Back to Journals » Clinical Interventions in Aging » Volume 15
The Serum Concentration of Anti-Aging Proteins, Sirtuin1 and αKlotho in Patients with End-Stage Kidney Disease on Maintenance Hemodialysis
Authors Zbroch E , Bazyluk A , Malyszko J , Koc-Zorawska E, Rydzewska-Rosolowska A , Kakareko K, Hryszko T
Received 10 November 2019
Accepted for publication 17 February 2020
Published 16 March 2020 Volume 2020:15 Pages 387—393
DOI https://doi.org/10.2147/CIA.S236980
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Walker
Edyta Zbroch, 1 Angelika Bazyluk, 1 Jolanta Malyszko, 2 Ewa Koc-Zorawska, 1 Alicja Rydzewska-Rosolowska, 1 Katarzyna Kakareko, 1 Tomasz Hryszko 1
1 2-nd Department of Nephrology and Hypertension with Dialysis Centre, Medical University, Bialystok, Poland; 2Department of Nephrology, Dialysis and Internal Medicine, Medical University of Warsaw, Warsaw, Poland
Correspondence: Edyta Zbroch
2-nd Department of Nephrology and Hypertension with Dialysis Unit, Medical University, Sklodowskiej Curie 24a, Bialystok 15-276, Poland
Tel +48 85 8317872 Email [email protected]
Introduction: Sirtuin1 (SIRT1) acts as an anti-aging protein due to anti-apoptotic, anti-oxidative and anti-inflammatory effect and is implicated in several diseases including diabetes or cardiovascular problems. SIRT1 renal overexpression indicates oxidative stress. Similarly, αKlotho was primarily exposed as anti-aging factor. It is primary produced in kidney. It’s deficiency is associated with progression of chronic kidney disease and heart disorders.
Purpose: The aim of the study was to assess the serum concentration of sirtuin1 and αKlotho in hemodialysis (HD) patients compared to healthy volunteers in regard to age, blood pressure control, residual kidney function (RKF), diabetes, cardiovascular disease, dialysis vintage and type of dialyzer.
Patients and Methods: The serum level of SIRT1 and αKlotho was evaluated using ELISA tests in 103 HD patients, median age 67 years and in 21 volunteers. Blood pressure, RRF, echocardiography and dialysis parameters were assessed. HD group was divided according to the presence/absence of RKF.
Results: The serum SIRT1 level was higher (28.4 vs 2.71ng/mL, p< 0.0001) and αKlotho was lower (433.9 vs 756.6pg/mL, p< 0.0001) in HD then in control group. αKlotho was lower in those without RKF (387.2 vs 486.2pg/mL, p=0.028). SIRT1 positively correlated with hemodialysis vintage. αKlotho negatively correlated with left ventricular posterior wall thickness. There was no significant relationship between SIRT1 and αKlotho level and age, blood pressure control, type of dialyzer, Kt/V and diabetes. Multivariate analysis revealed association of SIRT1 with ejection fraction (B − 0.72; p=0.32).
Conclusion: Elevated SIRT1 and lower αKlotho concentration are associated with impaired kidney function. The decrease in levels of αKlotho may also indicate heart hypertrophy in hemodialysis patients. The role of anti-aging proteins, particularly SIRT1 as biomarkers/predictors of oxidative stress, inflammation and cardiovascular diseases need further examination.
Keywords: sirtuin1, αKlotho, chronic kidney disease, hemodialysis
Plain Language Summary
For decades people have tried to slowdown aging processes and extend their lifespan. Sirtuin1 and αKlotho are considered anti-aging factors. SIRT1 is implicated in several diseases including diabetes or cardiovascular problems. Klotho is primarily produced in kidney. Its deficiency is associated with progression of kidney and heart disease. As renal patients are in a state resembling accelerated aging, we designed this study to assess the serum concentration of sirtuin1 and αKlotho in hemodialysis patients comparing to healthy volunteers in regard to age, blood pressure control, residual kidney function, diabetes, cardiovascular disease, dialysis vintage and type of dialyzer. Results revealed higher serum SIRT1 and lower αKlotho level in hemodialysis group comparing to the control one. αKlotho was also lower in those without residual kidney function. We concluded that elevated SIRT1 and lower αKlotho concentration are associated with impaired kidney function. Decreased level of αKlotho may also be a marker of heart hypertrophy in hemodialysis patients.
