Back to Journals » Patient Preference and Adherence » Volume 11
The prevalence of medication nonadherence in post-myocardial infarction survivors and its perceived barriers and psychological correlates: a cross-sectional study in a cardiac health facility in Malaysia
Authors Ganasegeran K , Rashid A
Received 7 September 2017
Accepted for publication 7 November 2017
Published 8 December 2017 Volume 2017:11 Pages 1975—1985
DOI https://doi.org/10.2147/PPA.S151053
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Johnny Chen
Kurubaran Ganasegeran, Abdul Rashid
Department of Public Health Medicine, Penang Medical College, George Town, Malaysia
Background: Although evidence-based practice has shown the benefits of prescribed cardioprotective drugs in post-myocardial infarction (MI) survivors, adherence rates remain suboptimal. The aim of this study was to determine the prevalence and factors associated with medication nonadherence among post-MI survivors in Malaysia.
Materials and methods: This cross-sectional study was conducted from February to September 2016 among 242 post-MI survivors aged 24–96 years at the cardiology outpatient clinic in a Malaysian cardiac specialist center. The study utilized an interviewer-administered questionnaire that consisted of items adapted and modified from the validated Simplified Medication Adherence Questionnaire, sociodemographics, health factors, perceived barriers, and novel psychological attributes, which employed the modified Confusion, Hubbub, and Order Scale and the Verbal Denial in Myocardial Infarction questionnaire.
Results: The prevalence of medication nonadherence was 74%. In the multivariable model, denial of illness (AOR 1.2, 95% CI 0.9–1.8; P=0.032), preference to traditional medicine (AOR 8.7, 95% CI 1.1–31.7; P=0.044), lack of information about illness (AOR 3.3, 95% CI 1.1–10.6; P=0.045), fear of side effects (AOR 6.4, 95% CI 2.5–16.6; P<0.001), and complex regimen (AOR 5.2, 95% CI 1.9–14.2; P=0.001) were statistically significant variables associated with medication nonadherence.
Conclusion: The relatively higher medication-nonadherence rate in this study was associated with patient-, provider-, and therapy-related factors and the novel psychological attribute denial of illness. Future research should explore these factors using robust methodological techniques to determine temporality among these factors.
Keywords: medication nonadherence, myocardial infarction, perceived barriers, psychology
Background
Evidence-based practice has shown the benefits of prescribed cardioprotective drugs in post-myocardial infarction (MI) survivors.1 Despite proven efficacy, adherence rates remain suboptimal, ranging 40%–70% globally.2,3 Asian countries report higher medication-nonadherence rates, 40%–80%,4 while in Malaysia the medication-nonadherence rate in post-MI patients averages 50%.5 This relatively poor adherence to medication has predisposed patients to greater clinical complications, recurrent hospital admissions, increased mortality, and escalated health-care expenditure.6,7
The World Health Organization (WHO) defines “adherence” as “the extent to which a person’s behavior – taking medications, following a diet, and/or executing lifestyle changes, corresponds with agreed recommendations from a health care provider”.8 Established correlates of medication nonadherence include demographic factors,9,10 socioeconomic factors like health coverage,11 and disease comorbidities.10,11 Doctor’s prescriptions and follow-up plans are often challenged when patients launch a series of justifications for not wanting to adhere, including cost, accessibility, nuisance, potential side effects, lack of knowledge of current health condition, preference for traditional medicines, and a complex regimen.12–16 Other reasons for lack of adherence to medications include poor communication, poor rapport with health-care providers, and a lack of continuity in care.13,15,17
Recent literature has hypothesized potential psychological factors to influence medication nonadherence. One such psychological factor postulated to influence medication adherence is life chaos.15 Chaotic lifestyles and environment encompass myriad factors, including variability in daily routines, ability to plan and anticipate the future, punctuality, ability to maintain financial stability, employment, housing, and ability to maintain appointments.15 Two recent preliminary investigations from the US found an association of life chaos with medication nonadherence among post-MI survivors.15,18
There are conflicting reports on the influence of denial of medication nonadherence. Some argued that denial is associated with faster recovery, better psychosocial readjustment, and lower mortality, but evidence for these claims is potentially confounded by many medical, psychological, and social factors.19–23 One study that explored post-MI coping experiences through a grounded-theory approach postulated that it was not easy to integrate treatment-related behavior patterns into one’s everyday life or to give up old habits, as the need for change is influenced by both behavioral and psychosocial factors.24 The influence of MI on patients depends on their perception of the illness and coping abilities. Kentsch et al reported that a quarter of patients in their study overtly said that they did not want to acknowledge their symptoms, admitting that they used denial as a coping strategy.25 This coping strategy appears to be related to delayed treatment-seeking behavior, because they wait for symptoms to go away and they do not take post-MI care seriously, not wanting to bother anybody, and they tend to self-medicate.22 No published studies have investigated the association of denial and medication nonadherence in post-MI patients.
The health-belief model postulates that patient engagement in health-related behaviors like medication adherence is often influenced by various “modifying factors”. These factors determine how patients perceive the threat of their health problems, which subsequently determines their likelihood to adhere to agreed treatment recommendations. Previous evidence has largely been derived from national health registries, clinical data repositories, and administrative data sets. These revealed limited information about certain types of modifying factors related to individual psychology or behavior, knowledge deficits, and self-beliefs that influence medication taking. To address the existing gap, this study aims to evaluate associations among perceived barriers, established correlates, and new psychological factors with medication nonadherence among post-MI survivors in Malaysia.
