potential of Foeniculum vulgare Mill. plant and SeNP and utilize Foeniculum vulgare Mill.-derived SeNP to treat rheumatoid arthritis. Not only would this approach directly target the inflammatory pathways characteristic of rheumatoid arthritis but also fulfill the selenium deficiency which would consequently assist the selenoproteins dependent redox regulatory processes in restoring the body’s oxidative balance. To the best of our knowledge, previous studies have highlighted the individual benefits of these constituents, but their combined effect has not been checked before for the treatment of rheumatoid arthritis.
Materials And Methods
Preparation Of Foeniculum vulgare Mill. Seed Extract
After collection of dried Foeniculum vulgare Mill. seeds from a local source, their morphology was analyzed and verified using previously published literature. Plant seed extract was prepared by mixing 7 g of ground dried seeds in 100 mL deionized water which was kept in shaking incubator for 24 hrs at 40°C in dark conditions at a speed of 2000 rpm. Later, it was centrifuged at 6000 rpm at 4°C and then filtered.
Synthesis Of SeNP From Foeniculum vulgare Mill. Seed Extract
To prepare 100 mL (10 mM) nanoparticle reaction mixture, 173 mg of sodium selenite salt was homogenized in 90 mL deionized water. Then, 10 mL previously prepared plant seed extract was added to it. This reaction mixture was kept in shaking incubator for 72 hrs at 40°C at 2000 rpm in dark conditions. The reaction mixture was initially yellow and turned brick red by the end of 72 hrs. For purification, the supernatant was discarded and deionized water was used to give two washes to the nanoparticle pellet. This was done for 20 mins each at 6000 rpm in a centrifuge using 15 mL falcon tubes. The nanoparticle pellet settled down and was dried at 150°C on a hot plate in a china dish.
Characterization Of SeNP
SeNP were characterized by multiple techniques. UV-Visible (UV-Vis) analysis of plant seed extract and nanoparticle reaction mixture was carried out by spectrophotometer (A & E Lab, Guangzhou, Guangdong, China). Fourier transform infrared spectroscopy (FTIR) analysis was performed using spectrophotometer (Perkin-Elmer Spectrum-100 FTIR Spectrophotometer, Waltham, Massachusetts, United States). Sigma-Aldrich infrared spectrum table was used for finding functional groups corresponding to the frequency peaks. X-ray diffraction (XRD) analysis was performed on powdered nanoparticles in angle range of 10 to 20 theta (ϴ) using X-ray diffractometer (D8 ADVANCE BRUKER, AXS, Germany). Scanning electron microscopy (SEM) analysis was performed using SEM system (TESCAN VEGA 3 tungsten thermionic emission, model 51-ADD0007, sensor 51-1385-046, Kohoutovice, Czech Republic) at the resolution power 5.9 KeV. Energy-dispersive X-ray spectroscopy (EDS) analysis was performed using EDS instrument (Oxford X-act, Tubney Woods Abingdon, Oxfordshire, United Kingdom).
Toxicity Analysis Of Biosynthesized SeNP
Toxicity analysis was conducted on healthy mice to check the biosafety profile of synthesized SeNP. Twenty female Balb/c mice at age eight to twelve weeks were divided into five groups (four mice/group) as depicted in Table 1. One group consisted of healthy control mice while the rest of the four groups were given SeNP in their feed at 2.5 mg/kg, 5 mg/kg, 10 mg/kg and 20 mg/kg doses, respectively, for fourteen consecutive days. At the end of fourteen days, dissection was performed after overnight fasting. Organs comprising liver, kidney and spleen and serum of the mice were collected. Clinical chemistry parameters including liver function tests (total bilirubin, alanine aminotransferase (ALT), alkaline phosphate (ALK)) and renal function tests (urea and creatinine) were evaluated using the collected serum and histological examination was performed on spleen, liver and kidneys of mice. The purpose of conducting these tests was to check if SeNP caused any adverse effects on the enzyme levels or the aforementioned organs.