Introduction
Sirtuins (SIRT – silent information regulator) are a group of NAD+ - dependent histone deacetylases which has an impact on many different biological processes by promoting chromatin silencing and transcriptional repression.1,2 Thus they are involved in the cellular energy metabolism, biogenesis of mitochondria, response on stress, apoptosis, inflammatory reaction and fibrosis. The family of sirtuins contains seven isoforms: SIRT1 - SIRT7.3 Sirtuin1, which is also called the longevity gene, is mostly studied one.4,5
Klotho is a transmembrane protein that shares sequence homology with family I β-glycosidases.6,7 There is merely one mammalian αKlotho gene that encodes three isoforms of Klotho protein, namely the transmembrane, a shed soluble and a truncated soluble form generated by alternative Klotho mRNA splicing.6,8
For decades people have tried to slow down aging processes and extend their lifespan. Sirtuin1 and αKlotho are considered anti-aging factors.1,6 SIRT1 due to an anti-apoptotic, anti-oxidative and anti-inflammatory effect is involved in the pathogenesis of several diseases such as diabetes or cardiovascular disorders. It improves glucose tolerance, reduces hyperinsulinemia and enhances systemic insulin sensitivity.1,9,10 By far, SIRT1 is an important nephroprotective factor, especially in the diabetic kidney disease.11,12 Its renal overexpression is an answer to oxidative stress. Klotho is mainly produced in the kidneys.6 Its deficiency is associated with the progression of chronic kidney disease and heart disease. Low levels of Klotho in patients with chronic kidney disease can also promote vessel calcification and salt-dependent hypertension.13,14 Animal studies indicate a nephroprotective role of Klotho probably by influence on the activity of renin-angiotensin-aldosterone system.15
As renal patients are in a state resembling accelerated aging, we designed this study to assess the serum concentration of sirtuin1 and αKlotho in hemodialysis (HD) patients comparing it to healthy volunteers and to analyze the effect of age, blood pressure control (BP), residual kidney function (RKF), diabetes, cardiovascular disease, dialysis vintage and type of dialyzer on both SIRT1 and αKlotho levels.
Patients and Methods
Participants
In this cross-sectional study, we included 103 hemodialysis (HD) patients (median age of 67–51% male). The median hemodialysis vintage was 36 months. All patients were informed about the aim of the study and gave their written informed consent, according to the Declaration of Helsinki. The study was approved by the Medical University Ethic Committee. The clinical trial registration number is: R-I-002/455/2016.
Methods
Blood pressure (BP) was measured before and after hemodialysis session in the sitting position using automatic manometer. The arithmetic average of three measurements taken on different days was used in the analysis. Well-controlled blood pressure level was defined according to Kidney Disease Outcomes Quality Initiative guidelines16 as lower then 140/90 mmHg before HD session and lower than 130/80 mmHg after it. Body weight gain was calculated according to the dry weight and the weight measured before the HD session (the arithmetic average from three hemodialysis sessions). Residual kidney function was based on a 24 hr urine collection. The presence of RKF was defined as a 24 hr urine output above 100 mL. Echocardiography was performed in the day between two dialysis according to current guidelines,17 Data on hypotensive drugs used in and individual patient and the type of dialyzer (low flux or high flux) was collected from individual prescription cards. Samples for the estimation of serum SIRT1 and αKlotho concentration were taken once before the HD session in the mid-week dialysis session (when also BP and weight were assessed). Enzyme-linked immunosorbent assay (ELISA) with the use of a monoclonal antibody specific to sirtuin1 - Cloud-Clone Corp., Houston, Texas, USA and αKlotho -Human soluble α-Klotho-IBL (Immuno-Biological Laboratories Co.), Japan was applied. This study was conducted with the use of equipment purchased by Medical University of Bialystok as part of the RPOWP 2007–2013 funding, Priority I, Axis 1.1, contract No. UDA- RPPD.01.01.00-20-001/15-00 dated 26.06.2015. To obtain normal ranges for sirtuin1 and αKlotho in these ELISA assays, 21 healthy volunteers were studied. The study cohort was divided into two groups according to presence/absence of residual renal function.
