Back to Journals » Clinical Ophthalmology » Volume 16
The Relationship Between the Thickness of cpRNFL in Segments and Intraocular Pressure
Authors Lešták J , Fůs M , Král J
Received 6 September 2022
Accepted for publication 12 October 2022
Published 9 November 2022 Volume 2022:16 Pages 3673—3679
DOI https://doi.org/10.2147/OPTH.S388936
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Jan Lešták, Martin Fůs, Jakub Král
Faculty of Biomedical Engineering, Czech Technical University in Prague, Kladno, Czech Republic
Correspondence: Jan Lešták, Faculty of Biomedical Engineering, Czech Technical University in Prague, nám. Sítná 3105, Kladno, 272 01, Czech Republic, Tel +420 602 336 770, Email [email protected]
Purpose: The aim of this study was to investigate whether the retinal nerve fibre layer (RNFL) in some segments of the optic nerve disc in pathological intraocular pressure is more damaged in eyes without antiglaucoma treatment.
Patients and Methods: The cohort consisted of 69 subjects (122 eyes), 32 males (6x one, 26x both eyes) aged 21 to 76 years and 37 females (4x one and 30x both eyes) aged 22 to 75 years, who were measured to have IOP greater than 21 mmHg (21– 36) in routine ambulatory care. Measurements were performed using the Ocular Response Analyser, taking into account corneal hysteresis. RNFL thickness was measured using the Avanti RTVue XR and was assessed in 8 segments (1-IT, 2-TI, 3-TS, 4-ST, 5-SN, 6-NS, 7-NI, 8-IN). The visual field was examined with a fast threshold glaucoma program using the Medmont M700. The overall defect (OD) was evaluated. Pearson’s correlation coefficient r was used to assess the dependence between the selected parameters.
Results: The largest peripapillary changes in RNFL were observed in segments 1, 4, 5 and 8. It should be emphasized that segments 1 and 4 have been temporarily shifted. Segments 5 and 8 then corresponded to the upper (at no. 12) and lower (at no. 6) sectors.
Conclusion: The most important result of this study is the finding that the greatest changes in the RNFL layer were observed in pathological IOP at segment 5 (r=− 0.3) and 8 (r=− 0.28), at the point where the fibres of the magnocellular retinal ganglion cells enter the retina.
Keywords: IOP, OCTA, RNFL, overall defect of visual field
Introduction
Intraocular pressure (IOP) is a major risk factor for the development and progression of glaucoma.1,2 After an increase in IOP, the ganglion cells of the retina are altered. Studies of different animal models of glaucoma have revealed a higher sensitivity of magnocellular ganglion cells.3,4
Magnocellular ganglion cells of the retina are also referred to in the literature as alpha, M, Y or parasol. Shou et al also showed in an experiment that the loss of magnocellular cells is greater than that of parvocellular cells. Cell density of all ganglion cell types in the retina decreased with glaucoma duration, and cell loss was more significant in large ganglion cells than in beta cells (parvocellular).5
Weber et al described that the first changes after IOP increase occur in ganglion cells themselves (shrinking of the dendritic tree and the cell’s body) and that their axons only subsequently narrow.6 Similarly, Naskar et al showed in an experiment that changes at the level of ganglion cells occur earlier than changes in their axons.7 The retinal axon interruptions were found localised in a narrow transverse line at the level of the posterior part of the lamina cribrosa and immediately behind it. Retinal axons in front of this zone were mostly intact.8
This was demonstrated in an experiment by Soto et al, who found in mouse models that retinal ganglion cell degeneration in glaucoma has two separate stages: the first involves ganglion cell atrophy and the second involves damage to the ganglion cell axons. The retrolaminar degeneration of axons takes place before the degeneration of their intraretinal parts.9 The study by Quigley et al confirmed that these are predominantly magnocellular fibres. Larger diameter fibres died faster than smaller fibres, although no fibre size was completely spared at any stage of atrophy.10 Although retinal magnocellular cells die before their axons in hypertensive glaucoma, their diagnosis is difficult. Obviously, in the early stages of hypertension glaucoma (HTG), when the first changes occur in the retinal ganglion cells, we cannot even theoretically detect a decrease in sensitivity in the central part of the visual field.11
Therefore, it is more appropriate and accessible to examine their axons on the optic nerve disc, where their concentration is the highest. Since RNFLs are assessed using their average values, we wondered whether their decline would be more pronounced in some segments. This would also be crucial for early diagnosis and thus early treatment. In our previous study (where the effect of IOP on RNFL and vessel density was investigated), no correlation was found between IOP and RNFL in eyes with normal IOP (r=−0.06), but a moderate correlation was found for IOP above 20 mmHg (−0.418<r>-0.59).12 For that reason, the aim of this study was also to define the dependence of RNFL in the individual segments, on the level of IOP.
