Back to Journals » Risk Management and Healthcare Policy » Volume 14
The Relationship Between Metabolic Parameters, Age, and Thyroid Status: A Cross-Sectional Study-Based National Survey of Iodine Nutrition, Thyroid Disease
Authors Deng B, Yuan Y, Zhong M, Ren R, Deng W, Duan X
Received 13 February 2021
Accepted for publication 31 March 2021
Published 23 April 2021 Volume 2021:14 Pages 1723—1730
DOI https://doi.org/10.2147/RMHP.S306122
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Marco Carotenuto
Bo Deng,1,* Yi Yuan,1,* Miao Zhong,1,* Rui Ren,1 Wuquan Deng,1 Xiaodong Duan2
1Department of Endocrinology, Chongqing University Central Hospital, Chongqing Emergency Medical Center, Chongqing, 400014, People’s Republic of China; 2Department of Rehabilitation, The Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, 646000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaodong Duan Email [email protected]
Aim: The relationship between thyroid status and metabolic factors was investigated as well as iodine nutrition in the general population and among the elderly population.
Methods: We performed a cross-sectional survey of 2483 subjects to assess the status of national iodine nutrition and incidence of thyroid disease. The general and elderly populations were divided into normal thyroid function (NTF) and subclinical hypothyroidism (SCH) groups. The anthropometric parameters and biochemical indicators were then analyzed.
Results: Overall, 327 participants were diagnosed with thyroid diseases, 73 (22.32%) of whom were 65 years or older. For the general population, compared with the NTF group, individuals in the SCH group were older, presented with higher systolic blood pressure, fasting blood glucose, serum cholesterol, and urinary iodine concentration (all p< 0.05) but lower blood uric acid (UA) (p< 0.05). Linear regression analysis further revealed that age and triglyceride (TG) serum levels positively correlated with serum thyroid-stimulating hormone (TSH) levels. Multiple regression analyses revealed that age, TG, waist circumference (WC), and body mass index were independent predictors for abnormal TSH serum levels. For the elderly population, compared with NTF group, individuals in the SCH group were substantially older (p< 0.05) but presented with lower UA (p< 0.05). Pearson linear regression analysis revealed a positive correlation between age (p=0.003) as well as TG levels (p< 0.001) and serum TSH levels. In contrast, WC (p=0.003) was negatively related to TSH serum levels. Further multiple regression analysis revealed that age, TG, WC and heart rate were independent predictors of blood TSH.
Conclusion: The large-scale national study of iodine nutrition, thyroid disease has shown a vital relationship between metabolic indicators and serum TSH levels. Age and metabolic diseases increase the likelihood of developing thyroid diseases, both the general population and among the elderly.
Keywords: thyroid function, metabolic status, aging
Introduction
Thyroid disease is a common disease affecting people of all ages around the world.1 Subclinical hypothyroidism (SCH), characterized by high levels of serum thyroid stimulating hormone (TSH) but normal serum-free thyroxine levels, is very common in the general population. Even though the global incidence of SCH is estimated to be between 5% and 10%, the disease is more prevalent in the elderly and among women.2 In the past decade, the prevalence of SCH has increased from 3.22% to 16.7%, as same as the proportion of people with positive thyroid peroxidase antibodies from 9.81% to 11.5%.3 SCH can be divided into two categories based on the TSH level: Mild SCH is characterized by less than 10 mIU of TSH per liter of blood, whereas severe SCH is characterized by more than 10 mIU of TSH per liter of blood.4 Mild SCH accounts for 90% of the SCH cases.5 In euthyroid subjects with a TSH in the upper normal range (2.5–4.5mIU/L) were more obese, had higher triglycerides, and had an increased likeliness for the metabolic syndrome.6 Patients with SCH are at a higher risk of developing coronary heart disease, particularly when TSH concentrations is above 10 mIU/L. TSH levels higher than 7 mIU/L further increase the risk of death from cardiovascular complications.7 Also, higher levels of TSH in SCH patients increase the risk of heart failure.8 Meanwhile, hypothyroidism is associated with reversible dementia,9 whereas SCH adversely affects lipid metabolism.10 Also, hypothyroidism increases the death risk of patients on dialysis.11 The prevalence of cerebrovascular diseases in type 2 diabetic patients is higher in hypothyroidism than hyperthyroidism patients even after adjustment for age and sex. In a related study, the prevalence of hypothyroidism in diabetics was found to be 6.8%, with the majority of them presenting with underlying SCH.12
Several studies have demonstrated the high serum TSH in elderly population. We hypothesize that all or some of the SCH-related diseases are also closely associated with age-related metabolic imbalance12,13. However, reports on the association between SCH and metabolic disease are not entirely consistent.14–16 This may be attributed to differences in the race, sampling and sample sizes as well as the non-community crowd. Therefore, we conducted a population-based cross-sectional survey to explore the relationship between serum TSH levels and blood glucose, blood lipids, blood pressure, UIC, circulating thyroid antibodies and other metabolic parameters.