Materials and methods
Study setting and population
This cross-sectional single-center study was conducted from February to September 2016 among post-MI patients who were on follow-up at the Cardiology Outpatient Department of Serdang Hospital, Selangor, Malaysia. Serdang Hospital is one of the three premier cardiac centers in Malaysia, along with the National Heart Institute in Kuala Lumpur and Sarawak General Hospital in Borneo. The hospital is the main cardiac center in Selangor, one of the 14 states in Malaysia, and coupled with the National Heart Institute they share an equal burden for cardiac referral cases throughout Peninsular Malaysia.26 Eligibility for sample recruitment included MI survivors prescribed post-MI treatment regimen that included antiplatelets, statins, anti-ischemic agents like β-blockers, ACE inhibitors, or angiotensin-receptor blockers, as documented by a physician based on the diagnostic criteria of the Malaysian clinical practice guidelines.27 Patients had to be aged 18 years or older and discharged from the hospital at least 1 month post-MI. Patients were excluded if they had multiple significant comorbidities likely to cause mortality, such as a combination of disease clusters in addition to the diagnosis of MI (hypertension, diabetes, and heart failure; hypertension, diabetes, and stroke; cardiac arrhythmias; or other acquired cardiac illness or terminal illnesses that do not share common risk factors or pathogenic pathways of MI) that were evidenced to have greater odds of dying,28 diagnosed with stable or unstable angina, cognitively impaired, or with an active mental illness.
Sample size
Based on a sample-size calculation for a study of finite population,29 with roughly 500 post-MI survivors from Serdang Hospital,30 a sample size of 217 patients was calculated to represent a cross-section of the population and to allow the study to determine the prevalence of medication nonadherence with a margin of error of ±5%.31 An additional 20% were included in the calculated sample32 to compensate for possible missing data or nonresponse, for a final sample size of 261.
Ethics statement
This study complied with the guidelines covered in the Declaration of Helsinki. Ethical approval was obtained from the Medical Research Ethics Committee, Ministry of Health Malaysia (government approval NMRR-15-2210-28696-IIR). The study objectives and benefits were explained verbally and documented in a form attached to the questionnaires. Respondents’ confidentiality and anonymity was assured. Written consent was obtained from those who agreed to participate.
Study instruments
Data for this study were collected using an interviewer-assisted questionnaire comprised of five parts, while clinical health information was derived from patient medical records. The first part consisted of sociodemographic information (sex, age-group, marital status, education level, monthly household income, employment status, and residence–hospital distance). Age and marital status were categorized according to current population estimates for Malaysia.33 Income level was categorized according to Malaysia’s 2014 median household income and basic amenities survey.34
In the second part, the respondent’s self-rated general health status was measured using the single validated item, “How would you rate your current health status?”, with five response alternatives: poor (5), fair (4), good (3), very good (2), and excellent (1).35 These items were reversed and categorized as good (1–3) and poor (4–5), consistent with previous reported scoring rules.36,37
The primary outcome measure, medication nonadherence, was evaluated in the third part. The authors adapted and modified items from the validated six-item Simplified Medication Adherence Questionnaire (SMAQ), developed by Knobel et al.38 The modified SMAQ utilized in this study was intended to measure medication nonadherence for all prescribed medications based on the Malaysian clinical practice guidelines for a post-MI treatment regimen. Two items intended to measure frequency of medication nonadherence in the original scale were excluded. The modified SMAQ that measured nonadherence using four items in the current study identified two main subtypes of nonadherence: unintentional nonadherence (when patients wish to adhere to medication, but are prevented from taking it for some reason; based on forgetfulness and clarification regarding the medical regimen) and intentional nonadherence (when patients deliberately do not take their medications; based on withdrawing medications when feeling better or worse).39,40 Items in the adapted and modified SMAQ scale included: Do you ever forget to take your medicine?; Are you careless at times about taking your medicine?; Sometimes if you feel worse, do you stop taking your medicines?; and Did you decide to self-omit or alter prescribed doses of your medications? As the SMAQ was dichotomous, any answer in the sense of nonadherent was considered nonadherent.38 The four-item scale had a scoring scheme of yes =1 and no =0. The items were summed to give a range of scores of 0–4. Total scores were dichotomized, with a score of 0 as adherent and scores of 1–4 as nonadherent.
The fourth part explored barriers to medication taking. Three domains with five items in accordance with the WHO framework of medication adherence8 were evaluated: patient factors (prefer taking traditional medicine, lack of transportation),16 provider or health-system factor (lack of information about illness),16 and therapeutic factors (complex regimen, fear of side effects)13 using validated single-item reported measures adapted from previous studies. Respondents were asked, “Thinking of your current cardiac health situation, what would be the possible barriers for you for not wanting to take prescribed medications?” Respondents were given the response option of yes =1 or no =0.