Materials and Methods
The prospective cohort study consisted of 69 subjects (122 eyes), 32 males (6x one, 26x both eyes) aged 21 to 76 years and 37 females (4x one and 30x both eyes) aged 22 to 75 years, who were measured to have IOP greater than 21 mmHg (21–36) in routine ambulatory care. IOP was the result of the average of three measurements with the Ocular Response Analyser (ORA). The inclusion criteria were as follows: IOP greater than 21 mmHg, visual acuity of 1.0 with possible correction of less than ±3 dioptres, approximately equal changes in visual fields in all eyes, no other ocular or neurological disease, and no prior treatment for hypertensive glaucoma. RNFL thickness was measured using the Avanti RTVue XR in the inter-circular range (2–4 mm radius from the centre of the papilla). We evaluated their thickness in 8 peripapillary segments. Because the segments did not conform to anatomical terminology, we labeled them with numbers 1–8. The first began with the inferior temporal IT-1, TI-2, TS-3, ST-4. Similarly, nasal SN-5, NS-6, NI-7, IN-8 (see Figure 1). Values were adjusted according to the age of the patient. The visual field was examined using the Medmont M 700 rapid threshold glaucoma program to evaluate the overall defect (OD) parameter. This examination also served to exclude other influences on the changes in the visual field. To assess the relationship between individual intraocular pressure ranges and RNFL values, we used Pearson’s correlation coefficient r (r = 0.00–0.19 very weak, r = 0.20–0.39 weak, r = 0.40–0.59 moderate, r = 0.60–0.79 strong, r = 0.80–1.00 very strong).
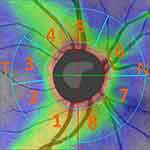 |
Figure 1 Designation of individual segments. Abbreviations: T, temporal; N, nasal. |
Results
The values of correlation coefficients of RNFL (retinal nerve fibre layer) parameters in each evaluated segment are shown in Table 1. Result shows that the largest changes in RNFL were observed in segments 1 (r=−0.23), 4 (r=−0.24), 5 (r=−0.3) and 8 (r=−0.28). The differences between these segments are rather small. The highest correlation was observed for segment 5 (see Figure 2) and no correlation for segment 2 (Figure 3). Dependence of IOP on OD (r=−0.15, p=0.09) was not observed. OD showed very weak dependence only in segment 8 (r=−0.18), IOP increased with increasing age (r=0.24, p=0.006).
 |
Table 1 Aggregate Data Mainly on the Relationship Between IOP and RNFL in Each Segment. 1-IT, 2-TI, 3-TS, 4-ST, 5-SN, 6-NS, 7-NI, 8-in |
 |
Figure 2 RNFL dependence on IOP in segment number 5. |
 |
Figure 3 RNFL dependence on IOP in segment number 2. |
Discussion
As mentioned in the Introduction, early changes in retinal magnocellular cells occur after pathological IOP elevation. Perimetric examination of magnocellular ganglion cells, which are localised in the periphery of the retina, does not have the same validity as RNFL examination. Because the ganglion cell fibres converge to the optic disc, their examination is much more accessible and has a higher sensitivity than examination of the visual field.
