Back to Journals » Neuropsychiatric Disease and Treatment » Volume 15
The Relationship Between Course of Illness and β-Endorphin Plasma Levels in Patients with Schizophrenia
Authors Urban-Kowalczyk M , Kotlicka-Antczak M, Strzelecki D , Rudecka E, Śmigielski J
Received 30 July 2019
Accepted for publication 21 November 2019
Published 3 January 2020 Volume 2019:15 Pages 3609—3614
DOI https://doi.org/10.2147/NDT.S225321
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Małgorzata Urban-Kowalczyk,1 Magdalena Kotlicka-Antczak,1 Dominik Strzelecki,1 Ewa Rudecka,2,3 Janusz Śmigielski4
1Department of Affective and Psychotic Disorders, Medical University of Łódź, Łódź, Poland; 2Babiński Memorial Hospital in Łódź, Łódź, Poland; 3Faculty of Human Nutrition and Consumer Sciences, Warsaw University of Life Sciences SGGW, Warsaw, Poland; 4State High Vocational School in Konin, Konin, Poland
Correspondence: Małgorzata Urban-Kowalczyk
Department of Affective and Psychotic Disorders, Medical University of Łódź, Czechosłowacka 8/10, Lodz 92-216, Poland
Tel +48 42 675 73 71
Fax +48 42 675 74 03
Email [email protected]
Objective: Extensive investigations have been conducted into predictors of schizophrenia outcome. The heterogeneity of the illness implies that many factors should be taken into account. Some studies have reported the relationship between increased β-endorphin concentration and predominant negative symptoms.
Methods: We included 77 outpatients with schizophrenia and 74 healthy controls. Data referring to duration and course of illness, hospitalization number and treatment were collected on the basis of clinical interviews and medical documentation analysis. The β-endorphin concentrations were assessed once in all participants, at the onset of the study.
Results: A chronic course of illness was found in 44 of the 77 schizophrenics. Patients with schizophrenia, especially those with a chronic course of illness, revealed significantly higher β-endorphin concentrations than those with an episodic course and controls (mean 29.70 vs 19.86 pmol/L; p=0.0001). Increased levels of β-endorphin were related to longer duration of illness (R=0.294, p=0.009) and frequent psychiatric hospitalization (R=0.346, p=–0.002).
Conclusion: Endorphins may be potential biological predictors of persistent negative symptoms and final outcome in schizophrenia.
Keywords: β-endorphin, dopamine, opioid peptides, schizophrenia
Introduction
Despite extensive research into the etiopathology and treatment of schizophrenia, all domains of patient functioning may still be impaired not only during acute episodes but, in many cases, also permanently. Hence, the disturbance of reality processing involved in this illness can have serious consequences for patients, their families and the community. It should be underlined that schizophrenia seems to be a heterogeneous rather than a homogeneous illness complex, which additionally complicates the treatment and prognosis of outcomes. Neurochemical, genetic, epigenetic, developmental and environmental factors can interfere with normal brain development and maturation.1 Although the biology of schizophrenia has been intensively investigated for a long time, there are still a lot of questions over the symptoms leading to pronounced social disability and deterioration of everyday functioning, i.e. negative symptoms and cognitive impairment. Scientific research has attempted to identify the course and outcome of schizophrenia. When taking into account schizophrenia episodes, not only positive symptoms but also impairment of social functioning and negative symptoms should be considered. Approximately 10–15% of patients recover after first episode psychosis, although the course of schizophrenia usually fluctuates, with acute exacerbation of psychotic symptoms overlapping with poorly controlled negative, neurocognitive and social cognitive symptoms. A 15-year follow-up in the MUFUSSAD study2 in first hospitalized patients found that 57% of schizophrenics presented with chronic and 39% with an episodic–remitting course of illness. The chronic course was predicted by severe positive symptoms at admission to hospital and by negative symptoms at discharge. In a follow-up study by Harrison et al,3 33.6% of patients with schizophrenia had been continuously ill over the past 2 years. Moreover, negative symptoms were prominent in half of the individuals with such a pattern of schizophrenia course. Knowledge of potential predictors of poor outcome or persistent psychopathological symptoms may be very useful for planning individual treatment and rehabilitation for the improvement of patients functioning in various domains.