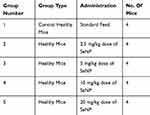 |
Table 1 Experimental Grouping For Toxicity Analysis |
Collagenase Type 2-Induced Arthritic Animal Model Construction
Thirty female Balb/c mice were divided into five groups of six mice each as depicted in Table 2. Arthritis was induced in mice in four of these groups. In the first step of arthritis induction protocol, 0.1 M acetic acid was mixed in Hartman solution in 2:1. Then, 2 mg of collagenase type 2 (Worthington Biochemical Corporation, Lakewood, New Jersey, United States) was added to 1 mL of the above prepared solution to make 1 mL collagenase type 2 solution. This mixture was kept at 4°C overnight in a shaking incubator. Bovine serum albumin (BSA) was dissolved in Hartmann solution (1:1). Collagenase type 2 was mixed in complete Freund’s adjuvant (Sigma-Aldrich, St. Louis, Missouri, United States) in 1:1 and vortexed for 2 to 3 mins. Then, BSA was added into it in 2:1. This mixture was thoroughly mixed in an Eppendorf tube with the help of a pipette. Then, 0.2 mL (2 µl) of this solution was filled into each syringe. Three injections containing the above mixture were administered subcutaneously in the tail on day 0, 7 and 14. The fourth and fifth injections comprised 150 µL of complete Freund’s adjuvant and were administered in the right hind paws of mice on day 21 and 28. The protocol lasted twenty-eight days. Paw volume of all mice was measured at day 0, 7, 14, 21 and 28 using a vernier caliper. By the end of RA induction protocol, swelling and difficulty in motility were visible in mice. Inflammation severity was graded, and grade 3 and 4 scoring mice were further experimented on.21 Previously, Han et al have used collagen type 2 and complete Freund’s adjuvant for induction of arthritis in Balb/c mice.22
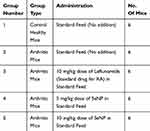 |
Table 2 Experimental Grouping For Therapeutic Analysis |
Administration Of Therapeutic SeNP Doses In Arthritic Mice
Following arthritis induction in mice, the first group containing healthy mice and second group now categorized as untreated arthritic control mice were given standard laboratory feed for fourteen consecutive days. Third group was classified as the one receiving standard drug and was given leflunomide in 10 mg/kg dose for fourteen consecutive days. Fourth and fifth groups comprised the experimental treatment groups. They were administered 5 mg/kg and 10 mg/kg SeNP in their feed, respectively, for fourteen consecutive days. During this time, paw volume of all mice was measured on day 0, 7 and 14 of feed administration. At the end of fourteen days, the mice were dissected following overnight fasting and specimen (serum, spleen, liver and hind paws) were collected, their clinical chemistry parameters were measured using serum and histological analysis of paw tissues was carried out. Spleen indices were calculated to check if the nanoparticles caused splenomegaly.21 For the determination of antioxidant activity, 2,2-diphenyl-1-picrylhydrazyl (DPPH 95%, Alfa Aesar, Ward Hill, MA, USA) assay was performed on treated mice serum. Change of DPPH color from violet to yellow is an indication of free radicals being scavenged. Catalase activity was checked in mice liver tissues for further evaluation of antioxidant activity of SeNP.23–26
Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics version 21 and GraphPad Prism 5. Variance among groups was evaluated using one-way ANOVA followed by Student T test and two-way ANOVA along with Bonferroni Post-test. Values with differences P < 0.05, P < 0.01 and P < 0.001 were considered to be statistically significant.
Results And Discussion
Synthesis Of Foeniculum vulgare Mill.-Derived SeNP
The SeNP reaction mixture that was kept in shaking incubator for 72 hrs, changed color from yellow to brick red. This served as a primary visual indication of successful SeNP synthesis (Figure 1). The characteristic red color of biosynthesized SeNP has been reported by previous studies as well.27
 |
Figure 1 Color transformation of selenium nanoparticle reaction mixture from yellow to brick red depicting successful synthesis of selenium nanoparticles. |
Characterization Of Biosynthesized SeNP
A comparative UV-Visible spectroscopy analysis of absorption spectra of fennel seed extract (Figure 2A) and SeNP (Figure 2B) showed that plant seed extract had multiple constituent compounds which were evident through the multiple peaks, but the SeNP reaction mixture showed a single sharp peak at 275 nm. The absence of any other peak in the SeNP reaction mixture absorption spectrum also indicated that the synthesized nanoparticles were made of pure selenium and there was no impurity.
Similarly, FTIR spectroscopy analysis of fennel seed extract (Figure 3A) compared to SeNP reaction mixture (Figure 3B) showed change in absorption peaks. This proved that chemically active compounds in fennel seed extract caused reduction of sodium selenite ions into elemental selenium which consequently resulted in formation of SeNP.