It was already confirmed in the last century that changes in the RNFL outpace changes in the perimeter.13–16 The present studies are consistent with this conclusion.17 The current issue is to diagnose early the magnocellular cell fibres, which are more damaged in HTG.
Retinal Ganglion Cells
Magnocellular retinal cells are morphologically characterised not only by large somata and dendritic tree, but also by thicker axons.18
Of the total number of ganglion cells in the human fovea, there are midget cells (about 90%), parasol cells (about 5%) and small bistratified cells (about 1%). In the periphery, midget cells make up about 40–45% of the total, parasol cells about 20% and small bistratified cells about 10%. Thus, from the periphery to the central retina, the number of midget ganglion cells gradually increases compared to the parasol and small bistratified types.19
In both young and old adults, the magnocellular cells are regularly distributed in a Gaussian fashion along radii that extend from the perimacula toward the far periphery. Dawson et al did not find these cells in the central retina. Magnocellular cells may be particularly susceptible to damage in the early stages of inner retinal disease.20
The dendritic fields of both parasol and midget ganglion cells are smaller in the nasal retina than in the temporal retina, which is equidistant from the fovea.21
Because the number of ganglion cells is smaller in the temporal retinal periphery, their dendritic tree must also be larger to cover the retina than in the nasal periphery.
This is consistent with the study by Gurcio and Allen, who found in the peripheral retina that the densities in the nasal retina exceeded those in the corresponding eccentricities in the temporal retina by more than 300%; the superior exceeded the inferior by 60%.22
Retinal Ganglion Cell Axons
The location of nerve fibres from the periphery of the temporal parts of the retina, by avoiding the macular fibres, to the upper and lower sectors of the optic nerve disc have been described for almost a century.23
This was confirmed by Hunter et al, who followed the in vivo course of myelinated nerve fibres in the retina of 47 eyes.24
Analysis by Drenhaus et al revealed distinct groups of axon diameters, with the following mean axon diameters and proportions. The group of small axons with a diameter of 0.55 micrometres accounted for 70%. The group of medium-sized axons with a diameter of 1.39 microns is 10%.25
Inferior and/or nasal RGC axons were on average larger than superior and/or temporal axons. The inferior retina contained some very large axons. Foveal axons were on average smaller than non-foveal axons. Peripheral axons were significantly larger, in contrast to inferior temporal retinal samples or samples nasal to the optic disc.26
Pathology of Nerve Fibres
Post-mortem examinations have shown that the most susceptible fibres of the optic papilla appear to fall within an hourglass-shaped zone, with the two widest portions located at the position of 12 o’clock and 6 o’clock.13,27 Visual field defects associated with glaucoma usually initially occur in the upper visual field (corresponding to defects at the lower pole of the disc).28–30 Optic nerve fibres of larger than average diameter died more rapidly than smaller fibres, although no fibre size was completely spared at any stage of atrophy.10
Another study by Quigley et al also demonstrated significant thinning of the RNFL in the lower quadrant in eyes with intraocular hypertension (OHT) compared to healthy eyes. The finding that initial RNFL thinning occurs in the lower quadrant of eyes with OHT is particularly interesting. Optic nerve defects associated with glaucoma often occur initially at the lower pole.31,32 RNFL defects measured by OCT in the lower quadrant show the closest association with glaucoma status.33
Glaucomatous neuroretinal rim loss occurred in a sequence of sectors. It generally started in the inferotemporal region of the disc and then progressed to the superotemporal, temporal horizontal, inferior nasal and finally superior nasal sectors. This finding may be important for “early” diagnosis of glaucoma.34
RNFL thinning associated with increased IOP in monkeys was observed around the optic nerve disc from the positions of 6 o’clock to 9 o’clock after laser treatment, and the degree of RNFL thickness reduction varied between peripapillary sectors. Correlations between cumulative IOP increase and RNFL thickness reduction were statistically significant for the temporal-superior (p=0.024), nasal-inferior (p=0.044), and temporal (p=0.049) sectors.35