In the past, the involvement of β-endorphin (BE) in the pathogenesis of schizophrenia was extensively examined; however, further research was discontinued without unambiguous conclusions having been reached. Endorphin deficiency, the presence of abnormal endorphin and an excess of endorphins were considered as endogenous opioid disturbance in schizophrenia.4 The most popular hypothesis is endogenous “hypermorphinergic pathology” manifesting as an increase in BE concentration among patients. The imbalance in modulatory effects of endogenous opioids on the dopaminergic system may be related to the pathogenesis of schizophrenia.5 Further research studies investigated BE concentration in drug-naïve patients and in those after pharmacological treatment. Some of them failed to find significant differences in BE concentration between drug-naïve schizophrenics and controls, although there was a trend toward higher values in the patients.6 The majority of previous studies on pharmacologically treated patients included very small and non-homogeneous patient samples.7,8 The study methodology also varied in terms of the biological material used to assess BE content, i.e. peripheral blood, cerebrospinal fluid and peripheral blood mononuclear cells, as well as postmortem brain samples. More recent studies found an excess of BE in patients, especially those with negative symptoms, in comparison to healthy controls.9,10 Considering the previous findings, we hypothesized that BE concentration may be related to the clinical course of schizophrenia and, secondarily, may determin the outcome of the illness.
Methods
The study comprises 77 patients with schizophrenia and 74 healthy controls. The patients were recruited from the Affective and Psychotic Department outpatient unit at the Medical University of Łódź, Poland. Staff and students at the Medical University of Łódź were included in the control group. Sample characteristics are presented in Table 1. The study groups did not differ with regard to age (p=0.436) or gender composition (Chi2=0.14; p=0.708). Schizophrenia was diagnosed by a senior psychiatrist according to ICD-10 criteria. The data were collected from the patient’s personal interview as part of routine psychiatric assessment and retrospectively from the hospital and psychiatric outpatient unit. Information about duration of illness (DOI), frequency of psychiatric hospitalizations (hospitalization number, Hn), course of schizophrenia and kind of treatment were noted. The pattern of psychosis course was divided into episodic and chronic, according to Harrison et al.3 An episodic course means that the patient had no psychotic episode lasting longer than 6 months. A chronic course was described in those with no remission lasting longer than 6 months. DOI means the time after the onset of a psychiatric disorder according to the ICD-10 criteria. Patients were under neuroleptic treatment (first and second generation antipsychotics), in a few cases in co-medication with valproate, lamotrygine or benzodiazepines. BE plasma concentrations were measured in both study groups.
 |
Table 1 Sample Characteristics for Patients with Schizophrenia and Healthy Comparison Group |
The exclusion criteria for all groups were as follows: history of drug abuse, history of psychiatric disorders (other than schizophrenia for the patient sample), neurological disorders, head trauma and/or loss of consciousness.
All participants gave their written informed consent prior to their inclusion in the study. The study was approved by the Ethics Committee of the Medical University of Łódź (nr RNN/92/12/KE). The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2013.
Biochemical Assay
The BE assay was performed using a DRG Diagnostics β-Endorphin Kit (RIA 3023). A sample of 3 mL of peripheral blood was collected from every participant once, at the inclusion to the study. All blood samples were drawn directly into EDTA and then centrifuged. The plasma samples were stored at –20°C until assay. All blood samples from patients and controls were taken in the morning, at 7–8 a.m.
Statistical Analysis
The data were verified for normality (Shapiro–Wilk test) of distribution and equality of variances. To compare the means, the Student’s t-test was used when the distribution was normal; in other cases, the Mann–Whitney U-test was used to compare the average values. Correlations between the age, DOI, Hn and BE were analyzed with Spearman’s rank correlation coefficient. The statistical analysis was performed using the Statistica 13th CSS program. The results of the quantitative variables are presented as mean ± SD (standard deviation) and minimum and maximum values. The limit of statistical significance was set at p<0.05 for all analyses.
