Back to Archived Journals » Open Access Animal Physiology » Volume 7
The naked mole-rat as an animal model in biomedical research: current perspectives
Authors Schuhmacher L, Husson Z, Smith ESJ
Received 10 May 2015
Accepted for publication 30 June 2015
Published 11 August 2015 Volume 2015:7 Pages 137—148
DOI https://doi.org/10.2147/OAAP.S50376
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Peter Koulen
Laura-Nadine Schuhmacher, Zoé Husson, Ewan St. John Smith
Department of Pharmacology, University of Cambridge, Cambridge, UK
Abstract: The naked mole-rat (NMR) is a subterranean rodent that has gained significant attention from the biomedical research community in recent years as molecular mechanisms underlying its unusual biology start to be unraveled. With very low external mortality, NMRs have an unusually long lifespan while showing no signs of aging, such as neurodegeneration or cancer. Furthermore, living underground in large colonies (100 to 300 animals), results in comparatively high carbon dioxide and low oxygen levels, from which NMRs have evolved extreme resistance to both hypoxia and hypercapnia. In this paper we have summarized the latest developments in NMR research and its impact on biomedical research, with the aim of providing a sound background that will inform and inspire further investigations.
Keywords: naked mole-rat, longevity, cancer, hypoxia, nociception, pain
Introduction
The naked mole-rat (NMR) (Heterocephalus glaber) is a subterranean mammal, which has recently gained interest from scientists across a variety of research fields. Unlike the majority of mammals, NMRs are poikilothermic and eusocial, ie, are cold-blooded and have a single breeding female within a colony.1 In addition to these features, which have limited biomedical translatability, NMRs have also evolved several physiological adaptations to habituate to their extreme environmental conditions, which have led researchers to study this mammal with the hypothesis that by understanding the extreme biology of NMRs, more will be understood about normal mammalian physiology. Although studied for several decades, it has been recent advances in sequencing technology that have helped fuel the current surge in research dedicated to understanding more about the molecular mechanisms underlying NMR biology, and thus making it a major model in biomedical research.
In this paper, we will firstly describe the unusual physiological features of NMRs compared to other mammals, before focusing on recent findings that demonstrate the significant impact that this relatively new model species is having on biomedical research in areas such as longevity, cancer, hypoxic brain injury, and nociception. Finally, we will discuss the limitations of NMRs as a laboratory model in biomedical studies, as well as highlighting some future perspectives for further understanding the extraordinary biology of NMRs.
NMR eusociality and poikilothermy
NMRs belong to the Bathyergidae African mole-rat family and are found in arid regions of East Africa, mainly in Somalia, Kenya, and Ethiopia. The bathyergids are endemic to Sub-Saharan Africa and live in a variety of habitats that differ with regards to their relative humidity, soil types, and vegetation diversity.2 According to recent molecular-based phylogenetic analysis, the bathyergids can be divided into six main genera: Bathyergus, Georychus, Heliophobius, Fukomys, Cryptomys and Heterocephalus.3 Species among these genera can be split into either solitary species (eg, the Cape mole-rat, Georychus capensis and the silvery mole-rat, Heliophobius argenteocinereus), social species (eg, the Natal mole-rat, Cryptomys hottentotus natalensis, and the Mahali mole-rat, Cryptomys hottentotus mahali), and eusocial species: (eg, the NMR, H. glaber, and the Damaraland mole-rat [DMR], Fukomys damarensis).2,4
Like social insects, such as bees or termites, NMRs are eusocial and live in colonies of up to 300 animals.1,4,5 Strict eusociality, defined by overlapping generations within a colony, cooperative offspring care, and division of labor between reproductive and non-reproductive groups of animals, is extremely rare among mammals and only NMRs and DMRs fulfill all of these characteristics.1 Within each colony, a single breeding female gives birth to pups and remains reproductively active into old age.6,7 Morphologically, the breeding female is usually larger than the other mole-rats of the colony,8 and actively suppresses the reproductive ability of both male and females colony members,9 except for one to three breeding males. Importantly, although maintained in a prepubescent stage while subjected to reproduction suppression by the breeding female,10 non-breeder individuals are able to effectively mate and reproduce when the opportunity arises, eg, after the death of the breeding female, or when split off as a breeding pair in captivity.7
As will be discussed in the following sections, this highly social lifestyle has an important impact on the biology of NMRs, as eusociality is thought to favor the occurrence of longevity, but can also worsen hypoxic/hypercapnic conditions encountered by NMRs within the burrows.