Tu et al worked on a similar model. They found that the most sensitive quadrants to IOP increase were the lower and upper quadrants of the RNFL, with the rate of RNFL change almost parallel to the IOP level.36
Similar conclusions in humans were reached by Bowd et al. The mean RNFL was significantly thinner in ocular hypertension than in normal eyes, at 72.8 micrometres (66.4–78.1 micrometres) and 85.8 micrometres (80.2–91.7 micrometres), respectively. More specifically, the RNFL was significantly thinner in ocular hypertension than in normal eyes in the inferior quadrant, 84.8 micrometres (75.6–94.0 micrometres) vs 107.6 micrometres (99.3–115.9 micrometres); and in the nasal quadrant, 44.1 micrometres (37.5–51.7 micrometres) vs 61.8 micrometres (53.0–65.6 micrometres). The retinal nerve fibre layer was significantly thinner in glaucoma eyes than in ocular hypertension and normal eyes throughout 360 degrees and in all quadrants.37
The basis is not known for these observations as described by Bowd et al. It is possible that RNFL thinning in the lower quadrant of eyes with OHT is an early form of glaucoma that precedes detectable optic nerve and/or visual field defects. Another possibility is that the RNFL in eyes with OHT may initially be thin in the lower quadrant, making these eyes particularly susceptible to the effects of elevated IOP.37
This review shows the most common RNFL changes in the vertical quadrants of the optic nerve disc. This study can also be used to specify precisely the individual segment.
It is clear from Gurcio’s study that the lowest number of ganglion cells in the periphery of the human retina is found in the inferior temporal quadrant, followed by the superior temporal quadrant. This corresponds to the inputs to the optic nerve disc in the inferior temporal and superior temporal sectors. Even the results of our study are consistent with this fact. Taking into account the fact that magnocellular cells in HTG do not die selectively, but to a lesser extent also with parvocellular cells, their loss is more noticeable due to the thickness of their axons.
We have shown that with increasing IOP, there are changes in RNFL in segments 1, 4, 5 and 8. It is highest for no. 5, which corresponds to the upper segment (r=−0.3, p=0.0006) and no. 8, which corresponds to the lower segment (r=−0.2785, p=0.002). That is, just at the point where the fibres of the magnocellular ganglion cells enter the optic nerve disc. The loss of these axons relative to their thickness gives us a great chance to diagnose their early damage.
Conclusion
The unequivocal conclusion of this study is the finding of significant RNFL damage in the lower and upper segments. These are axons of predominantly magnocellular cells.
Ethics Approval
The present study was performed according to the Declaration of Helsinki and was approved by the internal ethics committee of the Ophthalmology Clinic JL (Prague, Czech Republic). All details, medical records, figures, medical history or test results were used with the written consent for publication from the patient, which is available from the corresponding author on reasonable request. All data used were anonymized.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bengtsson B, Heijl A. Diurnal IOP fluctuation: not an independent risk factor for glaucomatous visual field loss in high-risk ocular hypertension. Graefe’s Arch Clin Exp Ophthalmol. 2005;243:513–518. doi:10.1007/s00417-004-1103-8
2. Guo L, Moss SE, Alexander RA, Ali RR, Fitzke FW, Cordeiro MF. Retinal ganglion cell apoptosis in glaucoma is related to intraocular pressure and IOP-induced effects on extracellular matrix. Invest Ophthalmol Vis Sci. 2005;46:175–182. doi:10.1167/iovs.04-0832
3. Morgan JE, Uchida H, Caprioli J. Retinal ganglion cell death in experimental glaucoma. Br J Ophthalmol. 2000;84:303–310. doi:10.1136/bjo.84.3.303
4. Morgan JE. Retinal ganglion cell shrinkage in glaucoma. J Glaucoma. 2002;11:365–370. doi:10.1097/00061198-200208000-00015
5. Shou T, Liu J, Wang W, Zhou Y, Zhao K. Differential dendritic shrinkage of alpha and beta retinal ganglion cells in cats with chronic glaucoma. Invest Ophthalmol Vis Sci. 2003;44:3005–3010. doi:10.1167/iovs.02-0620
6. Weber AJ, Kaufman PL, Hubbard WC. Morphology of single ganglion cells in the glaucomatous primate retina. Invest Ophthalmol Vis Sci. 1998;39:2304–2320.
