Back to Journals » Therapeutics and Clinical Risk Management » Volume 14
The outcome of pediatric patients undergoing congenital cardiac surgery under pulsatile cardiopulmonary bypass in different frequencies
Authors LI G , Jiang W, Zhang Y, Zhang X, Chen J, Zhuang J, Zhou CB
Received 10 April 2018
Accepted for publication 21 June 2018
Published 3 September 2018 Volume 2018:14 Pages 1553—1561
DOI https://doi.org/10.2147/TCRM.S170642
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Deyun Wang
Guanhua Li,1,* Wen Jiang,2,* Yu Zhang,3,* Xiaohua Zhang,1 Jimei Chen,1 Jian Zhuang,1 Chengbin Zhou1
1Department of Cardiovascular Surgery, Guangdong Cardiovascular Institute, Guangdong Provincial Key Laboratory of South China Structural Heart Disease, Guangdong General Hospital, Guangdong Academy of Medical Sciences, Guangzhou, Guangdong, People’s Republic of China; 2Department of Thoracic Surgery, The First People’s Hospital of Yunnan Province, Kunming, Yunnan, People’s Republic of China; 3Department of Pathology, Guangdong Provincial Hospital of Chinese Medicine, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, Guangdong, People’s Republic of China
*These authors contributed equally to this work
Purpose: To investigate the influence and possible pathophysiological mechanism of pulsatile cardiopulmonary bypass (CPB) in various frequencies in pediatric patients undergoing congenital cardiac surgery.
Patients and methods: Clinical data and hemodynamic parameters were collected in 80 patients who underwent congenital cardiac surgeries and were perfused in different settings: pulsatile perfusion (PP) in frequencies of 30 beats/min, PP 60 beats/min, PP 100 beats/min and non-pulsatile perfusion (NP). Serum proteins, plasma-free hemoglobin (PFH), endothelin-1 (ET-1) and nitric oxide (NO) were collected to study possible pathophysiological changes, possible hematological injury and oxidative status under different perfusing conditions.
Results: Patients in all groups had similar baseline characteristics, aortic cross-clamping time and CPB duration. More effective pulse gradient (PG), energy-equivalent pressure (EEP) and surplus hemodynamic energy (SHE) were observed in pulsatility with lower frequency setting, under which more patients achieved physiologically normal mean arterial pressure (MAP), without the support of inotropic agents during bypass. Significant between-group differences of serum proteins and PFH were absent the whole time during and after bypass, while a relatively lower percentage of perioperative requirement of diuretics was observed in the low frequency pulsatile group. A better performance to oxidative stress was seen in the low frequency group with higher levels of NO and lower concentration of ET-1, and both intergroup differences were found (P<0.01). Satisfactory clinical outcome was obtained on post procedure course in all groups.
Conclusion: Pulsatile perfusion with low frequency setting in pediatric patients undergoing congenital cardiac surgery showed better hemodynamic profiles, potential protective effects on vital organs, better oxidative status and satisfactory clinical outcome.
Keywords: pulsatile perfusion, cardiopulmonary bypass, frequency, congenital cardiac surgery
Introduction
Cardiopulmonary bypass (CPB) may lead to varying degrees of vital organ injuries, of which the incidence is reported to be 1%–5% in pediatric patients.1 Several investigators have recommended pulsatile cardiopulmonary bypass, offering pulsatile perfusion (PP) that mimics physiological waveforms which is supposed to be more beneficial in vital organ protection than non-pulsatile perfusion (NP) during extracorporeal circulatory support.2,3
The advantage of PP is mainly based on the fact that it provides extra stimulus which hemodynamically affects tissue microcirculation. Previous studies have demonstrated that PP has more positive effects on postoperative recovery of the respiratory system compared with NP, which results in unbalanced flow distribution, compromising peripheral and vital organ circulation.4 Investigations on tissue perfusion have confirmed advantageous effects of pulsatile pattern, as deduced by the alleviation of systemic inflammatory response after CPB.5
However, the comparison of pulsatile and non-pulsatile flow mode with regard to the damage of blood components remains controversial,6 and moreover, some other researchers have claimed that no superiority has been observed in postoperative recovery or surgical prognosis.7,8 Studies on pulsatile vs non-pulsatile patterns rarely reach consensus to support or oppose PP as conflictions exist in mortality, surgical complications, impairments of vital organs and neurologic dysfunction.9 Similarly, to the best of our knowledge, few studies have so far focused explicitly on the impact of pulsatile frequency on pathophysiological changes during CPB.