XRD analysis showed sharp specific diffraction peaks (Figure 4) at 2ϴ values 23.28, 29.50, 41.26, 43.51, 45.24, 51.29, 55.61, 61.50 and 65.29. These 2ϴ values corresponded to reflections at 100, 101, 110, 012, 111, 201, 003, 022 and 210, respectively, from crystalline SeNP (JCPDS number: 03-065-1876). Similar reflection values have been reported by previous studies conducted on biosynthesis and characterization of SeNP.28
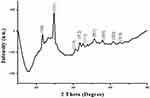 |
Figure 4 X-ray diffraction pattern of selenium nanoparticles derived from Foeniculum vulgare Mill. seed extract. |
SEM analysis showed spherical to irregular morphology of SeNP (Figure 5). The average nanoparticle size measurement from SEM image turned out to be 47.14 nm. Previous studies reveal that nanoparticles that range in size from 10 to 60 nm exhibit maximum cellular uptake in non-phagocytic cells.29
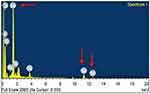
EDS analysis highlighted specific selenium peaks at three points (Figure 6). The presence of elements like carbon, calcium, oxygen and sodium in the graph can be attributed to plant (organic) source of nanoparticle synthesis.
Clinical Chemistry Parameters Of SeNP-Administered Healthy Mice
Comparison of ALT, total bilirubin, creatinine and urea values of healthy control mice and the groups administered four doses of SeNP showed insignificant differences. However, ALK value of groups that received 10 mg/kg and 20 mg/kg dose of SeNP showed significant difference (P <0.05) from the healthy mice (Table 3). This may be attributed to the fact that these two doses increased the selenium levels in the serum and this excess selenium ultimately caused deviation in the ALK values. Previously, Hoffman has reported variation in ALK values due to selenium toxicity.30
 |
Table 3 Clinical Chemistry Parameters Of Healthy Mice Administered Four Doses Of SeNP (Toxicity Analysis) |
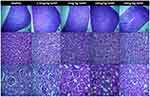
Histopathological Analysis Of SeNP-Administered Healthy Mice
Spleen, liver and kidney sections of healthy mice and the mice comprised in the groups that received four different doses of SeNP are depicted in Figure 7. Histopathological analysis of spleen showed intact red and white pulp in all groups (Figure 7A–E). The liver of healthy mice and all four groups given SeNP showed intact hepatocellular morphology (Figure 7F–J). The kidney sections of healthy mice and those that received 5 mg/kg, 10 mg/kg and 20 mg/kg doses of SeNP showed intact glomerular structures (Figure 7K–O). Whereas, the group that received 2.5 mg/kg dose of SeNP showed somewhat distorted glomeruli (Figure 7L). Glomerular distortion has been reported in the past by Kumar, Gautam and Sinha in selenium-administered broiler birds.31
Paw Swelling In RA-Induced Mice
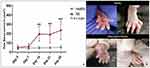
A significant increase in paw volume (P < 0.001) at day 14, 21 and 28 was evident in experimental mice compared to healthy control mice (Figure 8A). The difference in swelling can be witnessed in Figure 8B. In addition, redness was also observed. These observations indicated that RA was successfully induced in mice.
Reduction In Paw Volume Of Treated Arthritic Mice
Paw volume of untreated arthritic mice kept increasing till day 14 as evident in Figure 9A. In contrast, the groups of mice treated with SeNP showed decrease in paw volume. Mice that were treated with 5 mg/kg dose of SeNP showed comparatively less decrease in paw volume (P < 0.05). Mice treated with 10 mg/kg dose of SeNP showed greater decrease in paw volume (P < 0.01), while the group of mice that were treated with standard arthritic drug leflunomide showed greatest decrease in paw volume (P < 0.001). A pictorial depiction of gross changes in treated and untreated mice groups is shown in Figure 9B.
Histopathological Analysis Of Treated Arthritic Mice Paw Tissues
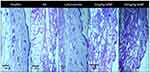
Histopathological analysis of untreated arthritic mice paw tissue showed bone erosion and distorted cellular morphology (Figure 10), whereas, photomicrographs of paw tissues from SeNP-treated groups (5 mg/kg and 10 mg/kg) as well as leflunomide-treated group showed restored cellular morphology and no signs of erosion. Paw tissue from healthy mice is also shown for comparison (Figure 10).