The underground network of chambers and tunnels where NMRs live can extend for up to three kilometers, depending on the food availability and the number of individuals composing the colony.11 Like many subterranean mammals, NMRs chiefly feed on roots, tubers, and corms of various geophyte plant species.11 This subterranean habitat, in addition to offering good protection against predation, also provides stable climatic conditions, allowing for an ambient temperature and humidity levels to be maintained in the burrows; temperature varies from 28°C to 32°C depending upon burrow depth with little seasonal change, while humidity is uniformly high (up to 90%).11,12 This high constant ambient temperature is a critical factor for NMRs, as they have a poor thermoregulatory capacity. NMRs exhibit a surprisingly low body temperature (Tb) (approximately 32°C),12 even in comparison to other fossorial mammals (eg, the lesser blind mole-rat, Spalax Leucodon: 37°C; the silvery mole-rat, H. argentiocinereus: 35°C; and the southeastern pocket gopher, Geomys pinetis: 36.1°C),12 which also exhibit lower Tb than other mammals, due to higher rates of heat loss and increased risks of overheating and water loss during digging activities in the relatively higher temperatures of burrows than aboveground.12,13 When exposed to temperatures outside their thermoneutral zone (31°C to 34°C), NMRs do not maintain their Tb, and thus Tb follows the ambient temperature12,14 (a fact that anyone receiving a shipment of NMRs can attest to – a box of barely moving animals is transformed after 30 minutes in a 32°C incubator, into a box of highly energetic animals). NMRs are thus defined as poikilotherms,14 and indeed one might predict this phenotype when considering some of their morphologic characteristics such as thick, hairless skin, and little subcutaneous fat.15 However, they possess brown adipose tissue which is a similar property of homoeothermic species,16 and use their social environment to regulate their Tb, notably through huddling in groups.17
To our knowledge, NMRs are the only poikilothermic mammal. This poor thermoregulatory capacity, possibly due to their low thyroid hormone levels,18 may have significant implications for other aspects of NMR physiology, such as longevity or hypoxia tolerance.
The physiological adaptations to their extreme living conditions, as well as their highly unusual mammalian traits, such as eusociality and poikilothermy, establish NMRs as a unique animal model for biomedical studies.
Insights into longevity and healthy aging
Across species, maximum lifespan (MLSP) is positively correlated with body mass, such that as body mass increases, extrinsic mortality (eg, predation) decreases, and species can invest more in maintaining fecundity and long-term survival.19 The NMR seems to be one of the few exceptions to this rule: similar in mass to the laboratory mouse, C57/BL6, Mus musculus (adult NMR: 35–75 g, and adult C57/BL6 mouse: 25–35 g), NMRs have an MSLP of 32 years compared to 4 years for mice.20 It has been suggested that the fossorial lifestyle of NMRs, and other long-lived rodents, is a contributor to the extended lifespan, since it also strongly decreases extrinsic mortality. A recent analysis of age and body mass data of vertebrates, taking into account habitat and ecology, showed a clear correlation between fossorial, or volant lifestyles, and longer life.19 However, it has been argued that a more important driver of longevity is eusociality. Using data that included loosely eusocial species (such as wolf, jackal, and coyote), as well as NMR and DMR, it was concluded that while body mass accounts for about 30% of the variation in MLSP, sociality affected 3.3%, while habitat only explained 0.01% of the effect.21