Recently, we launched a study to investigate the clinical profile and possible mechanism in pediatric patients undergoing congenital cardiac surgery during CPB in a heart-lung machine running in various pulse frequencies or conventional non-pulsatile fashion.
Materials and methods
Patients and surgical preparation
Clinical data were collected in 80 cases of pediatric patients undergoing congenital cardiac surgeries under PP in different pulse frequencies matched with control cases in NP. Random allocation was done consecutively via “Research Randomizer Program” (http://www.randomizer.org). Cases were randomly divided into four groups: PP with pulse frequency 30 beats/minute (Group A, n=20); PP with pulse frequency 60 beats/minute (Group B, n=20); PP with pulse frequency 100 beats/minute (Group C, n=20); and NP control group (Group D, n=20). Cases allocated in the current study had non-cyanotic congenital cardiac malformations, including ventricular septal defect (52 cases), ventricular septal defect combined with atrial septal defect (16 cases), ventricular septal defect with patent ductus arteriosus (7 cases) and pulmonary stenosis (5 cases). Patients with cyanotic or complex cardiac anomalies, severe pulmonary hypertension, severe multiple organ dysfunction or aortic cross-clamp period less than 40 minutes were all excluded in this study.
The study protocol was approved by the ethical committee of Guangdong General Hospital, and all patients provided written informed consent for this study.
CPB establishment and surgery
Median sternotomy was performed under general anesthesia in every case. After arterial cannulation of ascending aorta and bicaval cannulation of the right atrium, CPB was started routinely via a Stöckert S5 roller pump (Stöckert Instruments, Sorin Group, Munich, Germany). CPB was connected to a membrane oxygenator (CAPIOX SX10R Oxygenator; Terumo Medical Co., Ann Arbor, MI, USA), in which the extracorporeal circuit was primed with 150–250 mL of lactated Ringer’s solution, 10 mg of heparin, 1 mg/kg furosemide, 0.5 g of MgSO4 and red blood cells 100 mL. CPB was maintained at a pump flow rate of 100 to 150 mL/kg/min, with rectal temperature ranging from 30°C to 34°C after the heart was arrested. Anterograde crystalloid cardioplegic solution was used in each case, with activated clotting time (ACT) maintained above 480 second during bypass. PP was commenced after aortic cross-clamp, with frequencies according to group settings (30 beats/minute, 60 beats/minute, 100 beats/minute or non-pulsatile blank control), base flow 30% and pulse width set at 30%. Fluid level was supervised intensively during CPB, and hemodynamic parameters were recorded at 3-minute intervals. Mean arterial pressure (MAP) was maintained between 25 and 60 mmHg, and phenylephrine shots were given in case of hypotension (<20 mmHg). Lactated Ringer’s solution was added to the reservoir when fluid loss was indicated through the fluid gauge. After removal of the aortic cross-clamp, the heart was re-perfused, and PP setting was terminated when acceptable contractility was reached. The patient was re-warmed until nasopharyngeal temperature and rectal temperature reached 36.0°C and 34°C, respectively. After discontinuation of extracorporeal circulation (ECC), 4 mg/kg of protamine was administered for heparin neutralization.