Analysis
Statistica 10.0 StatSoft Poland was used for statistical analysis. Continuous data are reported as median and interquartile range (IQR). The Shapiro–Wilk test was used to determine the normal distribution and t-Student test or U Mann–Whitney test for comparison of the two groups depending on meeting the assumptions. Associations between sirtuin1 and variables of interest were evaluated with Spearman correlation. A two-tailed p level value below 0.05 was considered significant. Multivariate analysis was performed to determine significant factors associated with SIRT1.
Results
Studied Population
The basal characteristic of the studied groups, divided according to the presence/absence of residual renal function, is presented in Table 1. Dialysis vintage was significantly longer in patients without residual renal function (64.5 [36.0–114.3] vs 27.3 [12.0–48.0] months, p=0.0003. There were no significant differences in blood pressure control and type of dialyzer between two groups – with and without RKF.
 |
Table 1 The Clinical Characteristics of Studied Groups (Divided According to the Presence of Residual Renal Function) |
Healthy Volunteers
Twenty-one persons in median age of 58 (48% male), with no history of chronic disease, including hypertension, other cardiovascular disease, chronic kidney disease, diabetes mellitus. Median BP – 110/70 mmHg, median creatinine level 0.8 mg/dl.
Concentration of Anti-Aging Proteins and Their Associations with Other Variables
Serum SIRT1 level was higher (28.4 [13.5–42.8] vs 2.71 [1.9–5.0] ng/mL, p<0.0001) (Figure 1) and αKlotho was lower (433.9 [362–580] vs 756.6 [632.7–931] pg/mL, p<0.0001) in HD group comparing to the control one (Table 2). No differences were found in SIRT1 levels in patients with diuresis ≥100 mL and <100 mL per day (24.9 [11.6–39.3] vs 30.3 [14.6–46.7] ng/mL, p>0.05). αKlotho was declined in those without RKF (387.2 [314.7–596.1] vs 486.2 [400.8–592.6] pg/mL, p=0.028) (Table 3). There was a positive correlation between SIRT1 and hemodialysis vintage (R 0.24; p=0.03) (Figure 2). We also found a negative correlation between αKlotho concentration and left ventricular posterior wall thickness (R=−0.31; p=0.021) (Figure 3). There was no significant relationship between SIRT1 or αKlotho level and age, blood pressure control, type of dialyzer, Kt/V, diabetes presence. Multivariate analysis revealed association of SIRT1 with ejection fraction (B −0.72; p=0.32).
 |
Table 2 Anti-Aging Proteins Concentration in the Study Group Comparing to Control Group |
 |
Table 3 Anti-Aging Proteins Concentration in the Studied Groups (Divided According to the Presence/Absence of Residual Renal Function) |
 |
Figure 1 Serum sirtuin1 in the study and control group. |
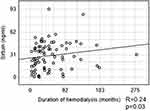 |
Figure 2 Relationship between sirtuin1 and the duration of hemodialysis. |
 |
Figure 3 Relationship between αKlotho and left ventricular posterior wall thickness. |
Discussion
Sirtuin1 and Klotho are known as anti-aging proteins due to protective effects against age-related diseases.1,6,18 Study of Zhong et al19 on healthy subjects revealed significantly lower SIRT1 level after 65 years of age. The same finding was made regarding Klotho level by Xiao et al20 - they reported lower serum Klotho concentration with each age group in humans after 40, in a screened population with age range from 0 to 91 years. Among many risk factors leading to the development of renal insufficiency, aging is an independent and strong predictor for progressive of chronic kidney disease. Hypertension and cardiovascular disease is also diagnosed more often in elderly population.13