Psychological factors were assessed in the fifth part. Perceived life chaos was explored through the validated six item of the Malay version of the Confusion, Hubbub, and Order Scale (CHAOS-6).41 This scale was designed to evaluate chaos in one’s life that addressed consistency of daily routine, ability to plan and anticipate future activities, and being on time.15,42,43 This six-item scale constituted the following items: “My life is organized”, “My life in unstable”, “My routine is the same from week to week”, “My daily activities from week to week are unpredictable”, “Keeping a schedule is difficult for me”, and “I don’t like to make appointments too far in advance because I don’t know what might come up.” Response choices were recorded on a 5-point Likert scale ranging from “definitely true” to “definitely false.” Scoring of the CHAOS scale was performed by summing item responses and ranged 6–30, such that a higher score indicated a more chaotic lifestyle.15,18
Subsequently, respondent acceptance of cardiac illness was measured using the validated eight-item Verbal Denial in Myocardial Infarction questionnaire.20 This scale encompasses three domains: denial of illness (two items), denial of impact (four items), and suppression (two items). These scale items were rated on a 5-point Likert scale ranging from “disagree completely” to “agree completely”. Scoring of the total scale and the three subscales was performed by summing the item responses such that higher scores indicated a greater denial of MI.20
Clinical health information, such as time from MI event and disease comorbidities (diabetes, hypertension, or hypercholesterolemia), were derived from patient records. Baseline parameters are defined as follows: patients with fasting plasma glucose level 7 mmol/L (126 mg/dL) or above and prescribed oral hypoglycemic agents or insulin regimen, as documented in medical records, were classified as having diabetes;44 patients were labeled hypertensive if they had been previously diagnosed with hypertension and administered antihypertensives, as documented in medical records;44 hypercholesterolemia was defined as total cholesterol more than 5.2 mmol/L with high plasma triglyceride concentration (>1.7 mmol/L), low high-density-lipoprotein cholesterol concentration (<1 mmol/L for men, <1.3 mmol/L for women), and increased concentration of low-density-lipoprotein cholesterol (>2.6 mmol/L with cardiac risk factors) with patients currently on statins, as documented in medical records.45 Table 1 briefly describes the study instrument.
  | Table 1 Summary of the study instrument |
Statistical analyses
Analysis was conducted using STATA version 13.0 (StataCorp, College Station, TX, USA). To check the validity of the modified SMAQ among the Malaysian population, an exploratory factor analysis was performed using principal-component analysis by the direct oblimin method. The Kuder–Richardson test was performed to test the internal consistency and reliability of the modified SMAQ binary-measure scale in this study sample. Cronbach’s α-value for the Verbal Denial of Myocardial Infarction scale was also used to test the internal consistency of the scale. Descriptive statistics were obtained for all variables in the study. Cross-tabulation analysis was used to obtain the proportions. Simple logistic regression analysis was used to obtain ORs and to assess the association between medication nonadherence and categorical variables. Multiple logistic regression analysis using stepwise backward elimination was performed to obtain the final model and AORs. All independent variables with significant associations in the univariate analyses were included in the multivariate analysis. The significance level was set to 0.05 (P<0.05).
Results
Sample characteristics
A total of 261 consecutive patients were invited to participate in the study, and 242 (92.7% response rate) participated. The sample constituted 208 (85.9%) men and 34 (14.1%) women. The mean (±SD) age of the respondents was 55 (±11) years, with a range of 24–96 years. Most respondents were married (85.9%), had attained secondary education or less (90.1%), and employed (62.8%), with a monthly household income of less than RM4,500 (US$1 = RM4.40) (89.4%). The mean (±SD) of residence–hospital distance was 26.8 (±33.7) km, and the distance ranged 1–250 km (Table 2). The mean (±SD) time from MI event was 22 (±24.5), range 1–204 months. The bulk of respondents perceived themselves to have “good” health status (73.1%). With regard to disease comorbidities, 67.8% of the respondents had hypertension, 62.8% hypercholesterolemia, and 47.5% diabetes (Table 2).
  | Table 2 Sociodemographics and health attributes of respondents (n=242) |
Medication nonadherence and perceived barriers
Exploratory factor analyses using the direct oblimin method of the modified SMAQ yielded one factor with given eigenvalue greater than 1 (2.9). The one-factor solution accounted for 51% of the variance. Factor loading ranged from 0.67 to 0.8. The Kuder–Richardson internal consistency and reliability test for the binary measure of the modified-SMAQ scale was 0.65, suggesting acceptable internal consistency for the study sample. The prevalence of medication nonadherence in this study was 74%. Reasons cited included fear of side effects (39.3%), complex regimen (27.7%), lack of information about illness (22.3%), lack of transport (12.8%), and preference for traditional medicine (12.4%) (Figure 1).
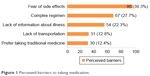  | Figure 1 Perceived barriers to taking medication. |
Psychological attributes
Cronbach’s α-coefficient for the Verbal Denial in Myocardial Infarction Questionnaire was 0.62, suggesting acceptable internal consistency suitable for the Malaysian population. The mean (±SD) perceived life-chaos score was 15 (±5.3), ranging 6–30. The mean (±SD) denial-of-illness score was 4.5 (±2.5), and the total sub-domain score ranged 2 to 10. The mean (±SD) denial of impact score was 10.7 (±4), and the total subdomain score ranged 4–20. The mean (±SD) suppression score was 6.9 (±2.6), ranging 2–10. The mean (±SD) of the total cardiac denial sum score was 22.1 (±6.2), ranging 8–37.