Clinical documentation and tests
Hemodynamic parameters were monitored and recorded during surgical course. To quantify pulsatility, arterial pressure gradient was used to evaluate native pulsatile flow and was equivalent to pulse pressure. Arterial pressure gradient was calculated according to the formula: Arterial pressure gradient = systolic pressure − diastolic pressure. The concepts of energy-equivalent pressure (EEP) and surplus hemodynamic energy (SHE) were employed in this study to more accurately evaluate the energy gradient induced by pulsatile flow. According to Shepard’s model,10 EEP was calculated following this formula: EEP = ∫QPdt/∫Qdt, in which Q is the blood flow (mL/s), P is the instantaneous pressure (mmHg), and t is the time (s). SHE was calculated according to the formula: SHE = 1,332 (EEP − MAP) (ergs/cm3).10
Arterial blood samples were taken from an arterial catheter at baseline (before CPB), and at the time of aortic cross- clamping, separation of CPB and decannulation, 12 hours and 24 hours after surgery. Analysis of hematocrit, acid–base variables, electrolytes and level of lactic acid were performed on blood gas analysis. Serum proteins such as creatine kinase MB (CK-MB), lactic dehydrogenase (LDH), alanine aminotransferase (ALT), bilirubin, blood urea nitrogen (BUN) and creatinine (Cr) were subjected to blood chemistry workups for the investigation of vital organ function changes during and after CPB. Plasma-free hemoglobin (PFH) was tested using a HemoCue Plasma/Low Hb photometer (HemoCue AB, Ängelholm, Sweden), for the possibility of hematologic damage relevant to CPB. Endothelin-1 (ET-1) and nitric oxide (NO) were also measured to study the status of oxidative stress. ET-1 was determined through the endothelin radioimmunoassay kit (Science and Technology Development Center, PLA General Hospital, Beijing, China), and results were described as ng/L. The measurement of NO was based on the nitrate reductase method, using the NO assay kit (Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China) according to the manufacturer’s instructions, in which results were stated in μmol/L.
One consistent team of physicians in the intensive care unit (ICU) took charge of postoperative management of patients enrolled in the present study. Clinical data were thoroughly documented during the whole operative course, including duration of the aortic cross-clamp, CPB time, duration of mechanical ventilation support, length of ICU stay and any complications which occurred.
Statistics
Continuous variables are displayed as mean ± standard deviation (SD), while categorical variables are presented in percentages. Prior to the comparison of differences between groups, test of normality and the Hartley test for homogeneity of variance were first applied. Analysis of variance (ANOVA) test was used to analyze within-group differences in continuous variables which were normally distributed, and when comparing within-group differences of quantitative variables at specific time checkpoints, repeated measures ANOVA was used – otherwise, one-way ANOVA was used. The chi-squared test was used to compare categorical variables between groups. A P-value less than 0.05 was considered as statistical significance. Software used for statistical analysis was SPSS version 10.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
There was no statistically significant difference in preoperative characteristics and patients in all groups had similar ages, body weight and sex distribution. Corrective surgeries were carried out in every case. No procedure at the stage of palliation was performed in this study. Intra-operative variables were documented with respect to the duration of aortic cross-clamp and CPB, in which no significant statistical difference was observed between groups (Table 1).
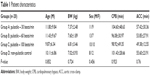  | Table 1 Patient characteristics |
Hemodynamic data
After the initiation of pulsatility, the mean arterial pressure gradients were 21.01±7.97, 12.46±6.53, 7.58±4.40 and 3.11±0.67 mmHg under frequencies of 30 beats/min, 60 beats/min, 100 beats/min and control group, respectively, and between-group differences were observed (Figure 1). Recorded hemodynamic variables are shown in Table 2.
  | Figure 1 Arterial pressure gradients induced by pulsatile perfusion under various frequencies. |
Mean energy-equivalent pressures (EEPs) from Group A to D were 38.23±21.08, 26.09±17.52, 10.63±5.96 and 3.18±1.20 mmHg, respectively; mean surplus hemodynamic energies (SHE) were 2,471±599, 1,619±70 5, 785±317 and 220±109 ergs/cm3, and between-group differences of both variables were observed. In Group A, ten cases of 20 (50%) achieved physiologically normal mean arterial pressure (MAP) without the support of inotropic agents during bypass, as compared to five cases (25%), two cases (10%), and one cases (5%) from Group B to D. As more effective pressure gradient and surplus hemodynamic energy could be much easier seen in lower pulsatility settings, MAP had a tendency to reach normal level.
