Back to Journals » Clinical Ophthalmology » Volume 15
The Role of Anterior Chamber Depth on Post-operative Refractive Error After Phacovitrectomy
Authors Katz G , el Zhalka F, Veksler R, Ayalon A, Moisseiev E
Received 5 March 2021
Accepted for publication 22 March 2021
Published 20 May 2021 Volume 2021:15 Pages 2111—2115
DOI https://doi.org/10.2147/OPTH.S309302
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Gabriel Katz, 1, 2,* Fidaa el Zhalka, 3,* Ronel Veksler, 1 Anfisa Ayalon, 3 Elad Moisseiev 2, 3
1Department of Ophthalmology, Sheba Medical Center, Ramat Gan, Israel; 2Sackler School of Medicine, TelAviv University, TelAviv, Israel; 3Department of Ophthalmology, Meir Medical Center, Kfar Saba, Israel
*These authors contributed equally to this work
Correspondence: Elad Moisseiev
Department of Ophthalmology, Meir Medical Center, 59 Tshernichovsky St., Kfar Saba, 4428164, Israel
Tel +97297471527
Fax +97297472427
Email [email protected]
Purpose: To investigate the effect of phacovitrectomy on the post-operative anterior chamber depth (ACD) and refractive outcomes, and to analyze the potential differences between vitreous filling with BSS, air and gas.
Methods: Patients who underwent phacovitrectomy were included in this study and invited for repeated post-operative examination including refraction and biometry at least 3 months after the surgery. Data retrieved included demographic information, indication for phacovitrectomy, surgical details, type of vitreous filling (BSS, air or gas), pre-operative and post-operative biometric data including K-readings, axial length (AL), and ACD, as well as spherical equivalent (SE) values of the target and final refraction.
Results: Forty-three eyes of 43 patients were included in this study, including 10 eyes filled with BSS, 18 with air and 15 with gas. The mean difference between the final measured spherical equivalent (SE) and the SE of the intended target refraction was 0.61± 0.68 D (p = 0.019). Only 58.1% of eyes had a final SE within ± 0.5D of the target refraction. Following surgery, AL remained unchanged, while mean pre-operative ACD increased significantly from 3.11± 0.34 mm to 4.77± 0.47 mm (p < 0.001). There was no difference in refractive error between the vitreous fillings and no correlation with AL or ACD.
Conclusions: Phacovitrectomy is associated with lower accuracy of post-operative refraction compared to cataract surgery. This may be attributed to a significant change in ACD, influencing the effective lens position of the IOL, and may require adjustment of the pre-operative calculations.
Keywords: anterior chamber depth, ACD, phacovitrectomy, refractive error, tamponade
A Letter to the Editor has been published for this article.
A Response to Letter has been published for this article.
Introduction
Combined phacoemulsification with intraocular lens (IOL) insertion and pars plana vitrectomy (phacovitrectomy) is a commonly performed surgery. Combined surgery has similar efficacy and safety as the sequential strategy of cataract surgery after vitrectomy.1,2 There are several reasons that make the combined surgery more attractive to patients and surgeons, the most obvious being the reduced burden of visiting the operating room twice instead of once. Another important reason was emphasized recently in several studies, that showed that although final results were similar, patients undergoing combined surgery benefited from a greater area under the curve of visual acuity improvement. They attained best visual acuity 15 months sooner compared with conventional sequential surgeries, indicating a significantly faster time to recovery.3,4
In several large studies on cataract surgery alone, a refractive result within ±0.5 diopters was found in 72–74% of cases.5,6 In contrast to these results, there is a still a level of uncertainty regarding refractive results of combined cataract and vitrectomy. Several studies prospectively compared Phaco-Vit to standard phaco surgery and found a significant difference in the prediction error (PE). The addition of vitrectomy was associated with an induced myopia of approximately −0.4 to −0.5 diopter.4,7,8 The addition of gas at the end of surgery did not cause a significant change, and the PE was lower in patients that underwent vitrectomy for macular holes (MH).8 PE was also associated with shallower anterior chamber depth (ACD) at presentation, increased central macular thickness (>300μ), and worse baseline best-corrected visual acuity. On the other hand, other studies found no myopic shift in phacovitrectomy cases.9,10 In a review study focused on this issue, there was no consensus regarding the post-operative ACD and ELP following phacovitrectomy.11 The only consistent result of most of the studies reviewed was the small myopic shift observed with combined surgery – which has not been fully explained. Some studies looked at the difference between combined phacovitrectomy vs. sequential surgery of cataract post-vitrectomy or vice versa, with contradicting results. One study found a greater PE with phacovitrectomy12 whereas another found no difference between the two techniques.2
The purpose of our study was to investigate the effect of phacovitrectomy on the post-operative ACD and refractive outcomes, and to analyze the potential differences between vitreous filling with BSS, air and gas.