In addition to long life, NMRs also fulfill all criteria associated with negligible senescence: the queen reproduces life-long with fully maintained fecundity, and there is no age-related change in physiological functions or gradual change in mortality rate, as is usually observed in most other species which exhibit a gradual, life-long deterioration.22 NMRs also show no age-related changes in basal metabolic rate, body fat, bone mineral density, and only very small changes in cardiovascular and gastrointestinal function.23,24 NMRs do however, show a fast decrease in functions when they reach MLSP and age very rapidly just before death.22,25
Investigations into the molecular adaptions and metabolic changes of NMRs have unearthed some clues as to why this small rodent lives a long and healthy life. Contradictory to the notion that oxidative damage is detrimental to health, NMRs exhibit elevated levels of DNA oxidative damage from a young age, but appear able to deal with this damage more efficiently than other organisms.25 It has been suggested that the NMR genome has a low background mutation rate and low nucleotide diversity, which would point to more efficient DNA damage control, but it is unclear if this observation is not just a consequence of the small gene pool and extreme inbreeding, since it can also be observed in the eusocial DMR.26,27 Interestingly, in DMRs, it has been observed that oxidative damage is lower in longer-lived breeding females than in other colony members,28 which suggests that breeding status is likely of importance. Indeed, evidence indicates that the breeding female in DMR colonies live for longer than non-breeding females,29 and it would be interesting to determine if similar is true for NMRs.
Compared to mice, NMR proteins have higher levels of cysteine residues, which have been suggested to act as a buffer of oxidative damage. While aging organisms accumulate proteins that exhibit both irreversibly oxidized cysteine and polyubiquitination,30 NMRs show no age-related changes in their overall low levels of either, which indicates that their proteins are kept in a healthy state throughout their life.25 Also, the NMR genome shows an expansion of heat shock protein HSP70 and HSP90 protein families, which could play a role in preventing protein misfolding.31 Interestingly, protein unfolding in response to treatment with urea was much more pronounced in mouse than in NMR, and whereas the amount of unfolded protein increased with age in mice, it did not in NMRs.25 Furthermore, RING domain ubiquitin ligases have been shown to be enriched for pseudogenes in the NMR genome, suggesting that there are lower levels of ubiquitination.32 NMR hepatic cells also have a higher cytosolic proteasome activity than mice, which suggests enhanced protein degradation and turnover.33,34 In addition, NMR hepatic cells show increased autophagy, which have previously been associated with longer lifespans in birds, and might contribute to a healthier cell metabolism.35 A contributing factor to healthy protein metabolism might be the observed change in ribosome processing, where NMR 28S rRNA is cleaved into two molecules.36 Further to this break, NMR cells have higher translation fidelity, which is consistent with the earlier observation that there is less accumulation of ubiquitinated proteins or age-related aggregates of misfolded protein.36 It has been speculated that this change in 28S rRNA processing was present in the common ancestor of the hystricognath clade (to which Bathyergidae belong), since it is also present in the South American tuco tuco (Ctenomys brasiliensis), and Degu (Octodon degus), but not in the South American guinea pig or African DMR,27 suggesting that it was present before a geographical dispersion occurred even though it has been lost by species on both continents.