The MAP level was similar between groups at any time points following surgery; however, only six (30%) cases in Group A required continuous inotropic administration 12 hours postoperatively than other groups (30% vs 45%, 75% and 75%, P=0.006), and the proportion decreased to four (20% cases) at 24 hours checkpoint (20% vs 30%, 55% and 65%, P=0.013). For other examined parameters like central venous pressure (CVP) and urinary output, no significant intergroup differences were obtained at any time points. No patients required hemofiltration or peritoneal dialysis in ICU. Of the 20 cases in Group A, a diuretic was administrated in two patients (10%), with a relatively lower percentage than other groups at 12 and 24 hours postoperatively (10% vs 15%, 25% and 20%, P=0.630; 10% vs 10%, 30% and 15%, P=0.267). However, this did not reach statistical significance.
Laboratory findings and blood chemistry workups
Laboratory findings and results of blood chemistry workups at different time points are presented in Table 3. Hematocrit (HCT) levels dropped dramatically after the initiation of bypass, mainly because of hemodilution by primed fluid and remained at relatively low levels in each group during CPB (P<0.05); nevertheless, no significant within-group difference was obvious. Central venous oxygen saturation (SvO2) was significantly lower and lactic acid level was significantly higher after commencement of CPB in all groups (P<0.001), but again significant difference was absent between groups. Impaired peripheral perfusion during CPB might be the reason for the changes of SvO2 and lactic acid.
Serum CK-MB and LDH levels abruptly increased after CPB, compared to preoperative baseline (P<0.001), but inter-group differences of the two cardiac enzymes were statistically insignificant, at each time checkpoint. Concentration of serum CK-MB reached its peak after weaning off the bypass and gradually decreased in ICU, while serum LDH still increased abruptly after surgery and peaked at 24 hours postoperatively. It might be the effect of myocardial ischemic-reperfusion injury that led to these changes. Neither level of hepatic and renal serum markers (ALT, bilirubin, BUN and Cr) in comparison with the baseline nor between-group differences of these serum proteins were significant at any time during and after bypass, and no manifestations of hepatic or renal injuries were found in this study.
A certain degree of hematologic damage was confirmed in every group, as PFH temporarily increased during ECC compared with pre-CPB levels (P<0.001), but soon declined and recovered after transferring to ICU. Significant inter-group difference was absent for this marker at any time.
For the investigation of oxidative status, concentration of nitric oxide (NO) went up during surgery, and peaked at decannulation, then decreased postoperatively. A higher NO value was observed in Group A when coming off CPB (P<0.001). Analogous to NO, ET-1 increased after aortic cross-clamping and also peaked at CPB-off, during which the concentrations of ET-1 were 67.89±32.59, 157.93±51.40, 130.60±65.15 and 163.62±68.13 ng/L from Group A to D, respectively. Lower concentration of ET-1 was seen in lower frequency pulsatility and an intergroup difference was obtained (P<0.001).
Clinical outcome evaluation
Clinical outcome parameters are displayed in Table 4. There was no in-hospital mortality in this study. ECC was weaned off successfully in every patient; however, one patient in Group A presented with cardiomegaly and compromised contractivity, therefore the closure of the sternum was delayed for about 24 hours. Both the duration of mechanical ventilation and the length of ICU stay were similar in all groups. Few surgical-related complications were observed in this study. One patient was diagnosed surgical site infection in Group B and required debridement 20 days after operation. Pneumothorax was found in one case in Group A. These sporadic complications were all properly managed.
  | Table 4 Clinical outcome profiles |
Discussion
The main finding of the current study was that PP in extracorporeal circulation with low frequency was more favorable with regard to vital organ protection and hemodynamics in pediatric patients undergoing congenital heart surgery. The pulsatile CPB with a frequency of 30 beats per minute resulted in more effective pressure gradient and surplus hemodynamic energy, better performance to oxidative stress, potential protective effects on vital organs during CPB, and satisfactory clinical outcome, without evidence of hemolysis, as well as severe complications in postoperative course. The potential clinical benefits of low frequency PP were thus regarded as better extra-energy effects to provide pulse stimulus to tissue perfusion and peripheral vasculature.