The mechanism of Klotho action is better known when compared to sirtuin1. The main spot of Klotho activity is kidney and in the physiological state presumably the most important one.6 This idea is warranted by the study made on mice with a kidney-specific ablation of Klotho which demonstrated the phenotype of global Klotho knockout mice.21 The serum soluble Klotho was declined about 80% in those kidney-specific Klotho knockout mice so the authors concluded that under physiological conditions the great amount of soluble Klotho detected in blood came from kidneys.21,22 The pathogenetic role of Klotho in the development of hypertension and cardiovascular disease by the influence on salt sensitivity and renin-angiotensin system is also well documented.13,14,23 Gao et al15 found that serum Klotho concentration was approximately 45% lower in patients with arterial stiffness and primary hypertension compared to healthy persons. Moreover, Klotho deficiency caused down-regulation of SIRT1 expression and activity in aorta and then contributed to arterial stiffening and hypertension. The authors revealed significant rise in pulse wave velocity and blood pressure in Klotho-haplodeficient (KL+/−) mice.15 Then Kakareko et al24 found reduced soluble Klotho levels after nephrectomy in patients with remaining renal function before surgery. Our study confirms the above findings and shows lower serum αKlotho concentrations in patients with end-stage kidney disease on maintenance hemodialysis compared to healthy volunteers. What is even more interesting, it was lower in subjects without preserved kidney function. We also observed a reverse correlation between αKlotho and left ventricle posterior wall thickness. It confirms the finding of Marçais et al.23 They, in the prospective 2 years ARNOGENE study of a cohort of 769 hemodialysis patients without specific cardiac interventions, demonstrated that higher αKlotho level was associated with a better 2-year cardiovascular protection.
The mechanism of action of sirtuin1 is still unknown and it remains in the interest of many researchers. Due to a wide expression and impact on the action of various proteins and gene transcription it may affect kidney function in many different ways. Genetic studies revealed relationship between SIRT1 gene polymorphisms and diabetic kidney disease11,12 as well as association with carotid atherosclerosis in the SAPHIR cohort (The Salzburg Atherosclerosis Prevention Program in subjects at High Individual Risk),25 which involved 1770 healthy unrelated white persons aged 39–67. Studies on Japanese hemodialysis patients also proved a significant association between SIRT1 polymorphisms, rs7069102 and rs2273773 and abnormal cholesterol metabolism and coronary artery calcification.26 The crucial action of SIRT1 is its beneficial effect on oxidative stress, inflammation and apoptosis, the processes which are also involved in kidney injury.18,27 SIRT1 overexpression in renal inner medullary mesenchymal cells is associated with renal oxidative stress.28 Chuang et al29 in animal models of SIRT1 knock down mice revealed increased albumin urine excretion and mitochondrial dysfunction in diabetics environment. It suggests that SIRT1 plays a pivotal role in homeostatic maintenance of podocyte in the stress/injury of mitochondria. Going further, Hasegawa et al30 observed that downregulation of SIRT1 and upregulation of the tight junction protein Claudin-1 by SIRT1-mediated epigenetic setting in podocytes led to enhancement of urinary albumin excretion in diabetes. Otherwise, increased SIRT1 expression saved podocytes against apoptosis triggered by the end products of glycation. SIRT1 may also mitigate the inflammatory response in diabetic kidneys. Kitada et al31 observed that Wistar diabetic rats had reduced renal sirtuin1 expression, increased albuminuria, declined creatinine clearance rate, and significantly elevated amount of acetylated NF-κB p65 and factors of inflammation compared