Methods
This study was approved by the institutional review boards of the Meir Medical Center and Sheba Medical Centers, in accordance with the Declaration of Helsinki. Both centers have large ophthalmology departments with a high surgical volume. The records were reviewed and patients who underwent phacovitrectomy between January 1, 2016 and December 31, 2019 were identified.
Included patients underwent uncomplicated combined phacovitrectomy, with a posterior-chamber IOL implanted in the capsular bag. All patients underwent sutureless 23G or 25G vitrectomy, with a final vitreous filling with BSS, air or gas (SF6 or C3F8). Patients who underwent silicone oil filling were excluded, as well as patients who had an IOL implanted in the sulcus, anterior chamber or fixated to the iris or sclera. All patients had the same type of one-piece hydrophobic IOL implanted. Patients with documented intraoperative complications were excluded, as well as any who required additional surgeries following phacovitrectomy. All patients were 18 years or older, and had at least 3 months of follow up after phacovitrectomy.
Patients who met the inclusion criteria were contacted and invited for a complete ocular examination, including refraction and repeated biometry. All patients gave their informed consent for participation in the study, and signed a consent form. Recorded parameters included demographic information, indication for phacovitrectomy, surgical details, type of vitreous filling (BSS, air or gas), pre-operative biometric data including K-readings, axial length (AL), ACD, target refraction, and post-operative K-readings, AL, ACD and refraction.
Biometry measurements were performed using IOL Master version 5, and SRKT formula was used for IOL calculations. The primary outcome measure of the study was the predictive error, calculated by the difference between the spherical equivalent (SE) values of the pre-operative target refraction and the post-operative measured refraction.
Correlations between continuous variables were analyzed using Pearson’s correlation coefficient and T-tests, chi-square test were used to analyze associations between categorical parameters. Data was analyzed using SPSS for windows version 21. A p-value of 0.05 was used to declare statistically significant difference between groups.
Results
The study included 43 eyes of 43 patients, 22 (51.1%) women and 21 (48.9%) men, with a mean age of 66.1±7.7 years at the time of surgery. Mean post-operative follow up time was 10.2±7.3 months.
Indications for surgery included 23 (53.5%) cases of epiretinal membrane (ERM), 5 (11.6%) cases of MH, 11 (25.6%) cases of vitreous hemorrhage and 4 (9.3%) cases of retinal detachment (RD). Final filling was performed with BSS in 10 (23.2%) cases, air in 18 (41.9%) cases and gas in 15 (34.9%) cases (10 cases with SF6 and 5 cases with C3F8, which were grouped together).
The mean difference between the final measured SE and the SE of the intended target refraction was −0.31±0.86 D, ranging from −1.92 D to +3.87 D. In terms of absolute values, the mean difference between the final measured SE and the SE of the intended target refraction was 0.61±0.68 D. The difference between the SE of the intended target refraction and the final measured SE was statistically significant (p=0.019).
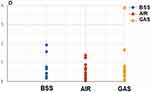
The mean absolute value of the difference between the final measured SE and the SE of the intended target refraction was 0.71±0.58 D in eyes filled with BSS, 0.49±0.38 D in eyes filled with air and 0.68±0.99 D in eyes filled with gas. The distribution of refractive error is presented in Figure 1. No statistically significant difference was found between these groups. However, it is important to note that three of the four cases with the largest difference (over 1.5D) between the intended and final SE occurred in eyes filled with gas.
Only 25 (58.1%) eyes had a final measured SE within ±0.5 D from the intended SE of the target refraction. This increased to 37 (86%) eyes with a final measured SE within ±1.0 D from the intended SE of the target refraction. The degree of refractive error did not correlate with AL or ACD.