Whether pulsatile CPB is superior to non-pulsatile CPB has been one of the long-lasting controversies in cardiac surgery for decades. PP is far from an ideal recipe. It may lead to hemolysis or blood spallation trauma in roller pumps owing to high speed ejection from the ascending aortic cannula. As many studies have demonstrated no benefit from employing pulsatility during CPB, the opponents of pulsatile CPB emphasize the dearth of irrefutable evidence for its clinical superiorities. Multiple factors may contribute to these disputes: patient selection criteria, patient characteristics, type of surgical procedures, preoperative vital organ function, means of obtaining pulsatility, and so forth. Some studies from literature are therefore incomparable and lack overwhelming evidences.
The optimal pulsatility should be more biomimetic or physiologic. It should contain the following features: a typical complete stroke volume delivered into the elastic ascending aorta over a typical systolic time and at an appropriate frequency.11 An appropriate pulsatile frequency enables more physiological flow, effective hemodynamic energy and pressure gradient down into the vital organs and peripheral vasculature, allowing for diastolic perfusion delivery. The pulsatility and the frequency should be in a reasonable manner, otherwise, the self-regulatory system might be impaired. Indeed, few studies have so far enunciated the influence of pulsatile frequency during CPB. In this study, more efficient pressure gradient was obtained in the low frequency pulsatility group, in which MAP came closer to physiological arterial pressure corresponding to infants at similar body weight. In spite of the similarity between high frequency pulsatility and relatively high heart rate in infants under physiological condition, high frequency setting triggers faster hemodynamic energy decaying in each cycle under fixed perfusion flow during CPB. Low frequency setting provides higher hemodynamic energy gradient, and thus imitates a more biomimetic fashion.
To create a successful PP, CPB parameters in the circuit – such as type of equipment, pump settings, aortic cannula, oxygenators, arterial line filters and CPB tube size – and the pulsatile flow delivered to circulation drops from these components should be modified. The shorter and smaller the CPB circuit, the less dampening the effect of pulsatile delivery it might generate. Various means can be chosen to establish a pulse, such as centrifugal and ventricular pumps; however, these methods still have not gained popularity in clinical practice. To clarify and confirm the clinical effects of these parameters contributing to pulsatile delivery, more comparable and well-designed studies are thereby worth carrying out.
Preoperative and postoperative functions of vital organs rely largely on perfusion quality. Especially in the kidney, both urinary volume and various serum proteins change sensitively according to the quality of perfusion, and postoperative renal function has proved to be better under PP in previous studies.5,12 Plenty of mechanisms describe the salutary impacts of PP on vital organ protection in both animal models and clinical investigations. It has been found that PP attenuates the activation of renin and angiotensin II,13,14 and also decreases sympathetic system activity, leading to lower blood concentration of catecholamine, which acts as vasoconstrictors of the renal vascular bed, causing a reduction in the glomerular filtration rate (GFR).15–18
Despite the limited number of included cases, there is still some evidence from our study to suggest that PP has some positive effects on vital organ protection during CPB. Albeit, in the absence of intergroup differences, a relatively lower percentage of perioperative requirements of diuretics was observed in the low frequency pulsatile group. Our study did not show any differences of hepatic or renal serum markers (ALT, bilirubin, BUN and Cr) between groups. However, our study included only patients without existing organ dysfunction before surgery, undergoing straightforward procedures with short aortic cross-clamping, and these markers might not be sufficiently raised to indicate organ impairments. If a case has higher risk, complex congenital malformation, presence of preoperative organ dysfunction, longer CPB duration, or more complicated surgical procedure, subsequent organ dysfunction might be detectable. The kidney is a sensitive organ in response to ischemic damage, and, in comparison with BUN and Cr, some available urinary markers are heralded as more powerful predictors of acute renal injury, such as N-acetyl-β-D-glucosaminidase (NAG), kidney injury molecule-1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL).19–21 Unfortunately, due to limitations in the laboratory settings, none of these biomarkers were employed in the current study. Certainly, further and deeper investigations are required to ascertain the detailed mechanism of organ protection.