Association of sociodemographic and health-related factors with medication nonadherence
Men had about threefold the odds of women of nonadherence to medications (OR 2.6, 95% CI 1.2–5.6; P=0.01). Similarly, patients aged 18–59 years had about double the odds of those aged ≥60 years of nonadherence to medications (OR 1.8, 95% CI 1.1–3.3; P=0.01). Patients with hypercholesterolemia had double the odds of those without hypercholesterolemia of nonadherence to medications (OR 2, 95% CI 1.1–3.5; P=0.022). A 1-month increase in time from the MI event was associated with a 10% increase in the odds of medication nonadherence (OR 1.1, 95% CI 0.9–1.2; P=0.041). A 1 km increase in distance between residence and hospital was associated with a 10% increase in the odds of medication nonadherence (OR 1.1, 95% CI 0.9–1.2; P=0.045) (Table 3).
Association between psychological attributes and medication nonadherence
Table 4 exhibits the associations between the psychological attributes and medication nonadherence. A 1-unit increase in life-chaos score was associated with a 10% increase in the odds of being nonadherent to medications (OR 1.1, 95% CI 0.9–1.2; P=0.003). A 1-unit increase in the denial-of-illness score was associated with a 20% increase in the odds of being nonadherent to medications (OR 1.2, 95% CI 1.1–1.4; P=0.003).
Association between perceived barriers and medication nonadherence
As shown in Table 5, medication nonadherence was higher among patients who lacked transport to the health facility (OR 5.9, 95% CI 1.4–25.5; P=0.008), those who preferred traditional medicines (OR 12, 95% CI 1.6–89.9; P=0.002), those lacking information about illness (OR 5.7, 95% CI 2–16.6; P<0.001), those fearing side effects (OR 9.4, 95% CI 3.9–22.9; P<0.001), and those with complex medication regimens (OR 4.9, 95% CI 2–12; P<0.001). These associations were statistically significant.
  | Table 5 Association between perceived barriers and medication nonadherence |
Factors associated with medication nonadherence using multiple logistic regression analyses
All statistically significant factors associated with medication nonadherence in the univariate analyses were included in the multivariate analyses. The multivariable model retained five statistically significant factors associated with medication nonadherence: denial of illness (AOR 1.2, 95% CI 0.9–1.8, P=0.032), preference for traditional medicine (AOR 8.7, 95% CI 1.1–31.7; P=0.044), lack of information about the illness (AOR 3.3, 95% CI 1.1–10.6; P=0.045), fear of side effects (AOR 6.4, 95% CI 2.5–16.6; P<0.001), and complex regimen (AOR 5.2, 95% CI 1.9–14.2; P=0.001) (Table 6). The adjusted R2 (Nagelkerke) showed that 39% of nonadherence was explained by this logistic model. The χ2 goodness of fit was not significant (P=0.862), showing that the model had an adequate fit.
Discussion
This study aimed to determine the prevalence and factors associated with medication nonadherence among post-MI survivors in Malaysia. The prevalence of medication nonadherence was 74%. The estimated rate of medication nonadherence in this study was slightly lower than that found in South Korea (79%),46 but relatively higher than that found in the UK (49.6%),47 US (43%),15 Portugal (50.4%),48 and Italy (37%).49 The remarkably greater magnitude of medication nonadherence could be attributed to variations in sample size and study methodologies executed in previous studies or differences in local clinical practice guidelines adopted across different health-care settings in other countries. Akeroyd et al4 stated that the striking differences in medication-nonadherence rates between European and Asian countries could be attributed to differences in the methodologies used, acknowledging economic feasibility, resource-limited countries, and developing nations in Asia primarily use self-report measures to assess medication nonadherence compared to the “gold standard” measurements, such as pharmacy-refill data, used in developed countries.
Contrary to previous studies that found women to be nonadherent,9,10,50 this study found that medication nonadherence was more prevalent in men. This finding should be interpreted with caution, as the statistically significant association between sex and medication nonadherence could be attributed to the relatively high number of men recruited in this sample. This situation is unavoidable, as the incidence of MI among the Malaysian population is more prevalent in men than in women.51,52 Another plausible explanation of medication nonadherence among men is the apprehension of the medication side effects (erectile dysfunction with β-blockers),18 as evidenced in rigorous clinical trials.53,54
Previous studies have shown mixed variations on the association between age and medication nonadherence: some argued that medication nonadherence was significantly higher in older groups as a consequence of impaired cognition, inadequate health literacy, and decline in physical or physiological functions,5,55 while others found significantly higher medication-nonadherence rates in younger groups that could be attributed to greater employment or social engagement.18,56 The statistically significant higher rates of medication nonadherence among younger groups in this study may agree with the latter notion. The authors found that medication nonadherence was higher among patients who traveled a longer distance to the health-care facility, and this association was statistically significant. Potential inhibitors may include difficult topography, lack of transport, or greater cost for travel.