7. Naskar R, Wissing M, Thanos S. Detection of early neuron degeneration and accompanying microglial responses in the retina of a rat model of glaucoma. Invest Ophthalmol Vis Sci. 2002;43:2962–2968.
8. Vrabec F. Glaucomatous cupping of the human optic disk: a neuro-histologic study. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1976;198:223–234. doi:10.1007/BF00410715
9. Soto I, Oglesby E, Buckingham BP, et al. Retinal ganglion cells downregulate gene expression and lose their axons within the optic nerve head in a mouse glaucoma model. J Neurosci. 2008;28:548–561. doi:10.1523/JNEUROSCI.3714-07.2008
10. Quigley HA, Dunkelberger GR, Green WR. Chronic human glaucoma causing selectively greater loss of large optic nerve fibers. Ophthalmology. 1988;95:357–363. doi:10.1016/s0161-6420(88)33176-3
11. Lešták J, Fůs M. Visual field assessment in hypertension glaucoma. Cesk Slov Oftalmol. 2021;77:20–24.
12. Kral J, Lestak J, Nutterová E. OCT angiography, RNFL and visual field at different values of intraocular pressure. Biomed Rep. 2022;16(5):36. doi:10.3892/br.2022.1519
13. Quigley HA, Addicks EM, Green WR. Optic nerve damage in human glaucoma, III: quantitative correlation of nerve fiber loss and visual field defect in glaucoma, ischemic neuropathy, papilledema, and toxic neuropathy. Arch Ophthalmol. 1982;100:135–146.
14. Quigley HA, Dunkelberger GR, Green WR. Retinal ganglion cell atrophy correlated with automated perimetry in human eyes with glaucoma. Am J Ophthalmol. 1989;107:453–464. doi:10.1016/0002-9394(89)90488-1
15. Sommer A, Katz J, Quigley HA, et al. Clinical detectable nerve fiber atrophy precedes the onset of glaucomatous field loss. Arch Ophthalmol. 1991;109:77–83. doi:10.1001/archopht.1991.01080010079037
16. Quigley HA, Enger C, Katz J, et al. Risk factors for the development of glaucomatous visual field loss in ocular hypertension. Arch Ophthalmol. 1994;112:644–649. doi:10.1001/archopht.1994.01090170088028
17. Fortune B, Burgoyne CF, Cull GA, Reynaud J, Wang L. Structural and functional abnormalities of retinal ganglion cells measured in vivo at the onset of optic nerve head surface change in experimental glaucoma. Invest Ophthalmol Vis Sci. 2012;53:3939–3950. doi:10.1167/iovs.12-9979
18. Rodieck RW, Binmoeller KF, Dineen J. Parasol and midget ganglion cells of the human retina. J Comp Neurol. 1985;233:115–132. doi:10.1002/cne.902330107
19. Dacey DM. Physiology, morphology and spatial densities of identified ganglion cell types in primate retina. Ciba Found Symp. 1994;184:12–28. doi:10.1002/9780470514610.ch2
20. Dawson WW, Hawthorne MN, Parmer R, Hope GM, Hueter R. Very large neurons of the inner retina of humans and other mammals. Retina. 1989;9:69–74. doi:10.1097/00006982-198909010-00009
21. Perry VH, Oehler R, Cowey A. Retinal ganglion cells that project to the dorsal lateral geniculate nucleus in the macaque monkey. Neuroscience. 1984;12:1101–1123. doi:10.1016/0306-4522(84)90006-x
22. Gurcio CA, Allen KA. Topography of ganglion cells in human retina. J Comp Neurol. 1990;300:5–25. doi:10.1002/cne.903000103
23. Hertel E. Lehrbuch und Atlas der Augenheilkunde begrundet von Th. Axenfeld [Textbook and Atlas of Ophthalmology, founded by Th. Axenfeld]. Jena, Verlag von Gustav Fischer; 1935:549.