NMRs show no signs of neurodegeneration, but they express higher levels of amyloid-β and a heavily phosphorylated version of tau protein, both of which are associated with Alzheimer’s disease, when compared with a transgenic mouse model of Alzheimer’s disease.37,38 However, no aggregations of these proteins or plaque formation has been observed, and it was suggested that high amyloid-β and phosphorylated tau might function as neural regulators in an environment of high oxidative stress.38 The maintenance of neuronal integrity has also been attributed to neuregulin 1 and its receptor ErbB4, which are both elevated in long-lived rodents, NMRs exhibiting the highest observed levels.39 Neuregulin 1 has also been indicated as cardio-protective,40 and it should be noted that NMRs exhibit no significant cardiovascular aging.24 Another transcription factor implicated in cardiovascular health and longevity is nuclear factor erythroid 2-related factor (NRF2).41,42 NRF2 regulates the transcription of antioxidants, chaperones, and other cytoprotective molecules.43 Two regulators of NRF2, Keap1 and βTrCP, which target NRF2 for degradation,44,45 are negatively correlated with MLSP and the high levels of NRF2 activity in underground species may have resulted from convergent evolution.24,46
Mouse models of longevity are associated with caloric restriction and lowered insulin-like growth factor signaling.47,48 These animals are usually smaller, which suggests that within a species, smaller body mass is correlated to a longer lifespan.49 Taking into account insulin-like growth factor 1 receptor (IGF1R) levels in tissues of 16 rodent species with different body sizes and MLSP, it was shown that IGF1R levels in the brain, but not in other tissues, are strongly negatively correlated with MLSP.50 Hystricognaths, including guinea pigs, crested porcupines, DMR, and NMR have evolved an alternative glucose metabolism that does not rely on insulin and insulin receptor, but instead makes use of IGF2R and its binding protein, which closely resembles a mode of glucose handling usually observed in the fetus,51 which is in agreement with the observation of lowered IGF1R signaling in calorie restricted rodent models.47
Molecular mechanisms of cancer resistance
According to Cancer Research UK,52 there were over 330,000 new cases of cancer in 2011 and over 160,000 cancer-related deaths in 2012 in the UK (~0.5% and ~0.2% of the UK population, respectively). These statistics indicate the importance of increasing our understanding of cancer pathogenesis in order to identify novel therapeutic targets. The NMR, alongside other cancer-resistant species, offers a unique insight into cancer pathogenesis, as a cancer resistant species, as it presumably has a plethora of different mechanisms that biomedical research might be able to leverage for the treatment of cancer in patients.
NMRs, and other long-lived rodents such as DMRs, and the blind mole-rat (BMR) (Spalax galili), appear not to develop cancer throughout their long lives, and furthermore, cancer cannot be artificially induced.53–55 Moreover, upon oncogene transformation with SV40 large T antigen (LT) and Ras G12V, which commonly leads to tumor formation in mice and rat cells, NMR cells undergo crisis when transplanted into immunodeficient mice (crisis is a terminal state resulting in necrosis due to DNA damage and chromosome dysfunction).56,57 It has thus been proposed that crisis might act as a tumor suppressor mechanism in NMR cells.57
One common mechanism of cancer prevention in long-lived organisms, such as humans, is replicative senescence, the suppression of telomerase activity in somatic cells during adult life.58 In replicative cells, telomerase prevents the shortening of the chromosomes’ telomere regions, and in organisms with replicative senescence this activity is restricted to adult stem cells. However, in rodents, telomerase activity seems to coevolve with body mass rather than with lifespan, and long-lived small rodents such as NMRs and the Eastern grey squirrel, Sciurus carolinensis, retain telomerase activity in somatic cells, suggesting that other anti-cancer mechanisms must exist.59 It has been observed that in most long-lived rodents (eg, NMR, muskrat, and Eastern grey squirrel), fibroblast proliferation rate in vitro negatively correlates with longevity, and fibroblasts from these animals exhibit slow proliferation rates, compared to short-lived small rodents such as mice.60,61