Subjects and Methods
Study Population
A total of 2663 individuals attending the National Survey of Thyroid Diseases and Diabetes in Chongqing of China between 2014 and 2017 were enrolled for this study. We remained with 2483 participants after excluding 180 individuals with incomplete data. Of the 2483 people, 336 (1.53%) of them were aged 65 or older (73±7.8 years old). To be included in the study, one must have fulfilled the following: (1) be 18 years or older; (2) of Han race; (3) have lived in respective a random community or village for more than 5 years; (4) not have undergone tests utilizing iodine as contrast agent or had taken amiodarone within the last three months; (5) details for treatment of underlying complication are availed and (6) not pregnant. The protocol for this study was approved by the Ethics Committee of Chongqing University Central Hospital. All procedures in the study were performed in accordance with the ethical standards of the 1964 Declaration of Helsinki. All participants were informed about the purpose of the study. The written informed consents were obtained from all individual participants included in the study.
Anthropometric Parameters
The major anthropometric parameters measured were blood pressure, height, weight, and waist circumference (WC). For height, the patients removed their shoes, stood upright with back against the weighing scale column, the heels close to each other and looked straight ahead. The head plate was then gently pulled down till it just touched the tip of the head, before the measurements were read. The accuracy of our height measurement scale was 0.1 cm. Before weight measurement, the participants fasted for xyz hours. Just before the weight was measured, the patients micturitated, took off the shoes and hat and stood at the center of the weighing scale. The weight was read after the pointer stabilized. The accuracy of the weighing machine used was 0.1 kg. The waist circumference was measured using a tape measure with an accuracy of 0.1 cm. The measurement was based on the distance between the midpoint of the horizontal line of the anterior superior iliac crest and the lower edge line of the 12th rib. Participants rested for 10 minutes before systolic and diastolic blood pressures were measured (Omron HEM7430, Omron Corporation).
Biochemical Indicators
Whole blood samples were drawn after overnight fasting. The urinary iodine concentration (UIC) was measured using inductively coupled plasma mass spectrometry (Agilent 7700×, Agilent Technologies, USA). The fasting blood glucose (FBG) level was measured using glucose-oxidase assay method. The glycosylated hemoglobin (HbA1c) levels were measured using high-performance liquid chromatography (BioRad VARIANT II Haemoglobin Analyzer). The cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglyceride (TG), and blood uric acid (UA) levels were measured using enzyme-based assays (Mindray BS-180 Analyzer). The anti-thyroglobulin antibodies (TgAb), TSH concentration, and thyroid peroxidase antibodies (TPOAbs) were detected and measured using immunochemiluminescence assays (Cobas 601 analyzer, Roche Diagnostics).
Assessment of Normal Functioning of Thyroid Glands and Thyroid Antibodies
The elderly participants were divided into NTF group and SCH group based on the level of several serum hormones: free thyroxine (FT4) (9–22 mmol/L), TSH (0.3–4.2 mmol/L), TPOAb (0–34 mmol/L), and TgAb (0–115 mmol/L). The thyroid gland is considered functioning normally if the FT4 and TSH levels are within the normal range. SCH is based on TSH higher than 4.2 mmol/L, even if FT4 is within the normal range.
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation. Differences between NTF and SCH groups were assessed using Student’s t-test (SPSS statistical software, version 22.0). The data were subjected to normal distribution analysis using the Kolmogorov–Smirnov test before statistical analysis. The correlation between different variables was assessed using Pearson correlation analysis whereas the association between TSH levels and likely influencing factors was assessed using multiple regression analysis and multiple stepwise regression analysis. P<0.05 was considered statistically significant for all of the analyses.