The preoperative impairment of vital organ function is relevant to inflammatory and oxidative stutus during CPB, meanwhile, the coagulation cascade could be activated by blood component injury induced by the mechanical force of the pump. Inflammatory and endothelial cytokines, such as interleukin-8 (IL-8), ET-1 and monocyte chemoattractant protein-1 (MCP-1), are considered to be downregulated during pulsatile CPB,5 while concentrations of anti-inflammatory markers like IL-10 are higher.22 Furthermore, pulsatility promotes the synthesis of NO, and thus regulates the auto-oxidative status.13,14 Nakano et al14 reported that pulsatile flow enhanced endothelium-derived NO release, yielding peripheral vasodilation and reduction of peripheral vascular resistance.5 Our results also revealed better performance to oxidative stress and lower level of ET-1, without hematological injury under low pulsatile settings, which are in accordance with many studies published previously.5,22 Pulsatility mimics the physiologic state, increases energy flow to the endothelium, and lowers vascular resistance, thus contributing to better perfusion in peripheral vasculature and microcirculation.
In addition to the positive protective impacts of pulsatility physiologically, satisfactory clinical outcomes were obtained in the postoperative course. Our study did not show significant differences with respect to surgical-related complications between groups. In the current study, we focused on clinical outcome parameters only in the early preoperative period, and unsafe or negative effects were not found during and after pulsatile CPB. However, the beneficial impacts on organ protection with respect to low frequency PP in this study did not reach statistical significance in patients with straightforward pediatric congenital cardiac operations. This might either be a result of well-compensated baseline organ function or the uncomplicated surgical process in relatively young age. Again, it should be noted that many investigations available in the literature are based on well-controlled animal experiments and are not comparable with clinical studies, which could be multifactorial and inconsistent. Murkin et al launched a double-blind, randomized study of 316 patients undergoing coronary bypass grafting and demonstrated that pulsatile setting decreased surgical mortality and the incidence of major complications.23 Similar clinically beneficial effects were also confirmed in other randomized studies.24–26 Based on the beneficial effects of low frequency pulsatility mentioned above, we may speculate that clinical outcome parameters in this study might reach significant differences in patients with higher surgical risks or advanced age.
Conclusion
Pulsatile perfusion with low frequency setting in pediatric patients undergoing congenital cardiac surgery showed more effective hemodynamic profiles, potential protective effects on vital organs during CPB and satisfactory clinical outcome, without evidence of hemolytic damage, as well as severe complications in postoperative course.
Acknowledgment
The corresponding author would like to acknowledge Dr Zhang Li for her valuable contribution to this study.
Disclosure
The authors report no conflicts of interest in this work.
References
Karkouti K, Wijeysundera DN, Yau TM, et al. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation. 2009;119(4):495–502. | ||
Moazami N, Dembitsky WP, Adamson R, et al. Does pulsatility matter in the era of continuous-flow blood pumps? J Heart Lung Transplant. 2015;34(8):999–1004. | ||
Alkan T, Akçevin A, Undar A, et al. Benefits of pulsatile perfusion on vital organ recovery during and after pediatric open heart surgery. Asaio J. 2007;53(6):651–654. | ||
Vasků J, Wotke J, Dobsák P, et al. Acute and chronic consequences of non-pulsatile blood flow pattern in long-term total artificial heart experiment. Pathophysiology. 2007;14(2):87–95. | ||
Sezai A, Shiono M, Nakata K, et al. Effects of pulsatile CPB on interleukin-8 and endothelin-1 levels. Artif Organs. 2005;29(9):708–713. | ||