The key to NMR cancer resistance might be a phenomenon termed early contact inhibition (ECI), that involves the retinoblastoma (Rb) and p53 pathways and p16 (Figure 1).54 The Rb pathway contains regulators of cell cycle control, specifically the G1 progression, and p53 is involved in apoptosis and cell cycle arrest; mutations in both oncogenes can be found in a majority of cancers.62 Mammalian cell lines grown in culture stop proliferating when they form a monolayer through a mechanism called contact inhibition, that uses the cyclin-dependent kinase inhibitor p27 to arrest the cell cycle in the G1 phase.63 NMR fibroblasts stop proliferation at a cell density that is three times lower than that of mouse fibroblasts, as soon as cell-cell contacts are established.54 This ECI is mediated by cell cycle arrest through the Rb and p16 pathway,64 and an apoptotic response using the p53 pathway.65 Inhibition of both of these factors using LT abolishes ECI in NMR cells, but when ECI is lost, NMR cells rely on the upregulation of p27 for contact inhibition; if either one of the factors is blocked, cells start growing more densely, but ultimately undergo apoptosis.54 Assuming that ECI is an important mechanism for cancer resistance in NMRs, then determining how it is triggered could be an important step forward in identifying a potential mechanism for inhibition tumor growth in humans. It has recently been suggested that ECI is initiated by hyaluronic acid (HA), an unbranched disaccharide glucuronic acid/N-acetylglucosamine polymer of the extracellular matrix.66 Isolated NMR fibroblasts produce a high molecular mass HA (HMM-HA) (NMR: 6 to 12 MDa, compared to mouse HA: 0.5 to 3 MDa), and an enzyme responsible for HA synthesis, HA synthase HAS2, is overexpressed in adult NMR skin fibroblasts compared to its levels in mouse and human fibroblasts.67 Furthermore, NMR HAS2 has two amino acid changes that might account for its high activity and the enzyme that breaks HA down, hyaluronidase, displays lower activity.67 It has been suggested that HMM-HA acts via its receptor, CD44, to activate p16 elevated expression of which coincides with ECI. Interestingly, the genes encoding CD44 and HMMR (another HA receptor), show signs of positive selection in NMRs,68 and NMR cells treated with CD44 blockers, or grown with hyaluronidase, show an absence of ECI and reduced levels of p16, while human embryonic kidney (HEK) cells overexpressing NMR HAS2 started to secrete HMM-HA.67 However, it has not been tested whether these HEK cells have elevated levels of p16 or show any ECI properties. The BMR has also been shown to secrete HMM-HA, though its cells do not exhibit ECI,69 and it is not known if they have elevated levels of p16 or p27. DMRs share one of two amino acid changes found in NMR HAS2, and also secrete HMM-HA, but whether they show ECI remains to be investigated.27 It has been suggested that HAS2 is undergoing purifying selection in other mammals, but whether or not this can be linked to cancer resistance remains to be investigated.70 BMRs do however, have a mutation in the DNA-binding domain of the p53 gene, which leads to decreased p53 activity and the downstream pro-apoptotic pathway,71 and which might contribute to the observed necrotic cell death. NMRs do not show changes in p53 expression, they do however show positive selection in the locus of p53 and additional proline motives in a proline-rich domain, which they share with humans and which might be a stabilizing mechanism co-evolved with an extended lifespan and enhanced DNA damage response.68 More recently, it has been found that NMRs express an additional product from the inhibitors of cyclin dependent kinase 4 (INK4) locus (Figure 1).72 In addition to p15, p16, and alternate reading frame, alternative splicing of the p15 exon 1, and p16 exons 2 and 3 creates pALT (named for alternative splicing), which is present in NMR cells and tissues, but is not found in mouse or human cells. Interestingly, its expression is strongly induced during ECI and stressors, such as ultra-violet radiation, and the INK4a/b locus was strongly upregulated by cell crowding and the build-up of HMM-HA,72 suggesting that it plays a role in the cellular defense mechanism of NMRs. When expressed in human cells, pALT achieved a stronger induction of cell-cycle than p15 or p16 alone.72 See Figure 1 for a summary of proposed cancer resistance mechanisms in NMR.