Results
Overall, 327 individuals were diagnosed with thyroid diseases. Eleven (0.44%), 17 (0.68%), 13 (11.5%), and 286 (11.5%) were diagnosed with hyperthyroidism, subclinical hyperthyroidism, hypothyroidism and SCH, respectively. In addition, 9% and 10.6% of the individuals presented with higher TgAb and TPOAb, respectively. Also, 336 of the study participants were 65 years or older. Of the elderly participants, 261 (77%) of them had NTF, whereas the rest, and 66 (20%) were diagnosed with SCH.
Table 1 shows the levels of anthropometric parameters and biochemical indicators of all the study participants. Compared with individuals in the NTF group, those in the SCH group were older and presented with higher SBP, FBG, TC and UIC (all p<0.05) but lower UA (p<0.05). There was no significant difference in the levels of the parameters between the two groups.
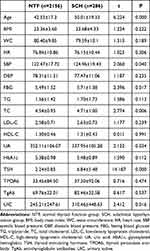 |
Table 1 The Anthropometric Parameters and Biochemical Indicators in the General Population |
Overall, as shown in Table 2, TSH levels positively correlated with age (r=0.106; p=0.000), SBP (r=0.085; p=0.000), FBG (r=0.044; p=0.031), TG (r=0.063; p=0.062), and TC (r=0.045; p=0.027). However, there was no association between serum TSH levels and BMI, WC, HR, DBP, LDL-C, HDL-C, UA, HbA1c, TPOAb, TgAb or UIC. Multiple linear regression analysis revealed age (p=0.000) and TG levels (p=0.009) positively correlated with TSH serum levels (Table 3). Multivariate stepwise regression analysis revealed that Age, TG, WC, and BMI were independent predictors of serum TSH levels (Table 4).
 |
Table 2 Pearson’s Correlation Analysis for TSH in the General Population |
 |
Table 3 Multiple Linear Regression Analysis for TSH in the General Population |
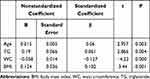 |
Table 4 Multiple Stepwise Regression Analysis of Metabolic Parameters and TSH in the General Population |
As shown in Figure 1, elderly SCH patients were significantly older (p=0.003) and displayed lower heart rate (p=0.04) than their NTF counterparts. There was no significant difference in the rest of the parameters between elderly NTF and SCH patients. There was also a strong correlation between TSH levels and age (r=0.193; p<0.001), SBP (r=0.134; p=0.015), waistline (r=−0.163; p=0.003), TG (r=0.160; p=0.004), and HbA1c (r=−0.011; p=0.044) (Figure 2). Multiple linear regression analysis revealed age (p=0.003) and TG (p<0.001) positively related with TSH levels, contrary to WC (p=0.003), which negatively correlated with TSH levels (Table 5). Age, TG, WC and heart rate were independent predictors of TSH levels (Table 6).
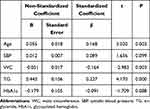 |
Table 5 Multiple Linear Regression Analysis in the Elderly Population |
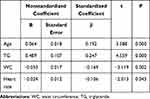 |
Table 6 Multiple Stepwise Regression Analysis of Metabolic Parameters and TSH Level in the Elderly Population |
 |
Figure 2 Pearson’s correlation analysis for TSH in the elderly population. Abbreviations: WC, waist circumference; SBP, systolic blood pressure; TG, triglyceride. |
Discussion
Higher TSH levels are closely associated with multiple metabolic disorders and cardiovascular diseases. In addition, older age increases the likelihood of developing thyroid dysfunctions. In Europe and US, the incidence of higher TSH level (>5.1 mIU/L) in individuals between 20 and 29 years was estimated to be approximately 9.5%.17 In this research, the incidence of higher TSH levels (>2.5 mIU/L) in the general population was 10.6%, compared with 40% for those 80 years or older. In one study, 14.5% of sampled individuals presented with TSH level of higher than 4.5 mIU/L.18 In this study, 295 (11.88%) of the sampled individuals had high serum TSH (TSH>4.2 mmol/L). Comparatively, 67 (20%) of the older population (≥65 years) presented with high TSH. The variation in the proportion level may be attributed to racial differences and indicator parameters used. However, the findings underline the burden of abnormal TSH production in elderly population.