Results
The majority of patients (57.14%) displayed a chronic course of illness. All patient samples revealed significantly higher BE plasma concentrations than controls (mean ± SD 25.48±12.64 pmol/L 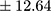 vs 17.50±7.21 pmol/L;
vs 17.50±7.21 pmol/L; 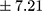 p=0.000). Patients with a chronic course of illness had much higher levels of BE than those with an episodic course (mean ± SD 29.70±13.45 pmol/L, min 12.99, max 64.25 pmol/L
p=0.000). Patients with a chronic course of illness had much higher levels of BE than those with an episodic course (mean ± SD 29.70±13.45 pmol/L, min 12.99, max 64.25 pmol/L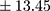 vs 19.86±8.88 pmol/L, min 9.46, max 46.01 pmol/L;
vs 19.86±8.88 pmol/L, min 9.46, max 46.01 pmol/L; 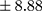 p=0.000). In patients with higher BE concentration, a longer duration of illness (Rs=0.294; p=0.009) (Figure 1) and more frequent hospitalizations were found (Rs=0.346; p=0.002) (Figure 2). There was no relationship between BE concentration and gender in patients (p=0.169) or in controls (p=0.679). Similarly, the age of participants had no impact on BE levels. Moreover, a chronic course of schizophrenia was related to significantly longer DOI than an episodic course (mean ± SD 9.30±6.93
p=0.000). In patients with higher BE concentration, a longer duration of illness (Rs=0.294; p=0.009) (Figure 1) and more frequent hospitalizations were found (Rs=0.346; p=0.002) (Figure 2). There was no relationship between BE concentration and gender in patients (p=0.169) or in controls (p=0.679). Similarly, the age of participants had no impact on BE levels. Moreover, a chronic course of schizophrenia was related to significantly longer DOI than an episodic course (mean ± SD 9.30±6.93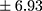 years vs 6.18±5.60 years;
years vs 6.18±5.60 years; 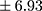 p=0.005) as well as more frequent hospitalizations (mean ± SD 4.66±3.43
p=0.005) as well as more frequent hospitalizations (mean ± SD 4.66±3.43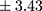 vs 2.94±2.41;
vs 2.94±2.41; 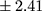 p=0.006) (Table 2).
p=0.006) (Table 2).
 |
Table 2 Comparison of Patient Samples in Relation to the Pattern of the Disease Course |
 |
Figure 1 The correlation between BE concentration and duration of illness (DOI) in patients with schizophrenia. |
 |
Figure 2 The correlation between BE concentration and number of hospitalizations in patients with schizophrenia. |
Discussion
The involvement of biological, genetic and environmental factors in the pathogenesis of schizophrenia is widely discussed, but nevertheless the trigger remains unknown. Antipsychotic agents are very effective in the treatment of positive symptoms of schizophrenia. However, none of this medication, including second generation antipsychotics, is effective enough for release from negative symptoms. It needs to be highlighted that impairment of social cognition, neurocognitive deficits and mainly negative symptoms are present in the prodromal phase of the illness and are pronounced with a high risk of schizophrenia transition.11,12 Moreover, it was found that DOI is negatively correlated with treatment response to both pharmacotherapy13 and electroconvulsive treatment.14
The phenomenon of increased BE levels, in general, is common among patients with schizophrenia. It seems that various clinical stages or manifestations may differ in their BE secretion pattern. Brambilla et al15 found higher BE plasma concentrations in 37 neuroleptic-free patients with chronic schizophrenia than in healthy controls. In line with these observations, increased basal BE concentrations were reported among pharmacologically treated individuals with chronic schizophrenia. Endorphin levels increased only in patients under long-term antipsychotic treatment.16 In our previous study, we found that inpatients during relapse of illness with predominant negative symptoms had higher BE concentrations than those with acute positive symptoms and healthy controls.9 Effective treatment resulted in a decrease in the final BE concentration to a level similar to that in healthy controls, despite the use of various individually tailored drugs. Moreover, in further studies we reported significantly increased concentrations in both inpatients and outpatients with schizophrenia.10 As for individuals with first episode psychosis, their BE levels were similar to healthy controls.17 Nevertheless, in that research, we found significant positive correlations between BE concentration and all PANSS (Positive and Negative Syndrome Scale) subscales, except for the positive symptoms subscale. It could be assumed that patients with schizophrenia exhibit a specific pattern of BE concentration relative to the predominant psychopathology. It is likely that patients with schizophrenia develop a specific pattern of BE secretion relative to the predominant psychopathology. Therefore, patients with predominant persistent negative symptoms do not achieve stable BE concentration “normalization”. The basic tenet of the following discussion is the need to update the potential role of BE in the neurobiology of schizophrenia. Patients with a long DOI may be biologically predisposed to symptom persistence/chronicity and delayed response to treatment.
The ABC Schizophrenia Study18 revealed that the mean frequency of psychotic relapse episodes was three per patient over the total course of 11.2 years (minimum of 0 and maximum of 29 relapses). Nevertheless, a high degree of heterogeneity in the illness courses was emphasized, rather independently of adherence to psychopharmacological treatment. The investigators observed a mean of three psychotic episode relapses per patient in the 11.2-year period. Although we did not analyze the frequency of relapses, but rather the number of psychiatric hospitalizations, the results indicate a significantly higher mean incidence of acute relapses among patients with a chronic course of illness than among those with an episodic course. Schultz et al19 did not find an age effect for negative symptoms, in contrast to positive and disorganized symptoms, which were less severe in older individuals.