Previous studies have deduced a significant association between medication nonadherence and time from MI event.5,57 This study found that patients with a longer time from the MI event were more likely to be nonadherent; however, the statistically significant association at the univariate level was eliminated in the final regression model. It is well known that post-MI patients are required to adhere to long-term cardioprotective drugs as a secondary prevention. This accelerates cost and possible side effects from prescribed medications, requiring adjustments of treatment regimen by cardiologists during follow-ups. This demotivates patients’ belief in the benefits of newer drugs with an increased pill burden, predisposing them to a higher nonadherence rate.57
This study found that the association between medication nonadherence with high-risk comorbidities (tendency to have high chances of post-MI fatality),28,58 like diabetes and hypertension, was not statistically significant. These findings were consistent with recent Italian9 and American57 studies, but inconsistent with South Asian56 and Malaysian5 studies. On the other hand, this study found a positive association between medication nonadherence and lower-risk comorbidity (tendency to have low chances of post-MI fatality),28,58 such as hypercholesterolemia. This finding is consistent with the South Asian59 and Malaysian5 studies, but inconsistent with the Italian study.9 This paradoxical finding could be attributed to the “sick-stopper bias” (sicker patients are more likely to disengage themselves from the agreed treatment plan), and “healthy-user bias”, which explains the better outcomes of less nonadherent patients.56
Consistent with recent efforts by Zullig et al15 and Crowley et al,18 this study found a positive association between perceived life chaos and medication nonadherence. Populations being mobilized within the nation’s fastest developmental phase and rapid urbanization are exposed to greater environmental noise, pollution, road traffic, employment competitiveness, rare neighborhood integration, and overcrowding.60 Such situations impact one’s health due to a chaotic lifestyle, apart from elevated frustrations of financial instability, loss of job, inappropriate housing, or inability to maintain clinic appointments as a sequel of MI,60,61 all of which form a risk for medication nonadherence.15
In contrast with previous studies, correlations between demographics and socioeconomic factors with medication nonadherence were eliminated in the final multivariable model. Perceived barriers were concurrent with the WHO domains-of-nonadherence framework.8 Patient-related factors (preference for traditional medicine), provider- and health system-related factors (lack of information about illness), and therapeutic factors (fear of side effects, complex regimen) showed positive associations with medication nonadherence and were statistically significant. This significance could be attributed to multiethnic Malaysians’ predilection for holistic health beliefs and complementary medicine use,14 in addition to physician adjustments to prescribed doses with newer synergistic therapies (in which patients may undermine the therapeutic efficacy or have greater concerns), can predispose patients toward medication-nonadherent behaviour.13
Denial of illness showed a positive association with medication nonadherence in the multivariate model. Lazarus62 initially proposed an intriguing hypothesis. He viewed denial as constructive at an early phase of the disease to overcome the emotional shock of the diagnosis, but found it destructive at a later course of disease progression, when active coping was needed. Fowers63 supported this notion. He argued that at an early stage of an illness, denial was perceived as a coping mechanism to allay anxiety in cardiac patients, but over time these mechanisms collapse, implicating poor knowledge of cardiac disease progression and outcomes, delay in seeking treatment, and not being adherent to prescribed medications.63
Study limitations
Limitations of this study should be acknowledged. The cross-sectional nature of this study could not ascertain temporal relationships. Although the adherence measure was validated, it may have been susceptible to social desirability bias as a self-report measure in this study, predisposing to overestimates of prevalence rates.
The relatively small sample from a single study site and the demographics of the study population (a majority men of younger groups) may limit the generalizability of the study findings, and thus extrapolation of the study findings is guarded. As noted in the results, the size of the study sample is reflected in its CI. The accuracy of the ORs, although significant, may not be wholly accurate. The CI reflects that the sample size was not adequate confidently to determine associations. Similarly, the findings that denial as a potentially new psychological factor was associated with medication nonadherence could be debated. The small sample could have been a reason for the statistically insignificant association between total denial score or other subdomain scores, like denial of impact and suppression with medication nonadherence. However, it should be noted that the sample-size calculation for this study was not done to predict this observation alone, but to involve other factors as well, including chaos, barriers to nonadherence, health attributes, and sociodemographics. Therefore, there is a high tendency for the total denial score and other subdomain scores not to predict the main outcome measure. It should be noted that medication nonadherence is a vast subject with many dynamic variables, and it is not possible to explore all factors in one study. However, this cohort offered advantages that helped to offset concerns relating to the sample size, allowing the authors to address an existing evidence gap by exploring new variables as potential modifying targets in the psychological domain.
Conclusion
The remarkably high prevalence rate of medication nonadherence in this study showed a statistical significant association with patient-, provider-, and therapy-related barriers in line with the WHO framework.8 A new psychological factor, denial of illness, also showed statistical significance.
Recommendations
While the results suggest that emerging psychological attributes and patient- and therapy-related barriers influence medication-nonadherence rates, health-care professionals should be more vigilant in screening patients with a higher risk of poor adherence, including those patients prescribed complex regimens that require a change in lifestyle behaviors. Stratifying patients based on their adherence level would enable early, targeted interventions. In addition, it would be interesting to know whether high multimorbidity burden negatively affects post-MI medication adherence. Further exploration in future research is needed to assess such relationships across subgroups of common and different pathogenic pathways of diseases in addition to MI to determine the best possible outcomes for better policy and practice implications.