Pre-operative mean AL was 23.99±1.08 mm, not significantly different from the final mean AL of 23.96±1.09 mm (p=0.88). However, mean pre-operative ACD was 3.11±0.34 mm and had significantly increased to 4.77±0.47 mm following surgery (p<0.001). The mean difference in ACD was 1.85±0.50 mm in eyes filled with BSS, 1.55±0.49 mm in eyes filled with air, and 1.77±0.93 mm in eyes filled with gas, with no significant difference between groups. These results are presented in Table 1. Importantly, a significant correlation was demonstrated between the refractive error and change in ACD (r=0.01).
 |
Table 1 Comparison of Baseline Characteristics, SE Difference and ACD Between Groups with Different Vitreous Filling in Phacovitrectomy |
Discussion
As the technologies of phacoemulsification and IOLs have advanced, cataract surgery is presently not only considered as a mean to improve vision but also an opportunity to control the refraction. Both patients and surgeons have very high expectations for accurate refractive results following cataract surgery. These expectations are intuitively extrapolated to cases undergoing combined phacovitrectomy – even if the visual acuity is limited by the retinal conditions requiring surgical intervention, the cataract surgery is often straightforward and calculations are made pre-operatively to achieve a specific refractive result.
Overall, our results are compatible with those of previously reported studies, with a slight myopic error of approximately −0.5 D.4,7,8 A recent meta-analysis comparing combined phacovitrectomy and sequential surgery concluded that there were no significant differences in visual and refractive outcomes between the two.13 However, some studies have reported that combined phacovitrectomy is associated with lower refractive accuracy.14,15 We note that a 58% rate of final SE within ±0.5D of the SE of the intended refraction is reasonable in these cases, which are considerably more complicated than phacoemulsification alone.
It has been suggested that patients with worse vision, greater central macular thickness, macula-involving RD and shallow anterior chambers are more prone to inaccurate pre-operative biometry.14,15 We note that IOL Master 5 measures the AL to the RPE, and therefore may be able to avoid measurement errors resulting from a detached retina. It has also been suggested that the final refractive inaccuracy may be due to changes in the IOLs’ effective lens position, as AL did not significantly change following surgery.16 This is supported by our results, as AL did not change due to surgery.
The formulas for IOL selection are all designed for non-vitrectomized eyes. Although some of the new-generation IOL formulas have been demonstrated to be accurate in vitrectomized eyes,17 this may not be the case with the simpler and more veteran formulas such as the SRK/T. In vitrectomized eyes, the difference in the final effective lens position is likely the result of two factors – the absence of the vitreous, and the possible effect of agents used to fill the vitreous cavity on IOL position. A recent study using swept-source OCT imaging has shown that in eyes filled with gas, IOLs are displaced forward, resulting in a greater myopic shift than eyes filled with BSS or air. Importantly, the IOL position and refractive error remained even after the gas had fully absorbed.18 We note that other studies did not find a significant difference on the PE after gas fill.19 In our study ACD was not significantly different in eyes filled with gas compared to those filled with BSS or air, but we note that a correlation was shown between change in ACD and the size of the refractive error. We suggest that in addition to a possible change in effective lens position, the reason for the myopic shift in eyes undergoing vitrectomy is due at least in part, but significantly, to the absence of the vitreous and change in the refractive index of the vitreous cavity. The refractive error was also not significantly different between the various groups of vitreous filling (BSS, air or gas), but the cases with the largest error in refraction were eyes filled with gas.
The present study is limited by its series size, which may have limited the ability to demonstrate significant differences between groups. Furthermore, there were variable indications for vitrectomy, multiple surgeons and degree of cataract was not recorded and analyzed. However, the surgical techniques were rather similar and we believe these factors did not influence the final outcomes. Additional limitations include using only the SRK/T formula, and the variability in post-operative follow up length.
In conclusion, it should be remembered that although combined surgery is appealing in patients who require retinal as well as cataract surgery, the accuracy of final refractive outcomes is slightly lower than in cataract surgery. This may be more significant in cases requiring gas filling. Care should be taken in pre-operative patient counseling and setting expectations, as well as in choosing the IOL. If using classic IOL formulas, it may be useful to compensate for a small expected myopic shift in these cases.
Disclosure
The authors reported no conflicts of interest for this work. None of the authors have any proprietary interest in this study. No funds or grants were received for this study.
References
1. Sizmaz S, Esen E, Isik P, Cam B, Demircan N. Outcome and complications of combined phacoemulsification and 23-gauge pars plana vitrectomy. J Ophthalmol. 2019;2019:7918237. doi:10.1155/2019/7918237
2. Hamoudi H, Christensen UC, Morten LC. Epiretinal membrane surgery: an analysis of 2-step sequential- or combined phacovitrectomy surgery on refraction and macular anatomy in a prospective trial. Acta Ophthalmol. 2018;96(3):243–250.