It is reported that a deterioration in social functioning is already present in the prodromal phase of schizophrenia. Along the course of the disease, 2–5 years after the first episode the symptom severity reaches a plateau; however, this occurs significantly earlier for positive than for negative symptoms.20 Hӓfner et al18,21 found that among a first episode psychosis sample, only 32.7% of individuals had regular employment after an average 12.3 years of illness duration. It should be obvious that direct investigation of the natural course of schizophrenia is currently not possible in an ethical manner. Therefore, the majority of patients are, at least in some periods, under psychopharmacological treatment, which affects the course of the illness in unclear ways.
The MUFUSSAD study2 revealed that severe positive symptoms on admission to hospital and significant negative symptoms at discharge predicted the development of a chronic course of schizophrenia. A significant increase in negative symptoms at the 15-year follow-up was also found, similar to this at first admission to hospital. Moreover, negative symptoms were chronic and persistent along the whole observation period. The authors emphasized that the patients evaluated in the study were treated almost exclusively with first generation antipsychotics, which are mainly effective for positive schizophrenia symptoms. Participants in our study were under first or second generation antipsychotic or combined treatment. Therefore, an additional confounding factor is the DOI and modifications to the medication during this period. However, the proportion of chronic and episodic courses of illness among our probands was similar to that published by Möller et al.2 It seems that the type of pharmacological treatment and its specific effectiveness are not enough to fully explain the predominance and persistence of negative symptoms.
Our study has some limitations. First of all, the data concerning course of illness were collected retrospectively, based on clinical interviews and medical documentation, and thus some information may have been missed. Moreover, the number of psychiatric hospitalizations was analyzed, but not the relapse index, which illustrates the frequency of the most severe relapses rather than the proportion of real remissions–psychotic episodes. The assignment of course pattern typology was arbitrary and simple, but clear from a pragmatic–clinical viewpoint. In addition, we did not obtain a fully unified evaluation of the psychopathological symptoms at the onset of disease, so it was unknown which patients primarily had predominant negative symptoms. It is highly likely that endorphin concentration, at least in some range, varies depending on the quality and severity of symptoms, as well as the effects of treatment. In our study, only current BE concentration was evaluated, depending on the course of the disease, but without noting the exact kind and severity of symptoms. It should be noted that peripheral blood BE assessment does not reflect directly the BE levels in the brain. Cerebrospinal fluid concentration would be a methodologically better choice but it requires much more invasive procedures and is less acceptable to participants. A prospective, naturalistic study with a long follow-up period would be much more informative. Further research should take into account changes in the clinical picture and their relationship with changes in BE concentrations at the beginning of and during treatment, but also the final outcome, including cognitive impairment and social functioning.
Acknowledgment
The study was supported by grant 502-03/1-155-02/502-14-269 from the Medical University of Łódź.
Disclosure
The authors report no conflicts of interest in this study.