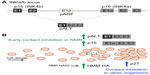  | Figure 1 Early contact inhibition (ECI) in the NMR. |
Hypoxia tolerance as an adaptation to a subterranean habitat
Hypoxia, a low level of oxygen, is involved in numerous pathological conditions, including cerebral ischemia (eg, stroke), heart defects, cancer, and neurodegenerative disorders, such as Alzheimer’s disease.73–75 One feature of ischemia is that it produces cell death because cells primarily use oxygen during aerobic metabolism to produce adenosine tri-phosphate (ATP), and thus oxygen deprivation starves cells of the ability to produce sufficient ATP. The central nervous system is particularly sensitive to hypoxic insults due to its high energetic requirements compared to its low energy reserves. Most of the ATP used by neurons is dedicated to maintenance of ion gradients and membrane potentials during synaptic transmission.76,77 Rapid reduction of cellular ATP levels during hypoxic/ischemic insults results in disruption of ion and neurotransmitter homeostasis,78,79 increased intracellular calcium levels,80 and leads to irreversible neuronal damage and cellular death.81 Moreover, accumulation of toxic byproducts created during anaerobic metabolism, such as lactic acid and protons, induce tissue acidosis which can worsen neurotoxicity through activation of acid-sensing ion channels (ASICs).82
Using hypoxia-sensitive animal models, such as laboratory mice and rats, has advanced our understanding of some of the mechanisms mediating hypoxia-induced neuronal damage and thus, has identified certain molecular therapeutic targets. However, it is equally sensible to study how hypoxia-tolerant organisms cope with low levels of oxygen, as these organisms have evolved successful physiological, cellular, and/or molecular strategies to survive hypoxic insults, and it is possible that mechanistic understanding of these strategies could identify new targets for the prevention, and/or treatment of hypoxia-related injury.
Numerous vertebrates experience periods of hypoxia as part of their normal activity,83,84 such as diving and hibernating mammals (eg, deep-diving hooded seals, Cystophora cristata, and arctic ground squirrels, Spermophilus parryii), reptiles (eg, the Western painted turtle, Chrysemys picta), fishes (eg, the crucian carp, Carassius carassius), and amphibians (eg, the common frog, Rana temporaria). Additionally, subterranean species have also received attention for their extreme resistance to hypoxia,84–86 including NMRs, which likely encounter oxygen levels as low as 6%,12,87 due to a combination of their subterranean, poorly ventilated habitat, and large colony size.
The ability to deal with sustained low levels of oxygen is aided by NMR hemoglobin, which has higher oxygen affinity than mice,88 thus securing oxygen delivery under low oxygen conditions. Moreover, NMR hypoxia-tolerance can also be explained by its surprisingly low basal metabolic rate (between 0.27 to 1 mL O2/g/h within its thermoneutral range),12,14,23,85,89 compared to the mouse (>1.2 mL O2/g/h),90,91 enabled by its low Tb. During 3% hypoxia, NMR metabolic rate is even further reduced.85 Metabolic suppression is a common physiological adaptation of hypoxia-tolerant species,92 in order to reduce ATP consumption, so that the supply matches demand when pools of available ATP are reduced during hypoxic periods.93 Moreover, existence of close interactions between Tb and hypoxia have been known about for several years.94 For example, hypothermia is thought to be neuroprotective in hypoxic insults, notably by reducing brain metabolic rates,95 although clinical benefits of therapeutic hypothermia are still debated.96 Thus, the peculiar poikilothermic thermoregulation of NMRs establishes them as a unique mammalian model to study metabolic suppression in hypoxia,97 and the role of thermoregulation in development of hypoxia-mediated pathological conditions.
In addition to physiological adaptations at the organism level, NMRs have developed hypoxia-tolerance at a neuronal level. In the hippocampus of hypoxia-sensitive mice, oxygen depletion induced a rapid decline in synaptic transmission while anoxia lead to neuronal death within ten minutes.98 By contrast, hippocampal slices from NMRs maintained synaptic activity during hypoxia and, more drastically, within the first 30 to 40 minutes of anoxia.98 Moreover, a significant number of NMR slices (approximately 75%), recovered functional synaptic activity upon returning to normoxia, whereas no recovery was observed in mouse slices.98 Additionally, less cell death occurs in organotypic hippocampal slices from NMRs compared to slices from rats after oxygen-glucose deprivation.99 These findings strongly support the fact that NMR neurons are able to face severe hypoxic insults with attenuated neurotoxicity compared to the mouse. In fact, NMR hippocampal slices perfused with hypoxic solutions accumulated less calcium than mouse slices,100 suggesting an adaptive mechanism to decrease intracellular calcium signaling and avoid the resulting neurotoxicity.