3. Port AD, Nolan JG, Siegel NH, Chen X, Ness SD, Subramanian ML. Combined phaco-vitrectomy provides lower costs and greater area under the curve vision gains than sequential vitrectomy and phacoemulsification. Graefes Arch Clin Exp Ophthalmol. 2020.
4. Falkner-Radler CI, Md TB, Susanne Binder MD. Accuracy of preoperative biometry in vitrectomy combined with cataract surgery for patients with epiretinal membranes and macular holes. Results of a prospective controlled clinical trial. J Cataract Refract Surg. 2013;39:942–947.
5. Lundström M, Dickman M, Henry Y, et al. Risk factors for refractive error after cataract surgery: analysis of 282 811 cataract extractions reported to the European Registry of Quality Outcomes for cataract and refractive surgery. J Cataract Refract Surg. 2018;44(4):447–452.
6. Manning S, Barry P, Henry Y, et al. Femtosecond laser-assisted cataract surgery versus standard phacoemulsification cataract surgery: study from the European Registry of Quality Outcomes for Cataract and Refractive Surgery. J Cataract Refract Surg. 42(12):1779–1790.
7. Vander Mijnsbrugge J, Fils JF, Jansen J, Hua MT, Stalmans P. The role of the vitreous body in effective IOL positioning. Graefes Arch Clin Exp Ophthalmol. 2018;256(8):1517–1520.
8. Wagenfeld L, Hermsdorf K, Stemplewitz B, Druchkiv V, Frings A. Refractive predictability in eyes with intraocular gas tamponade – results of a prospective controlled clinical trial. Clin Ophthalmol. 2017;11:993–998.
9. van der Geest LJ, Siemerink MJ, Mura M, Mourits MP, Lapid-Gortzak R. Refractive outcomes after phacovitrectomy surgery. J Cataract Refract Surg. 2016;42(6):840–845.
10. Sato T, Korehisa H, Shibata S, Hayashi K. Prospective comparison of intraocular lens dynamics and refractive error between phacovitrectomy and phacoemulsification alone. Ophthalmol Retina. 2020;4(7):700–707.
11. Hamoudi H, Morten LC. Refractive changes after vitrectomy and phacovitrectomy for macular hole and epiretinal membrane. J Cataract Refract Surg. 2013;39:942–947.
12. Tranos PG, Allan B, Balidis M, et al. Comparison of postoperative refractive outcome in eyes undergoing combined phacovitrectomy vs cataract surgery following vitrectomy. Graefes Arch Clin Exp Ophthalmol. 2020;258(5):987–993.
13. Farahvash A, Popovic MM, Eshtiaghi A, Kertes PJ, Muni RH. Combined versus sequential phacoemulsification and pars plana vitrectomy: a meta-analysis. Ophthalmol Retina. 2021.
14. Tranos PG, Allan B, Balidis M, et al. Comparison of postoperative refractive outcome in eyes undergoing combined phacovitrectomy vs cataract surgery following vitrectomy. Graefes Arch Clin Exp Ophthalmol. 2020;2e58(5):987–993.
15. Tan A, Bertrand-Boiché M, Angioi-Duprez K, Berrod JP, Conart JB. Outcomes of combined phacoemulsification and pars plana vitrectomy for rhegmatogenous retinal detachment: a comparative study. Retina. 2021;41(1):68–74.
16. Kang TS, Shin YI, Ryu CK, Kim JY. Two-year reproducibility of axial length measurements after combined phacovitrectomy for epiretinal membrane, and refractive outcomes. J Clin Med. 2020;9(11):3493.
17. Tan X, Zhang J, Zhu Y, et al. Accuracy of New generation intraocular lens calculation formulas in vitrectomized eyes. Am J Ophthalmol. 2020;217:81–90.
18. Shiraki N, Wakabayashi T, Sakaguchi H, Nishida K. Effect of gas tamponade on the intraocular lens position and refractive error after phacovitrectomy: a swept-source anterior segment OCT analysis. Ophthalmology. 2020;127(4):511–515.
19. Schweitzer KD, Garcıa R. Myopic shift after combined phacoemulsification and vitrectomy with gas tamponade. Can J Ophthalmol. 2008;43:581–583.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.