to Wistar nondiabetic rats. Administration of SIRT1 agonists considerably reduced the amount of albumin excretion in urine. One of the natural agonists of SIRT1 is resveratrol, the polyphenol detected in red wine and pomegranates which has been found to activate SIRT1 and extend the lifespan.32 It was detected in animal models that pretreatment with resveratrol reduced the rate of acute kidney injury in rats after glycerol administration.33 Resveratrol also has vasoprotective properties via SIRT1 dependent pathways.34 It was demonstrated by Wicinski et al35 that 4-week oral resveratrol treatment in rat models increased the serum levels of SIRT1and significantly reduced reactivity of resistant arteries partially independently of phosphodiesterase. An interesting study was conducted by Qi et al.36 They observed an association between the protective effect of intravenous vitamin C administration on hemorrhagic shock-related kidney injury and SIRT1 pathway. Most of th available researches regarding sirtuin1 studied its expression or serum activity, moreover majority of them are based on animal models. In our study, we assessed the serum SIRT1 concentration in humans-patients with end-stage kidney disease on maintenance hemodialysis. We found it was significantly higher when compared to healthy volunteers. We also report an interesting positive correlation of serum SIRT1 level and hemodialysis vintage and the association of sirtuin1 with ejection fraction. To our knowledge, there is no previous research concerning serum SIRT1 level in hemodialysis subjects. Moreover, we assessed the serum SIRT1 together with αKlotho concentration, which makes the study more valuable. In turn Olivares et al37 checked urinary SIRT1 values together with the expression of SIRT1 measured by quantitative PCR and immunoblot and clinical measures of lupus activity, including renal biopsy in a cohort of 40 patients with systemic lupus erythematosus (SLE). Then the authors compared all of studied parameters in patients with active lupus nephritis (LN), in remission and healthy controls. SIRT1 protein concentration was considerably augmented in all SLE patients when compared to healthy controls and it was 4.0-fold higher in those with the active LN compared to control group, 3.3-fold when compared to SLE patients without LN and 2.3-fold compared to patients with remission of LN. Interestingly they observed a strong correlation between SIRT1 mRNA and SIRT1 level in SLE which was even higher in patients with LN. The authors concluded that SIRT1 may be a valuable marker of renal injury. Recently an interesting research was published regarding serum SIRT1 concentration in 125 patients newly diagnosed with hypertension comparing to 40 healthy control subjects.38 The study population was also divided into two groups according to the presence/absence of left ventricular hypertrophy (LVH). Serum SIRT1 level was found to be significantly higher in patients with LVH compared to those without LVH and to healthy subjects; multivariate logistic regression analysis revealed that increased serum SIRT1 concentration independently predicted LVH in patients with hypertension. Another recent human study on SIRT1 concentration was reported by Kızıltunç et al.39 They assessed temporal changes of serum sirtuin1 in 40 patients with acute myocardial infarction (AMI) compared to subjects with normal coronary arteries and found no differences between AMI patients and the latter group. In our population of patients with end-stage kidney disease on maintenance hemodialysis no differences in sirtuin1 levels between patients with LVH or other known cardiovascular disease and without was found. This can be explained by the fact that most of hemodialyzed patients suffer from some kind of cardiovascular abnormality.