Radical Scavenging Activity Of Treated Arthritic Mice Serum
DPPH assay was conducted to measure antioxidant or radical scavenging potential of SeNP treated mice serum compared to untreated mice serum. Mice that received 10 mg/kg dose of SeNP showed significant increase in radical scavenging activity as compared to untreated arthritic mice serum (P < 0.05) as shown in Figure 11. The serum of mice treated with leflunomide also showed a considerable antioxidant potential compared to the arthritic group (P < 0.01). Biosynthesized SeNP showed moderate DPPH scavenging activity in a study conducted by Forootanfar et al.32
Catalase Enzyme Activity Of Treated Arthritic Mice Liver Tissues
Comparison of catalase enzyme activity of untreated and treated mice liver tissues showed no significant difference (Figure 12). This may be attributed to the fact that used liver tissues were preserved for a few days. Fresh tissue samples may exhibit different results. Ashouri et al reported significant increase in catalase activity in nano-selenium fed Cyprinus carpio liver tissues.33
Spleen Indices Of Treated Arthritic Mice
Spleen indices of treated as well as untreated groups showed no significant difference. This shows that biosynthesized SeNP did not have any adverse effects on spleen and did not cause splenomegaly (Figure 13). Hyperplasia of spleen has been reported previously in rats at some doses of SeNP.34
Clinical Chemistry Parameters Of Treated Arthritic Mice
Oral administration of SeNP in 5 mg/kg and 10 mg/kg doses showed no significant difference in the ALT, total bilirubin, urea and creatinine values of treated mice compared to healthy controls. However, mice treated with 5 mg/kg dose of SeNP and untreated arthritic mice showed significant difference in ALK values as compared to healthy control mice (P < 0.05). It indicates that 5 mg/kg dose of SeNP was not effective in restoring the ALK values that got increased in arthritic mice. This is depicted in Table 4.
 |
Table 4 Clinical Chemistry Parameters Of SeNP-Treated And SeNP-Untreated Arthritic Mice (Therapeutic Analysis) |
Biogenic SeNP find applications in various fields. They are utilized as anti-cancer, anti-oxidant and antimicrobial agents in medicine. Nanobiosensors employing SeNP have been reported to detect hydrogen peroxide. Another study reported use of amorphous biogenic SeNP for removing mercury from contaminated water.35
Conclusion
In a recent study conducted by Ren et al, commercially available SeNP dispersed in a specific type of phytochemical exhibited significant anti-inflammatory potential in arthritic rat model.36 Our study also reinforces the therapeutic nature of SeNP. According to our study, Foeniculum vulgare Mill. seed extract-derived SeNP retain a good biosafety profile in case of oral route of administration. They exhibited significant anti-arthritic and antioxidant activity which emphasizes their potential as a promising alternative treatment option for rheumatoid arthritis.
Abbreviations
RA, rheumatoid arthritis; SeNP, selenium nanoparticles; NSAIDs, non-steroidal anti-inflammatory drugs; DMARDS, disease-modifying anti-rheumatic drugs; MTX, methotrexate; UV-Vis, ultraviolet visible; FTIR, Fourier transform infrared; XRD, X-ray diffraction; SEM, scanning electron microscopy; EDS, energy dispersive X-ray spectroscopy; ALT, alanine aminotransferase; ALK, alkaline phosphatase; TB, total bilirubin; U/L, units per liter; mg/dL, milligrams per deciliter; mg/kg, milligrams per kilogram; DPPH, 2,2-Diphenyl-1-picrylhydrazyl; BSA, bovine serum albumin.
Ethics Approval Statement
All experimental protocols were approved by the institutional review board committee at Atta-ur-Rahman School of Applied Biosciences, National University of Sciences and Technology, Islamabad, Pakistan (Reference no: IRB-117). Mice were taken care of as per the principles stipulated in Guide for the Care and Use of Laboratory Animals.37
Disclosure
The authors report no conflicts of interest in this work.