For patients living far from a health-care facility or those lacking transport, the revolution of big health data for virtual interconnectedness through mobile health could prompt health-care professionals to interact with patients regularly through WhatsApp, WeChat, Line, or Tango by sending friendly reminders for taking medication and exploring possible difficulties throughout the course of the treatment.64 Community health nurses, pharmacists, doctors, and medical assistants could reach patients in rural areas far from current health-care facilities to provide follow-up care and prescribed medications through routine mobile health clinics.
Psychological factors like chaos and denial, which were tested for the first time in the Asian population, had significant or near-significant associations with medication nonadherence; however, some of these factors lost significance in the multivariate analyses. While the present study hypothesized these modifiable factors to be significantly associated with medication nonadherence, it was not powered to reject definitively the null hypothesis to claim these attributes as predictors of nonadherence in post-MI patients. Future research should explore these attributes using robust methodological techniques, such as prospective cohort designs, to determine temporality between these factors.
Acknowledgments
We thank Dr Kamaraj Selvaraj (deputy head of department, Cardiology Unit of Serdang Hospital), Dr Muralitharan Perumal (head of unit, Clinical Research Centre of Tengku Ampuan Rahimah Hospital), Dr Bahanordin Jaafar (head of Rehabilitation Unit, Tengku Ampuan Rahimah Hospital), Dr Rohisham Zainal Abidin (head of Anesthesiology Department, Tengku Ampuan Rahimah Hospital), and Mr Wilson Perianayagam (chief medical assistant for Selangor state, Selangor Health Department) for their support, encouragement, advice, and approval for conducting this study. This research project was funded by a mini-research seed grant from the Clinical Research Centre of Tengku Ampuan Rahimah Hospital (grant CRC-HTAR-01/16).
Author contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work. This study was part of a research-dissertation project performed by Dr Kurubaran Ganasegeran as fulfillment of his postgraduate Master’s in Health Research degree under the supervision of Professor Dr Abdul Rashid at Penang Medical College.
References
Mehta RH, Montoye CK, Faul J, et al. Enhancing quality of care for acute myocardial infarction: shifting the focus of improvement from key indicators to process of care and tool use. J Am Coll Cardiol. 2004;43:2166–2173. | ||
Rodriguez F, Cannon CP, Steg PG, et al. Predictors of long-term adherence to evidence-based cardiovascular disease medications in outpatients with stable atherothrombotic disease: findings from the REACH registry. Clin Cardiol. 2013;36:721–727. | ||
Mathews R, Wang TY, Honeycutt E, et al. Persistence with secondary prevention medications after acute myocardial infarction: insights from the TRANSLATE-ACS study. Am Heart J. 2015;170:62–69. | ||
Akeroyd JM, Chan WJ, Kamal AK, Palaniappan L, Virani SS. Adherence to cardiovascular medications in the South Asian population: a systematic review of current evidence and future directions. World J Cardiol. 2015;7:938–947. | ||
Kassab YW, Hassan Y, Aziz NA, Zulkifly HH, Iqbal MS. Trends in adherence to secondary prevention medications in post-acute coronary syndrome patients. Pak J Pharm Sci. 2015;28:641–646. | ||
Bosworth HB, Granger BB, Mendys P, et al. Medication adherence: a call for action. Am Heart J. 2011;162:412–424. | ||
Kolandaivelu K, Leiden BB, O’Gara PT, Bhatt DL. Non-adherence to cardiovascular medications. Eur Heart J. 2014;35:3267–3276. | ||
Sabate E. Adherence to Long-Term Therapies: Evidence for Action. Geneva: World Health Organization; 2003. | ||
Kirchmayer U, Agabiti N, Belleudi V, et al. Socio-demographic differences in adherence to evidence-based drug therapy after hospital discharge from acute myocardial infarction: a population-based cohort study in Rome, Italy. J Clin Pharm Ther. 2012;37:37–44. | ||
Kumbhani DJ, Fonarow GC, Cannon CP, et al. Predictors of adherence to performance measures in patients with acute myocardial infarction. Am J Med. 2013;126:74.e1–e9. | ||
Newby LK, La Pointe NM, Chen AY, et al. Long-term adherence to evidence-based secondary prevention therapies in coronary artery disease. Circulation. 2006;113:203–212. | ||
Rosenbaum L, Shrank WH. Taking our medicine: improving adherence in the accountability era. N Engl J Med. 2013;369:694–695. | ||
Desai NR, Choudhry NK. Impediments to adherence to post myocardial infarction medications. Curr Cardiol Rep. 2013;15:322. | ||
Ganasegeran K, Rajendran AK, Al-Dubai SA. Psycho-socioeconomic factors affecting complementary and alternative medicine use among selected rural communities in Malaysia: a cross-sectional study. PLoS One. 2014;9:e112124. | ||