In conclusion, elevated SIRT1 and lower αKlotho concentration is associated with impaired kidney function. Decreased αKlotho level may also be a sign of heart hypertrophy in maintenance dialysis patients. The role of anti-aging proteins, particularly SIRT1 as biomarkers/predictors of oxidative stress, inflammation and cardiovascular disease needs further examination.
Acknowledgment
This article has been supported by the Polish National Agency for Academic Exchange under Grant No. PPI/PZA/2019/1/00001/U/00001.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Watroba M, Szukiewicz D. The role of sirtuins in aging and age-related diseases. Adv Med Sci. 2016;61(1):52–62. doi:10.1016/j.advms.2015.09.003
2. Landry J, Sutton A, Tafrov ST, et al. The silencing protein SIR2 and its homologs are NAD-dependent protein deacetylases. Proc Natl Acad Sci U S A. 2000;97(11):5807–5811. doi:10.1073/pnas.110148297
3. Frye RA. Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem Biophys Res Commun. 2000;273(2):793–798. doi:10.1006/bbrc.2000.3000
4. Carafa V, Rotili D, Forgione M, et al. Sirtuin functions and modulation: from chemistry to the clinic. Clin Epigenetics. 2016;8:61. doi:10.1186/s13148-016-0224-3
5. Cohen HY, Miller C, Bitterman KJ, et al. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305(5682):390–392. doi:10.1126/science.1099196
6. Erben RG. Update on FGF23 and Klotho signaling. Mol Cell Endocrinol. 2016;432:56–65. doi:10.1016/j.mce.2016.05.008
7. Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390(6655):45–51. doi:10.1038/36285
8. Xu Y, Sun Z. Molecular basis of Klotho: from gene to function in aging. Endocr Rev. 2015;36(2):174–193. doi:10.1210/er.2013-1079
9. Yacoub R, Lee K, He JC. The role of SIRT1 in diabetic kidney disease. Front Endocrinol (Lausanne). 2014;5:166. doi:10.3389/fendo.2014.00166
10. Aditya R, Kiran AR, Varma DS, Vemuri R, Gundamaraju R. A review on SIRtuins in diabetes. Curr Pharm Des. 2017;23(16):2299–2307. doi:10.2174/1381612823666170125153334
11. Yue XG, Yang ZG, Zhang Y, Qin GJ, Liu F. Correlations between SIRT1 gene polymorphisms and diabetic kidney disease. R Soc Open Sci. 2018;5(6):171871. doi:10.1098/rsos.171871
12. Zhao Y, Wei J, Hou X, et al. SIRT1 rs10823108 and FOXO1 rs17446614 responsible for genetic susceptibility to diabetic nephropathy. Sci Rep. 2017;7(1):10285. doi:10.1038/s41598-017-10612-7
13. Kalaitzidis RG, Duni A, Siamopoulos KC. Klotho, the Holy Grail of the kidney: from salt sensitivity to chronic kidney disease. Int Urol Nephrol. 2016;48(10):1657–1666. doi:10.1007/s11255-016-1325-9
14. Zhou L, Mo H, Miao J, et al. Klotho ameliorates kidney injury and fibrosis and normalizes blood pressure by targeting the renin-angiotensin system. Am J Pathol. 2015;185(12):3211–3223. doi:10.1016/j.ajpath.2015.08.004
15. Gao D, Zuo Z, Tian J, et al. Activation of SIRT1 attenuates klotho deficiency-induced arterial stiffness and hypertension by enhancing AMP-activated protein kinase activity. Hypertension. 2016;68(5):1191–1199. doi:10.1161/HYPERTENSIONAHA.116.07709
16. Workgroup KD. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45(4 Suppl 3):S1–S153.
17. Mitchell C, Rahko PS, Blauwet LA, et al. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2019;32(1):1–64. doi:10.1016/j.echo.2018.06.004
18. Katsi VK, Pavlidis AN, Liakos CI, et al. The tree of sirtuins and the garden of cardiovascular youth. Curr Vasc Pharmacol. 2016;14(1):80–87. doi:10.2174/1570161113666150916093902
19. Zhong Y, Chen AF, Zhao J, Gu YJ, Fu GX. Serum levels of cathepsin D, sirtuin1, and endothelial nitric oxide synthase are correlatively reduced in elderly healthy people. Aging Clin Exp Res. 2016;28(4):641–645. doi:10.1007/s40520-015-0472-7
20. Xiao NM, Zhang YM, Zheng Q, Gu J. Klotho is a serum factor related to human aging. Chin Med J (Engl). 2004;117(5):742–747.