References
1. Smolen JS, Aletaha D, Barton A, et al. Rheumatoid arthritis. Nat Rev Dis Prim. 2018;4:18001. doi:10.1038/nrdp.2018.1
2. Weyand CM. Rheumatoid arthritis: a polygenic disease with multiple phenotypes. Arthritis Res. 2000;1(Suppl 1):S03. doi:10.1186/ar17
3. Silman AJ, Pearson JE. Epidemiology and genetics of rheumatoid arthritis. Arthritis Res. 2002;4 Suppl 3(Suppl 3):S265–S272. doi:10.1186/ar578
4. Sodhi A, Naik S, Pai A, Anuradha A. Rheumatoid arthritis affecting temporomandibular joint. Contemp Clin Dent. 2015;6(1):124–127. doi:10.4103/0976-237X.149308
5. West S. Clinical Overview of Rheumatoid Arthritis. Cham: Humana Press; 2018:1–18. doi:10.1007/978-3-319-68888-6_1
6. Majithia V, Geraci SA. Rheumatoid arthritis: diagnosis and management. Am J Med. 2007;120(11):936–939. doi:10.1016/j.amjmed.2007.04.005
7. Wang W, Zhou H, Liu L. Side effects of methotrexate therapy for rheumatoid arthritis: a systematic review. Eur J Med Chem. 2018;158:502–516. doi:10.1016/J.EJMECH.2018.09.027
8. Subesinghe S, Bechman K, Rutherford AI, Goldblatt D, Galloway JB. A systematic review and metaanalysis of antirheumatic drugs and vaccine immunogenicity in rheumatoid arthritis. J Rheumatol. 2018;45(6):733–744. doi:10.3899/jrheum.170710
9. Oliveira IM, Gonçalves C, Reis RL, Oliveira JM. Engineering nanoparticles for targeting rheumatoid arthritis: past, present, and future trends. Nano Res. 2018;11(9):4489–4506. doi:10.1007/s12274-018-2071-3
10. Silva GB, Reis BZ, Maria S, Cozzolino F. Pathology and clinical research micronutrients deficiencies in rheumatoid arthritis patients. Int J Pathol Clin Res. 2016;2(1):029. doi:10.23937/2469-5807/1510029
11. Yu N, Han F, Lin X, Tang C, Ye J, Cai X. The association between serum selenium levels with rheumatoid arthritis. Biol Trace Elem Res. 2016;172(1):46–52. doi:10.1007/s12011-015-0558-2
12. Tarp U, Overvad K, Hansen JC, Thorling EB. Low Selenium Level in Severe Rheumatoid Arthritis. Scand J Rheumatol. 1985;14(2):97–101. doi:10.3109/03009748509165490
13. Ueno M, Sekine-Suzuki MNE, Shimokawa T, Nakanishi I, Matsumoto K. Preparation of selenium-deficient mouse model and its glutathione peroxidase activity. Free Radic Biol Med. 2018;120:S141. doi:10.1016/J.FREERADBIOMED.2018.04.464
14. Hosnedlova B, Kepinska M, Skalickova S, et al. Nano-selenium and its nanomedicine applications: a critical review. Int J Nanomedicine. 2018;13:2107. doi:10.2147/IJN.S157541
15. Khurana A, Tekula S, Saifi MA, Venkatesh P, Godugu C. Therapeutic applications of selenium nanoparticles. Biomed Pharmacother. 2019;111:802–812. doi:10.1016/J.BIOPHA.2018.12.146
16. Thakkar KN, Mhatre SS, Parikh RY. Biological synthesis of metallic nanoparticles. Nanomedicine Nanotechnol Biol Med. 2010;6(2):257–262. doi:10.1016/J.NANO.2009.07.002