Zullig LL, Shaw RJ, Crowley MJ, et al. Association between perceived life chaos and medication adherence in a post-myocardial infarction population. Circ Cardiovasc Qual Outcomes. 2013;6:619–625. | ||
Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clin Proc. 2011;86:304–314. | ||
Ganasegeran K, Perianayagam W, Manaf RA, Jadoo SA, Al-Dubai SA. Patient satisfaction in Malaysia’s busiest outpatient medical care. ScientificWorldJournal. 2015;2015:714754. | ||
Crowley MJ, Zullig LL, Shah BR, et al. Medication non-adherence after myocardial infarction: an exploration of modifying factors. J Gen Intern Med. 2015;30:83–90. | ||
Benzer W. Cardiac rehabilitation after myocardial infarction: the influence of psychological disorders. In: Niebauer J, editor. Cardiac Rehabilitation Manual. Heidelberg: Springer; 2011:151–162. | ||
Havik OE, Maeland JG. Dimensions of verbal denial in myocardial infarction. Scand J Psychol. 1986;27:326–339. | ||
Stenström U, Nilsson A, Stridh C, et al. Denial in patients with a first-time myocardial infarction: relations to pre-hospital delay and attendance to a cardiac rehabilitation programme. Eur J Cardiovasc Prev Rehabil. 2005;12:568–571. | ||
Perkins-Porras L, Whitehead DL, Strike PC, Steptoe A. Causal beliefs, cardiac denial and pre-hospital delays following the onset of acute coronary syndromes. J Behav Med. 2008;31:498–505. | ||
Fang XY, Albarqouni L, Rothe AF, Hoschar S, Ronel J, Ladwig KH. Is denial a maladaptive coping mechanism which prolongs pre-hospital delay in patients with ST-segment elevation myocardial infarction? J Psychosom Res. 2016;91:68–74. | ||
Salminen-Tuomaala M, Astedt-Kurki P, Rekiaro M, Paavilainen E. Coping experiences: a pathway towards different coping orientations four and twelve months after myocardial infarction: a grounded theory approach. Nurs Res Pract. 2012;2012:674783. | ||
Kentsch M, Rodemerk U, Müller-Esch G, et al. Emotional attitudes toward symptoms and inadequate coping strategies are major determinants of patient delay in acute myocardial infarction. Z Kardiol. 2002;91:147–155. | ||
Ministry of Health Malaysia [website on the Internet]. Available from: www.moh.gov.my. Accessed April 15, 2017. | ||
Ministry of Health Malaysia. Clinical practice guidelines: management of acute ST segment elevation myocardial infarction (STEMI). 2014. Available from: http://www.moh.gov.my/penerbitan/CPG/CPG%20_%20Management%20of%20Acute%20ST%20Segment%20Elevation%20Myocardial%20Infarction%20STEMI%203rd%20Edition.pdf. Accessed January 15, 2017. | ||
McManus DD, Nguyen HL, Saczynski JS, Tisminetzky M, Bourell P, Goldberg RJ. Multiple cardiovascular comorbidities and acute myocardial infarction: temporal trends (1990–2007) and impact on death rates at 30 days and 1 year. Clin Epidemiol. 2012;4:115–123. | ||
Creative Research Systems. Sample size calculator. 2012. Available from: http://www.surveysystem.com/sscalc.htm. Accessed November 15, 2017. | ||
Selangor State Health Department [website on the Internet]. Available from: http://www.jknselangor.moh.gov.my/index.php/en. Accessed January 15, 2016. | ||
Rashid A, Conroy R, Ahmad Z. I Hate Statistics! George Town, Malaysia: Penang Medical College; 2012. | ||
Naing L, Winn T, Rusli BN. Practical issues in calculating the sample size for prevalence studies. Arch Orofac Sci. 2006;1:9–14. | ||
Malaysia Department of Statistics. Population and demography. 2017. Available from: https://www.dosm.gov.my/v1/index.php?r=column/ctwoByCat&parent_id=115&menu_id=L0pheU43NWJwRWVSZklWdzQ4TlhUUT09. Accessed January 15, 2017. | ||
Department of Statistics Malaysia. Report of household income and basic amenities survey 2014. 2015. Available from: https://www.dosm.gov.my/v1/index.php?r=column/cthemeByCat&cat=120&bul_id=aHhtTHVWNVYzTFBua2dSUlBRL1Rjdz09&menu_id=amVoWU54UTl0a21NWmdhMjFMMWcyZz09. Accessed November 15, 2017. | ||
Fernandez BR, Rosero-Bixby L, Koivumaa-Honkanen H. Effects of self-rated health and self-rated economic situation on depressed mood via life satisfaction among older adults in Costa Rica. J Aging Health. 2016;28:225–243. | ||
Ware JE Jr, Gandek B. Overview of the SF-36 health survey and the international quality of life assessment (IQOLA) project. J Clin Epidemiol. 1998;51:903–912. | ||
Zajacova A, Dowd JB. Reliability of self-rated health in US adults. Am J Epidemiol. 2011;174:977–983. | ||
Knobel H, Alonso J, Casado JL, et al. Validation of a simplified medication adherence questionnaire in a large cohort of HIV-infected patients: the GEEMA study. AIDS. 2002;16(4):605–613. | ||
Gadkari AS, McHorney CA. Unintentional non-adherence to chronic prescription medications: how unintentional is it really? BMC Health Serv Res. 2012;12:98. | ||
Oliveira-Filho AD, Morisky DE, Costa FA, Pacheco ST, Neves SF, Lyra DP. Improving post-discharge medication adherence in patients with CVD: a pilot randomized trial. Arq Bras Cardiol. 2014;103:502–512. | ||