Hickey PR, Buckley MJ, Philbin DM. Pulsatile and nonpulsatile cardiopulmonary bypass: review of a counterproductive controversy. Ann Thorac Surg. 1983;36(6):720–737. | ||
Saito S, Westaby S, Piggot D, et al. End-organ function during chronic nonpulsatile circulation. Ann Thorac Surg. 2002;74(4):1080–1085. | ||
Chow G, Roberts IG, Edwards AD, et al. The relation between pump flow rate and pulsatility on cerebral hemodynamics during pediatric cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1997;114(4):568–577. | ||
Doddakula K, Al-Sarraf N, Gately K, et al. Predictors of acute renal failure requiring renal replacement therapy post cardiac surgery in patients with preoperatively normal renal function. Interact Cardiovasc Thorac Surg. 2007;6(3):314–318. | ||
Shepard RB, Simpson DC, Sharp JF. Energy equivalent pressure. Arch Surg. 1966;93(5):730–740. | ||
Sunagawa G, Koprivanac M, Karimov JH, Moazami N, Fukamachi K. Is a pulse absolutely necessary during cardiopulmonary bypass? Expert Rev Med Devices. 2017;14(1):27–35. | ||
Alghamdi AA, Latter DA. Pulsatile versus nonpulsatile cardiopulmonary bypass flow: an evidence-based approach. J Card Surg. 2006;21(4):347–354. | ||
Taylor KM, Bain WH, Russell M, Brannan JJ, Morton IJ. Peripheral vascular resistance and angiotensin II levels during pulsatile and non-pulsatile cardiopulmonary bypass. Thorax. 1979;34(5):594–598. | ||
Nakano T, Tominaga R, Nagano I, Okabe H, Yasui H. Pulsatile flow enhances endothelium-derived nitric oxide release in the peripheral vasculature. Am J Physiol Heart Circ Physiol. 2000;278(4):H1098–H1104. | ||
Toda K, Tatsumi E, Taenaka Y, Masuzawa T, Takano H. Impact of systemic depulsation on tissue perfusion and sympathetic nerve activity. Ann Thorac Surg. 1996;62(6):1737–1742. | ||
Markham DW, Fu Q, Palmer MD, et al. Sympathetic neural and hemodynamic responses to upright tilt in patients with pulsatile and nonpulsatile left ventricular assist devices. Circ Heart Fail. 2013;6(2):293–299. | ||
Minami K, Körner MM, Vyska K, et al. Effects of pulsatile perfusion on plasma catecholamine levels and hemodynamics during and after cardiac operations with cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1990;99(1):82–91. | ||
Philbin DM, Levine FH, Kono K, et al. Attenuation of the stress response to cardiopulmonary bypass by the addition of pulsatile flow. Circulation. 1981;64(4):808–812. | ||
Liangos O, Tighiouart H, Perianayagam MC, et al. Comparative analysis of urinary biomarkers for early detection of acute kidney injury following cardiopulmonary bypass. Biomarkers. 2009;14(6):423–431. | ||
Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365(9466):1231–1238. | ||
McIlroy DR, Wagener G, Lee HT. Neutrophil gelatinase-associated lipocalin and acute kidney injury after cardiac surgery: the effect of baseline renal function on diagnostic performance. Clin J Am Soc Nephrol. 2010;5(2):211–219. | ||
Onorati F, Santarpino G, Tangredi G, et al. Intra-aortic balloon pump induced pulsatile perfusion reduces endothelial activation and inflammatory response following cardiopulmonary bypass. Eur J Cardiothorac Surg. 2009;35(6):1012–1019. | ||
Murkin JM, Martzke JS, Buchan AM, Bentley C, Wong CJ. A randomized study of the influence of perfusion technique and pH management strategy in 316 patients undergoing coronary artery bypass surgery. I. Mortality and cardiovascular morbidity. J Thorac Cardiovasc Surg. 1995;110(2):340–348. | ||
Baraki H, Gohrbandt B, del Bagno B, Haverich A, Boethig D, Kutschka I. Does pulsatile perfusion improve outcome after cardiac surgery? A propensity-matched analysis of 1959 patients. Perfusion. 2012;27(3):166–174. | ||
Kocakulak M, Aşkin G, Kuçukaksu S, Tarcan O, Pişkin E. Pulsatile flow improves renal function in high-risk cardiac operations. Blood Purif. 2005;23(4):263–267. | ||
Poswal P, Mehta Y, Juneja R, Khanna S, Meharwal ZS, Trehan N. Comparative study of pulsatile and nonpulsatile flow during cardio-pulmonary bypass. Ann Card Anaesth. 2004;7(1):44–50. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