References
1. Rapoport JL, Giedd JN, Gogtay N. Neurodevelopmental model of schizophrenia: update 2012. Mol Psychiatry. 2012;17:1228–1238. doi:10.1038/mp.2012.23
2. Möller H-J, Jäger M, Riedel M, Obermeier M, Strauss A, Bottlender R. The Munich 15-year follow-up study (MUFUSSAD) on first-hospitalized patients with schizophrenic or affective disorders: comparison of psychopathological and psychosocial course and outcome and prediction of chronicity. Eur Arch Psychiatry Clin Neurosci. 2010;260:367–384. doi:10.1007/s00406-010-0117-y
3. Harrison G, Hopper K, Craig T, et al. Recovery from psychotic illness: a 15- and 25-year international follow-up study. Br J Psychiatry. 2001;178:506–517. doi:10.1192/bjp.178.6.506
4. Volavka J, Davis LG, Ehrlich YH. Endorphins, dopamine, and schizophrenia. Schizophr Bull. 1979;5(2):227–239. doi:10.1093/schbul/5.2.227
5. Fricchione GL, Mendoza A, Stefano GB. Morphine and its psychiatric implications. Adv Neuroimmunol. 1994;4(2):117–131. doi:10.1016/S0960-5428(05)80006-3
6. Mauri MC, Rudelli R, Vanni S, et al. Cholecystokinin, beta-endorphin and vasoactive intestinal peptide in peripheral blood mononuclear cells of drug-naive schizophrenic patients treated with haloperidol compared to healthy controls. Psychiatry Res. 1998;20(1–2):45–50. doi:10.1016/S0165-1781(97)00145-5
7. Panza G, Monzani E, Sacerdote P, Penati G, Panerai AE. Beta-endorphin, vasoactive intestinal peptide and cholecystokinin in peripheral blood mononuclear cells from healthy subjects and from drug-free and haloperidol treated schizophrenic patients. Acta Psychiatr Scand. 1992;85:207–210. doi:10.1111/acp.1992.85.issue-3
8. Rapaport MH, Wolkowitz O, Kelsoe JR, Pato C, Konicki PE, Pickar D. Beneficial effects of nalmefene augmentation in neuroleptic-stabilized schizophrenic patients. Neuropsychopharmacology. 1993;9(2):111–115. doi:10.1038/npp.1993.49
9. Urban-Kowalczyk M, Śmigielski J, Strzelecki D. Comparison of beta-endorphin and CGRP levels before and after treatment for severe schizophrenia. Neuropsychiatr Dis Treat. 2016;12:863–868. doi:10.2147/NDT.S101647
10. Urban-Kowalczyk M, Śmigielski J, Kotlicka-Antczak M. Overrated hedonic judgment of odors in patients with schizophrenia. CNS Neurosci Ther. 2018;24(12):1156–1162. doi:10.1111/cns.12849
11. Millan MJ, Fone K, Steckler T, Horan WP. Negative symptoms of schizophrenia: clinical characteristics, pathophysiological substrates, experimental models and prospects for improved treatment. Eur Neuropsychopharmacol. 2014;24:645–692. doi:10.1016/j.euroneuro.2014.03.008
12. Schultze-Lutter F, Michel C, Schmidt SJ, et al. EPA guidance on the early detection of clinical high risk states of psychoses. Eur Psychiatry. 2015;30:405–416. doi:10.1016/j.eurpsy.2015.01.010
13. Brousse G, Meary A, Blanc O, Lancon C, Llorca PM, Leboyer M. Clinical predictors of response to olanzapine or risperidone during acute episode of schizophrenia. Psychiatry Res. 2010;179(1):12–18. doi:10.1016/j.psychres.2009.03.003
14. Chanpattana W, Sackeim HA. Electroconvulsive therapy in treatment-resistant schizophrenia: prediction of response and the nature of symptomatic improvement. J ECT. 2010;26(4):289–298. doi:10.1097/YCT.0b013e3181cb5e0f
15. Brambilla F, Facchinetti F, Petraglia F, Vanzulli L, Genazzani AR. Secretion pattern of endogenous opioids in chronic schizophrenia. Am J Psychiatry. 1984;141(10):1183–1189.
16. Brambilla F, Facchinetti F, Petraglia F, et al. Effects of neuroleptic treatments on peripheral opioid secretion. Neuropsychobiology. 1987;18(2):68–73. doi:10.1159/000118395
17. Urban-Kowalczyk M, Strzelecki D, Śmigielski J, Kotlicka-Antczak M. Odor perception and hedonics in chronic schizophrenia and in first episode psychosis. Neuropsychiatr Dis Treat. 2019;15:647–654. doi:10.2147/NDT.S192523
18. Hӓfner H, Maurer K, van der Heiden W. ABC Schizophrenia study: an overview of results since 1996. Soc Psychiatry Psychiatr Epidemiol. 2013;48(7):1021–1031. doi:10.1007/s00127-013-0700-4
19. Schultz SK, Miller Del D, Oliver SE, Arndt S, Flaum M, Andreasen NC. The life course of schizophrenia: age and symptom dimensions. Schizophrenia Res. 1997;23(1):15–23. doi:10.1016/S0920-9964(96)00087-4
20. Hӓfner H. What is schizophrenia? 25 years of research into schizophrenia - the Age Beginning Course Study. World J Psychiatry. 2015;5(2):167–169. doi:10.5498/wjp.v5.i2.167
21. Hӓfner H. From onset and prodromal stage to a life-long course of schizophrenia and its symptom dimensions: how sex, age, and other risk factors influence incidence and course of illness. Psychiatry J. 2019;2019:1–15. doi:10.1155/2019/9804836
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