Ganasegeran K, Selvaraj K, Rashid A. Confirmatory factor analysis of the Malay version of the Confusion, Hubbub and Order Scale (CHAOS-6) among myocardial infarction survivors in a Malaysian cardiac healthcare facility. Malays J Med Sci. 2017;24:39–46. | ||
Wong MD, Sarkisian CA, Davis C, Kinsler J, Cunningham WE. The association between life chaos, health care use, and health status among HIV-infected persons. J Gen Intern Med. 2007;22:1286–1291. | ||
Mochari H, Ferris A, Adigopula S, Henry G, Mosca L. Cardiovascular disease knowledge, medication adherence, and barriers to preventive action in a minority population. Prev Cardiol. 2007;10:190–195. | ||
Ganasegeran K, Renganathan P, Manaf RA, Al-Dubai SA. Factors associated with anxiety and depression among type 2 diabetes outpatients in Malaysia: a descriptive cross-sectional single-center study. BMJ Open. 2014;4:e004794. | ||
Ministry of Health Malaysia. Clinical practice guidelines: management of dyslipidemia 2011. 2011. Available from: https://www.malaysianheart.org/files/17706572750124a3988f0c.pdf. Accessed November 15, 2017. | ||
Shin ES, Hwang SY, Jeong MH, Lee ES. Relationships of factors affecting self-care compliance in acute coronary syndrome patients following percutaneous coronary intervention. Asian Nurs Res (Korean Soc Nurs Sci). 2013;7:205–211. | ||
Molloy GJ, Perkins-Porras L, Bhattacharyya MR, Strike PC, Steptoe A. Practical support predicts medication adherence and attendance at cardiac rehabilitation following acute coronary syndrome. J Psychosom Res. 2008;65:581–586. | ||
Dias A, Pereira C, Monteiro MJ, Santos C. Patients’ beliefs about medicines and adherence to medication in ischemic heart disease. Aten Primaria. 2014;46:101–106. | ||
Martino MD, Alagna M, Cappai G, et al. Adherence to evidence-based drug therapies after myocardial infarction: is geographic variation related to hospital of discharge or primary care providers? A cross-classified multilevel design. BMJ Open. 2016;6:e010926. | ||
Smolina K, Ball L, Humphries KH, Khan N, Morgan SG. Sex disparities in post-acute myocardial infarction pharmacologic treatment initiation and adherence problem for young women. Circ Cardiovasc Qual Outcomes. 2015;8:586–592. | ||
Lee CY, Hairi NN, Ahmad WA, et al. Are there gender differences in coronary artery disease? The Malaysian National Cardiovascular Disease Database – Percutaneous Coronary Intervention (NCVD-PCI) registry. PLoS One. 2013;8:e72382. | ||
Tee HL, Nordin R, Ahmad WA, et al. Sex differences in acute coronary syndrome in a multi-ethnic Asian population: results of the Malaysian National Cardiovascular Disease Database – Acute Coronary Syndrome (NCVD-ACS) registry 2015. Glob Heart. 2014;9:381–390. | ||
Keene LC, Davies PH. Drug-related erectile dysfunction. Adverse Drug React Toxicol Rev. 1999;18:5–24. | ||
Botros SM, Hussein AM, Elserafy AS. Effect of different beta blockers on penile vascular velocities in hypertensive males. Egypt J Radiol Nucl Med. 2015;46:749–754. | ||
Lenzi J, Rucci P, Castaldini I, et al. Does age modify the relationship between adherence to secondary prevention medications and mortality after acute myocardial infarction? A nested case-control study. Eur J Clin Pharmacol. 2015;71:243–250. | ||
Sanfélix-Gimeno G, Peiró S, Ferreros I, et al. Adherence to evidence-based therapies after acute coronary syndrome: a retrospective population based cohort study linking hospital, outpatient, and pharmacy health information systems in Valencia, Spain. J Manag Care Pharm. 2013;19:247–257. | ||
Faridi KF, Peterson ED, McCoy LA, Thomas L, Enriquez J, Wang TY. Timing of first postdischarge follow-up and medication adherence after acute myocardial infarction. JAMA Cardiol. 2016;1:147–155. | ||
Quintana HK, Janszky I, Gigante B, et al. Diabetes, hypertension, overweight and hyperlipidemia and 7-day case-fatality in first myocardial infarction. IJC Metab Endocr. 2016;12:30–35. | ||
Lai EJ, Grubisic M, Palepu A, Quan H, King KM, Khan NA. Cardiac medication prescribing and adherence after acute myocardial infarction in Chinese and South Asian Canadian patients. BMC Cardiovasc Disord. 2011;11:56. | ||
Stevenson M, Thompson J, de Sá TH, et al. Land use, transport, and population health: estimating the health benefits of compact cities. Lancet. 2016;388:2925–2935. | ||
Kleinert S, Horton R. Urban design: an important future force for health and wellbeing. Lancet. 2016;388:2848–2850. | ||
Lazarus RS. Costs and benefits of denial. In: Breznitz S, editor. The Denial of Stress. New York: International University Press; 1983. | ||
Fowers BJ. The Cardiac Denial of Impact Scale: a brief, self-report research measure. J Psychosom Res. 1992;36:469–475. | ||
Ganasegeran K, Renganathan P, Rashid A, Al-Dubai SA. The m-Health revolution: exploring perceived benefits of WhatsApp use in clinical practice. Int J Med Inform. 2017;97:145–151. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