Modulation of neuronal NMDA receptors (NMDARs), is a major player of excitotoxicity, in order to reduce calcium-mediated neurotoxicity, which is common in hypoxia-tolerant animals.101–105 In hypoxia-tolerant neonatal mice, differential expression of the GluN2 NMDAR subunits is associated with hypoxia sensitivity.106 Expression of the GluN2D subunit is transient during development and is thought to confer hypoxia-tolerance to neonates as it shortens the opening time of the channels, and decreases neuronal calcium entry.106 By contrast, NMRs maintain high levels of the GluN2D subunit during adulthood, implying that they retain the ability to reduce hypoxia-mediated calcium accumulation throughout their life.107 Similar up-regulation of GluN2D subunits is also found in the BMR,103 suggesting evolutionary convergence of these hypoxia-tolerance molecular mechanisms. Thus, NMRs are one of the few mammals known to modulate their glutamatergic activity to successfully deal with hypoxic challenges,84,103 although the molecular mechanisms of such regulation is not fully understood. Further investigations using hypoxia-tolerant NMRs will improve our knowledge of molecular mechanisms reducing glutamate-mediated and calcium-mediated neurotoxicity in hypoxic insults, and may open novel avenues for therapeutic strategies.
Neuropeptides and pain
Nociceptors are sensory neurons that can be activated by noxious stimuli, commonly perceived as pain, such as heat, cold, mechanical force, or chemicals.108–110 The ability of an organism to detect noxious stimuli is critical to its survival, a point validated by the commonality of nociceptors to organisms within the animal kingdom,109,111–114 but chronic pain, that often serves no survival benefit, is widespread with 19% of the adult human population expected to experience chronic pain at some point in their life, and the majority of these patients describe their pain medication as inadequate.115 Recent studies using the NMR as a novel model in nociception research, have helped to identify some of the molecular mechanisms that drive pain, which thus reinforces the validity in using this species in research with a biomedical focus, as will now be discussed.
In mammals, most nociceptors are unmyelinated C-fibers, but there are also thinly myelinated Aδ-fibers.109,110,116 C-fibers can be broadly categorized into non-peptidergic and peptidergic populations, the latter of which express the neurotransmitters substance P (SP), and calcitonin gene-related peptide.117 NMRs are peculiar in that their cutaneous nerves display a relative paucity of C-fibers,118 but more oddly still, cutaneous NMR C-fibers seem to completely lack SP and calcitonin gene-related peptide.119 Investigation of nocifensive behaviors has found that NMRs respond with avoidance behaviors to noxious thermal and mechanical stimuli similar to mice.120,121 NMRs develop mechanical, but not thermal hyperalgesia upon injection of complete Freund’s adjuvant, and do not display nerve growth factor-induced thermal hyperalgesia.121 NMRs are also behaviorally insensitive to capsaicin, ammonia fumes, and histamine-induced itch, all of which are known to activate peptidergic C-fibers in other mammals.122,123 Interestingly, isolated dorsal root ganglion (DRG) neurons respond to both capsaicin and histamine, suggesting that the lack of behavior is not down to insensitivity of sensory neurons, and capsaicin activates sensory neurons in the in vitro skin nerve preparation.121,123 However, intrathecal infusion of SP, prior to capsaicin or histamine administration, rescues nocifensive and scratching behaviors, respectively.121,124 These results suggest that SP neurokinin-1 receptors are present and functional in NMR spinal cord circuitry and, as in mice, neurokinin-1 receptors are expressed in the superficial dorsal horn of the NMR spinal cord (Figure 2).121
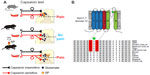  | Figure 2 Pain circuitry in the NMR. |
NMRs are also insensitive to acid-induced pain and acidic fumes.121,125 Using the in vitro skin-nerve preparation, acid failed to excite NMR sensory neurons,121 thus suggesting that the lack of nocifensive response to acid is that NMR neurons are insensitive to acid. Further investigation found that isolated NMR DRG neurons do respond to acid and that NMR proton sensors, such as ASIC1a, ASIC1b and transient receptor potential vanilloid 1 have similar proton sensitivity to mouse homologs.126 How then do NMR neurons not fire action potentials in response to acid? We identified that voltage-gated sodium (NaV) currents were more strongly inhibited by acid in NMR DRG neurons than mouse DRG neurons. Subsequent cloning and functional analysis demonstrated that NMR NaV1.7 has an amino acid variation resulting in more negatively charged amino acids that render the channel more sensitive to proton block:126 acid acts like an anesthetic, rather than an activator, of NMR sensory neurons. Interestingly, the acid-resistance NaV1.7 motif is shared between the NMR and other underground or cave-dwelling species,126,127 suggesting that this is an adaption to an environment that is high in carbon dioxide (Figure 2). In the blood, carbon dioxide dissociates into bicarbonate and protons, and an excess of carbon dioxide can lead to acidification of tissues,128 resulting, besides from other effects on metabolism, in acid pain.