24. Hunter SF, Leavitt JA, Rodriguez M. Direct observation of myelination in vivo in the mature human central nervous systém A model for the behaviour of oligodendrocyte progenitors and their progeny. Brain. 1997;120:2071–2082. doi:10.1093/brain/120.11.2071
25. Drenhaus U, Gunten A, Rager G. Classes of axons and their distribution in the optic nerve of the tree shrew (Tupaia belangeri). Anat Rec. 1997;249:103–116. doi:10.1002/(SICI)1097-0185(199709)249:1<103::AID-AR13>3.0.CO;2-T
26. FitzGibbon T, Taylor SF. Mean retinal ganglion cell axon diameter varies with location in the human retina. Jpn J Ophthalmol. 2012;56:631–637. doi:10.1007/s10384-012-0185-9
27. Quigley HA, Addicks EM. Regional differences in the structure of the lamina cribrosa and their relation to glaucomatous optic nerve damage. Arch Ophthalmol. 1981;99:137–143. doi:10.1001/archopht.1981.03930010139020
28. Drance SM. The glaucomatous visual field. Br J Ophthalmol. 1972;56:186–200. doi:10.1136/bjo.56.3.186
29. Werner EB, Drance SM. Early visual field disturbances in glaucoma. Arch Oph-Thalmol. 1977;95:1173–1175. doi:10.1001/archopht.1977.04450070071002
30. Hart WM, Becker B. The onset and evolution of glaucomatous visual field defects. Ophthalmology. 1982;89:268–279. doi:10.1016/S0161-6420(82)34798-3
31. Quigley HA, Addicks EM, Green WR, Maumenee AE. Optic nerve damage in human glaucoma, II: the site of injury and susceptibility to damage. Arch Ophthalmol. 1981;99:635–649. doi:10.1001/archopht.1981.03930010635009
32. Quigley HA, Hohman RM, Addicks EM, Massof RW, Green WR. Morphological changes in the lamina cribrosa correlated with neural loss in open-angle glaucoma. Am J Ophthalmol. 1983;95:673–691. doi:10.1016/0002-9394(83)90389-6
33. Schuman JS, Hee MR, Puliafito CA, et al. Quantification of nerve fiber layer thickness in normal and glaucomatous eyes using optical coherence tomography. Arch Ophthalmol. 1995;113:586–596. doi:10.1001/archopht.1995.01100050054031
34. Jonas JB, Fernandez MC, Sturmer J. Pattern of glaucomatous neuroretinal rim loss. Ophthalmology. 1993;100:63–68. doi:10.1016/S0161-6420(13)31694-7
35. Noguchi T, Shimazawa M, Hamaguchi K, Araki T, Horai N, Hara H. Relationship between elevated intraocular pressure and divided peripapillary sector retinal nerve fiber layer thickness in a cynomolgus monkey laser-induced ocular hypertension model. OphthalmicRes. 2017;58:99–106. doi:10.1159/000471884
36. Tu S, Li K, Ding X, Hu D, Li K, Ge J. Relationship between intraocular pressure and retinal nerve fibre thickness loss in a monkey model of chronic ocular hypertension. Eye. 2019;33:1833–1841. doi:10.1038/s41433-019-0484-1
37. Bowd C, Weinreb RN, Williams JM, Zangwill LM. The retinal nerve fiber layer thickness in ocular hypertensive, normal, and glaucomatous eyes with optical coherence tomography. Arch Ophthalmol. 2000;118:22–26. doi:10.1001/archopht.118.1.22
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