17. Parveen K, Banse V, Ledwani L. Green synthesis of nanoparticles: their advantages and disadvantages.
18. Badgujar SB, Patel VV, Bandivdekar AH. Foeniculum vulgare Mill: a review of its botany, phytochemistry, pharmacology, contemporary application, and toxicology. Biomed Res Int. 2014;2014:842674. doi:10.1155/2014/842674
19. Choudhary MK, Kataria J, Sharma S. A biomimetic synthesis of stable gold nanoparticles derived from aqueous extract of Foeniculum vulgare seeds and evaluation of their catalytic activity. Appl Nanosci. 2017;7(7):439–447. doi:10.1007/s13204-017-0589-4
20. Nigar Sulthana R, Rajanikanth A. Green synthesis of silver nanoparticles using seed extract of Foeniculum vulgare and their antibacterial activity. Int J Curr Res Biosci Plant Biol. 2018;5(7):77–83. doi:10.20546/ijcrbp.2018.507.010
21. Liu Y, Zhang L, Wu Y, et al. Therapeutic effects of TACI-Ig on collagen-induced arthritis by regulating T and B lymphocytes function in DBA/1 mice. Eur J Pharmacol. 2011;654(3):304–314. doi:10.1016/j.ejphar.2011.01.002
22. Han H-M, Hong S-H, Park H-S, et al. Protective effects of Fructus sophorae extract on collagen-induced arthritis in BALB/c mice. Exp Ther Med. 2017;13(1):146–154. doi:10.3892/etm.2016.3929
23. Jang D-Y, Kim S-J, Jeong J-H, et al. Protective effects of sodium selenite and selenium nanoparticles against experimental colon carcinogenesis in mice. J Prev Vet Med. 2016;40(3):101–108. doi:10.13041/jpvm.2016.40.3.101
24. Kaliaperumal J, Namasivayam E. Evaluation of the anti-tumor and antioxidant activity of amorphophallus paeonifolius on DMBA induced mammary carcinoma. Int J Chem Pharm Sci. 2010;1(2):40–50.
25. Sajeeth CI, Manna PK, Manavalan R. Antioxidant activity of polyherbal formulation on streptozotocin induced diabetes in experimental animals. Pelagia Res Libr. 2011;2(2):220–226. Available from: www.pelagiaresearchlibrary.com.
26. Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47(2):389–394. doi:10.1016/0003-2697(72)90132-7
27. Zhang W, Chen Z, Liu H, Zhang L, Gao P, Li D. Biosynthesis and structural characteristics of selenium nanoparticles by Pseudomonas alcaliphila. Colloids Surf B Biointerfaces. 2011;88(1):196–201. doi:10.1016/J.COLSURFB.2011.06.031
28. Srivastava N, Mukhopadhyay M. Biosynthesis and structural characterization of selenium nanoparticles mediated by Zooglea ramigera. Powder Technol. 2013;244:26–29. doi:10.1016/J.POWTEC.2013.03.050
29. Hoshyar N, Gray S, Han H, Bao G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine (Lond). 2016;11(6):673–692. doi:10.2217/nnm.16.5
30. Hoffman DJ. Role of selenium toxicity and oxidative stress in aquatic birds. Aquat Toxicol. 2002;57(1–2):11–26. doi:10.1016/S0166-445X(01)00263-6
31. Kumar D, Kumar Gautam A, Sinha MK. Histopathological alterations of selenium toxicity induced in broiler (Birds). Anim Res. 2018;52(4):599–604. doi:10.18805/ijar.v0iOF.7259
32. Forootanfar H, Adeli-Sardou M, Nikkhoo M, et al. Antioxidant and cytotoxic effect of biologically synthesized selenium nanoparticles in comparison to selenium dioxide. J Trace Elem Med Biol. 2014;28(1):75–79. doi:10.1016/J.JTEMB.2013.07.005
33. Ashouri S, Keyvanshokooh S, Salati AP, Johari SA, Pasha-Zanoosi H. Effects of different levels of dietary selenium nanoparticles on growth performance, muscle composition, blood biochemical profiles and antioxidant status of common carp (Cyprinus carpio). Aquaculture. 2015;446:25–29. doi:10.1016/J.AQUACULTURE.2015.04.021
34. He Y, Chen S, Liu Z, Cheng C, Li H, Wang M. Toxicity of selenium nanoparticles in male Sprague–Dawley rats at supranutritional and nonlethal levels. Life Sci. 2014;115(1–2):44–51. doi:10.1016/J.LFS.2014.08.023
35. Wadhwani SA, Shedbalkar UU, Singh R, Chopade BA. Biogenic selenium nanoparticles: current status and future prospects. Appl Microbiol Biotechnol. 2016;100(6):2555–2566. doi:10.1007/s00253-016-7300-7
36. Ren S-X, Zhan B, Lin Y, Ma D-S, Yan H. Selenium nanoparticles dispersed in phytochemical exert anti-inflammatory activity by modulating catalase, GPx1, and COX-2 Gene expression in a rheumatoid arthritis rat model. Med Sci Monit. 2019;25:991–1000. doi:10.12659/MSM.912545
37. National Research Council. Guide for the Care and Use of Laboratory Animals.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.