Herein, both the whole and older population, we found age was an independent predictor of TSH levels, consistent with previous findings.12,13 The likelihood of developing thyroid disease also increases with age. This is in part attributed to the high likelihood of autoimmune diseases in older patients. Also, physiological changes and intake of drugs for chronic disease drug, which are likely to occur with age, disrupt hypothalamus-pituitary-thyroid axis.19
Similar to age, TG level was also an independent predictor of TSH levels both in the general and elderly populations, consistent with previous findings.14,16 Researches show that lipotoxicity targets thyroid gland.20 Also, lipid metabolism disorders are often accompanied with cardiovascular diseases. For instance, higher serum TSH increases the risk of developing coronary heart disease and atherosclerosis.21 Converging evidence has demonstrated that lipid metabolism changes with age. In this study, we found no association between serum TSH and LDL-C, HDL-C and metabolism. However, separate studies have demonstrated the correlation between HDL, LDL as well as TC metabolism and serum TSH levels.10,15,16,22 Further research are needed to discern these disparities.
Except for age and TG, WC and BMI were independent predictors of serum TSH across the entire population. Research shows that SCH is associated with metabolic syndrome and related complications, particularly high BMI and obesity, though the relationship is weak.23 In a related study, Pesic et al found that SCH is associated with high BMI,16 consistent with our findings. In addition to age and TG, we found WC and heart rate were also independent predictors of TSH levels in the elderly. Meanwhile, Sakurai et al found no association between serum TSH level and waist circumference,25 contrary to our findings. Overall, our findings suggest that abnormal serum TSH level is a risk factor for developing metabolic syndrome.
Regarding limitations, first, being a cross-sectional not a follow-up study. Second, we did not adjust for several factors such as smoking and alcohol intake. Third, underlying comorbidities and use of certain drugs such as aspirin, non-steroidal anti-inflammatory drugs, glucocorticoids, etc., may affect production of thyroid hormones in the elderly.19,25 However, the large sample size and stratification of study participants based on age enhanced the credibility of our findings.
Abbreviations
NTF, normal thyroid function; SCH, subclinical hypothyroidism; SBP, systolic blood pressure; WC, waist circumference; DBP, diastolic blood pressure; TgAb, anti-thyroglobulin antibodies; TSH, thyroid stimulating hormone; TPOAb, thyroid peroxidase antibody; LDL-C, low-density lipoprotein cholesterol; UA, uric acid; TG, triglyceride; FBG, fasting blood glucose; HDL-C, high-density lipoprotein cholesterol; HbA1c, glycosylated hemoglobin; UIC, urinary iodine concentration; FT4, free thyroxine.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; BD, YY, MZ, RR, WD and XD took part in drafting the article and revising it critically for important intellectual content; All authors agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This work is funded by Chongqing Science and Technology Bureau and Health Commission Foundation Medical Research Funding (No.2020GDRC023 & No.2020FYYX122), Chongqing University for Fundamental Research Funds of Central Universities (2019CDYGYB020).
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Taylor PN, Albrecht D, Scholz A, et al. Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol. 2018;14(5):301–316. doi:10.1038/nrendo.2018.18
2. LeFevre ML, U.S. Preventive Services Task Force. Screening for thyroid dysfunction: U.S. preventive services task force recommendation statement. Ann Intern Med. 2015;162(9):641–650. doi:10.7326/M15-0483
3. Shan Z, Chen L, Lian X, et al. Iodine status and prevalence of thyroid disorders after introduction of mandatory universal salt iodization for 16 years in China: a Cross-Sectional Study in 10 Cities. Thyroid. 2016;26(8):1125–1130. doi:10.1089/thy.2015.0613
4. Duntas LH. Subclinical hypothyroidism: a misnomer in search of a new name. Thyroid. 2001;11:361–362. doi:10.1089/10507250152039091