21. Lindberg K, Amin R, Moe OW, et al. The kidney is the principal organ mediating klotho effects. J Am Soc Nephrol. 2014;25(10):2169–2175. doi:10.1681/ASN.2013111209
22. Lindberg K, Olauson H, Amin R, et al. Arterial klotho expression and FGF23 effects on vascular calcification and function. PLoS One. 2013;8(4):e60658. doi:10.1371/journal.pone.0060658
23. Marcais C, Maucort-boulch D, Drai J, et al. Circulating Klotho associates with cardiovascular morbidity and mortality during hemodialysis. J Clin Endocrinol Metab. 2017;102(9):3154–3161. doi:10.1210/jc.2017-00104
24. Kakareko K, Rydzewska-rosolowska A, Brzosko S, et al. The effect of nephrectomy on Klotho, FGF-23 and bone metabolism. Int Urol Nephrol. 2017;49(4):681–688. doi:10.1007/s11255-017-1519-9
25. Kedenko L, Lamina C, Kedenko I, et al. Genetic polymorphisms at SIRT1 and FOXO1 are associated with carotid atherosclerosis in the SAPHIR cohort. BMC Med Genet. 2014;15:112. doi:10.1186/s12881-014-0112-7
26. Shimoyama Y, Mitsuda Y, Tsuruta Y, Suzuki K, Hamajima N, Niwa T. SIRTUIN 1 gene polymorphisms are associated with cholesterol metabolism and coronary artery calcification in Japanese hemodialysis patients. J Ren Nutr. 2012;22(1):114–119. doi:10.1053/j.jrn.2011.10.025
27. Tucker PS, Scanlan AT, Dalbo VJ. Chronic kidney disease influences multiple systems: describing the relationship between oxidative stress, inflammation, kidney damage, and concomitant disease. Oxid Med Cell Longev. 2015;2015:806358. doi:10.1155/2015/806358
28. He W, Wang Y, Zhang MZ, et al. Sirt1 activation protects the mouse renal medulla from oxidative injury. J Clin Invest. 2010;120(4):1056–1068. doi:10.1172/JCI41563
29. Chuang PY, Xu J, Dai Y, et al. In vivo RNA interference models of inducible and reversible Sirt1 knockdown in kidney cells. Am J Pathol. 2014;184(7):1940–1956. doi:10.1016/j.ajpath.2014.03.016
30. Hasegawa K, Wakino S, Simic P, et al. Renal tubular Sirt1 attenuates diabetic albuminuria by epigenetically suppressing Claudin-1 overexpression in podocytes. Nat Med. 2013;19(11):1496–1504. doi:10.1038/nm.3363
31. Kitada M, Takeda A, Nagai T, Ito H, Kanasaki K, Koya D. Dietary restriction ameliorates diabetic nephropathy through anti-inflammatory effects and regulation of the autophagy via restoration of Sirt1 in diabetic Wistar fatty (fa/fa) rats: a model of type 2 diabetes. Exp Diabetes Res. 2011;2011:908185. doi:10.1155/2011/908185
32. Valenzano DR, Terzibasi E, Genade T, Cattaneo A, Domenici L, Cellerino A. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr Biol. 2006;16(3):296–300. doi:10.1016/j.cub.2005.12.038
33. Chander V, Chopra K. Protective effect of resveratrol, a polyphenolic phytoalexin on glycerol-induced acute renal failure in rat kidney. Ren Fail. 2006;28(2):161–169. doi:10.1080/08860220500531112
34. Ota H, Eto M, Ogawa S, Iijima K, Akishita M, Ouchi Y. SIRT1/eNOS axis as a potential target against vascular senescence, dysfunction and atherosclerosis. J Atheroscler Thromb. 2010;17(5):431–435. doi:10.5551/jat.3525
35. Wicinski M, Malinowski B, Weclewicz MM, Grzesk E, Grzesk G. Anti-atherogenic properties of resveratrol: 4-week resveratrol administration associated with serum concentrations of SIRT1, adiponectin, S100A8/A9 and VSMCs contractility in a rat model. Exp Ther Med. 2017;13(5):2071–2078. doi:10.3892/etm.2017.4180
36. Qi MZ, Yao Y, Xie RL, et al. Intravenous Vitamin C attenuates hemorrhagic shock-related renal injury through the induction of SIRT1 in rats. Biochem Biophys Res Commun. 2018;501(2):358–364. doi:10.1016/j.bbrc.2018.04.111
37. Olivares D, Perez-hernandez J, Forner MJ, et al. Urinary levels of sirtuin-1 associated with disease activity in lupus nephritis. Clin Sci (Lond). 2018;132(5):569–579. doi:10.1042/CS20171410
38. Duman H, Bahceci I, Cinier G, Duman H, Bakirci EM, Cetin M. Left ventricular hypertrophy is associated with increased sirtuin level in newly diagnosed hypertensive patients. Clin Exp Hypertens. 2019;41(6):511–515. doi:10.1080/10641963.2018.1510946
39. Kiziltunc E, Kosem A, Ozkan C, et al. Serum Sirtuin 1, 3 and 6 levels in acute myocardial infarction patients. Arq Bras Cardiol. 2019;113(1):33–39. doi:10.5935/abc.20190114
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