5. Vanderpump MP, Tunbridge WM. Epidemiology and prevention of clinical and subclinical hypothyroidism. Thyroid. 2002;12:839–847. doi:10.1089/105072502761016458
6. Ruhla S, Weickert MO, Arafat AM, et al. A high normal TSH is associated with the metabolic syndrome. Clin Endocrinol (Oxf). 2010;72:696–701. doi:10.1111/j.1365-2265.2009.03698.x
7. Rodondi N, den Elzen WP, Bauer DC, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010;304:1365–1374. doi:10.1001/jama.2010.1361
8. Gencer B, Collet TH, Virgini V, et al. Subclinical thyroid dysfunction and the risk of heart failure events: an individual participant data analysis from 6 prospective cohorts. Circulation. 2012;126:1040–1049. doi:10.1161/CIRCULATIONAHA.112.096024
9. Sorbi S, Hort J, Erkinjuntti T, et al. EFNS-ENS guidelines on the diagnosis and management of disorders associated with dementia. Eur J Neurol. 2012;19:1159–1179. doi:10.1111/j.1468-1331.2012.03784.x
10. Garduño-Garcia JJ, Alvirde-Garcia U, López-Carrasco G, et al. TSH and free thyroxine concentrations are associated with differing metabolic markers in euthyroid subjects. Eur J Endocrinol. 2010;163:273. doi:10.1530/EJE-10-0312
11. Rhee CM, Alexander EK, Bhan I, Brunelli SM. Hypothyroidism and mortality among dialysis patients. Clin J Am Soc Nephrol. 2012;8:593–601. doi:10.2215/CJN.06920712
12. Fei S, Bao C, Deng M, et al. The prevalence and determinants of hypothyroidism in hospitalized patients with type 2 diabetes mellitus. Endocrine. 2017;55:1–7. doi:10.1007/s12020-016-1162-8
13. Hadlow NC, Rothacker KM, Wardrop R, Brown SJ, Lim EM, Walsh JP. The relationship between TSH and free T(4) in a large population is complex and nonlinear and differs by age and sex. J Clin Endocrinol Metab. 2013;98:2936–2943. doi:10.1210/jc.2012-4223
14. Wang J-Y, Wang C-Y, Pei D. Association between thyroid function and metabolic syndrome in elderly subjects. J Am Geriatr Soc. 2010;58(8):1613–1614. doi:10.1111/j.1532-5415.2010.02998.x
15. Mora C, Navarro JF. The role of inflammation as a pathogenic factor in the development of renal disease in diabetes. Curr Diab Rep. 2005;5:399–401. doi:10.1007/s11892-005-0044-x
16. Pesic MM, Danijela R, Slobodan A, Radivoj K, Dobrila SD. Subclinical hypothyroidism: association with cardiovascular risk factors and components of metabolic syndrome. Biotechnol Biotechnol Equip. 2015;29:157–163. doi:10.1080/13102818.2014.991136
17. Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160:526–534. doi:10.1001/archinte.160.4.526
18. Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the U.S. population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab. 2007;92:4575–4582. doi:10.1210/jc.2007-1499
19. Chaker L, Cappola AR, Mooijaart SP, Peeters RP. Clinical aspects of thyroid function during ageing. Lancet Diabetes Endocrinol. 2018;6:733–742. doi:10.1016/S2213-8587(18)30028-7
20. Zhao M, Tang X, Yang T, et al. Lipotoxicity, a potential risk factor for the increasing prevalence of subclinical hypothyroidism? J Clin Endocrinol Metab. 2015;100:1887–1894. doi:10.1210/jc.2014-3987
21. Imaizumi M, Akahoshi M, Ichimaru S, et al. Risk for ischemic heart disease and all-cause mortality in subclinical hypothyroidism. J Clin Endocrinol Metab. 2004;89:3365–3370. doi:10.1210/jc.2003-031089
22. Iqbal A, Jorde R, Figenschau Y. Serum lipid levels in relation to serum thyroid-stimulating hormone and the effect of thyroxine treatment on serum lipid levels in subjects with subclinical hypothyroidism: the Tromso Study. J Intern Med. 2006;260:53–61. doi:10.1111/j.1365-2796.2006.01652.x
23. Knudsen N, Laurberg P, Rasmussen LB, et al. Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. J Clin Endocrinol Metab. 2005;90:4019–4024. doi:10.1210/jc.2004-222
24. Ren R, Ma Y, Deng F, et al. Association between serum TSH levels and metabolic components in euthyroid subjects: a nationwide population-based study. Diabetes Metab Syndr Obes. 2019;12:1563–1569. doi:10.2147/DMSO.S202769
25. Sakurai M, Nakamura K, Miura K, et al. Association between a serum thyroid-stimulating hormone concentration within the normal range and indices of obesity in Japanese men and women. Intern Med. 2014;53(7):669–674
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