NMRs also exhibit extreme changes in the opioid system compared to other mammals. In rats, opioids such as morphine or codeine induce a strong antinociceptive response, reducing for example response latency during a hotplate test.129 In NMRs, opioids lead to hyperactive and aggressive behavior, such as animals killing each other.120 Further investigations revealed that both mu and delta opioid agonists cause naloxone reversible (an opioid antagonist) hyperalgesia in NMRs in the hot plate test, while only a kappa opioid specific agonist caused analgesia,130 suggesting that some aspects of nociception are signaled by a non-opioid nociceptive system. In the formalin test however, morphine and synthetic agonists to mu, delta and kappa opioid receptors were antinociceptive,131,132 indicating that for some aspects of peripheral nociception, opioid systems play a role in the NMR.
Future perspectives and limitations
NMRs constitute an incredible resource for biomedical research with potential health care translatability due to what these mammals have evolved in order to cope with their extreme habitat (eg, hypoxia/hypercapnia), and by showing numerous adaptations to biological stressors (eg, longevity and cancer resistance). Although some of these phenotypes are shared with other vertebrate species (eg, tolerance to hypoxia/hypercapnia in hibernating mammals and others mole-rat species), NMRs possess a combination of characteristics, which designate them as a singular animal model (Figure 3). However, it should be noted that the majority of studies in current literature have compared NMRs with the mouse or rat, and that there is a relative lack of knowledge about species that, from a phylogenetic point of view, are more closely related to the NMRs, eg, the dassie rat (Petromus typicus), and the cane rat (Thyronomys), which, along with bathyergids, belong to the infraorder Phiomorpha. Further cross species comparisons will enable a better understanding of just how unique NMRs are, and will thus assist in establishing the molecular mechanisms that underlie NMR physiology.
Importantly, the physiological adaptations studied in NMR with respect to one particular biomedical field, often overlap with secondary research areas. For example, hypoxia-mediated pathways are involved in tumorigenesis,133 and hypoxic insults worsen neurodegenerative diseases;134 thus it could be profitable to explore these aspects in NMRs, which in the wild, live in a chronically hypoxic environment without developing these pathologies. In addition, NMR cellular tolerance to high oxidative stress, primarily examined with respect to their extreme longevity,25,135 may be involved in other aspects of its physiology, as reactive oxygen species, notably generated during hypoxia, act on molecular pathways involved in cancer, inflammation and neurodegenerative diseases.136,137 Some of the most striking interactions between NMR adaptations are summarized in Figure 3.
Nevertheless, using NMRs as a standard laboratory animal model has some experimental limitations. Firstly, like commonly used laboratory mice and rats, NMRs are rodents and therefore one can argue about the relative gap separating these species from humans, in order to develop appropriate translational therapeutic strategies. More specifically, breeding NMRs in the laboratory is more uncertain and time consuming than for mice/rat colonies, notably due to the fact that there is only one breeding female per colony and the gestational period is far longer than mice.7,138 Thus, development of transgenic NMRs would be hard to achieve in the near future, but with rapid advances in genome editing technology, such as CrispR, and the ever-increasing simplicity and decreasing costs of making transgenic mice, the potential for generating knock-in mice that express genes of interest from NMRs is a definite possibility; this line of investigation is aided by the NMR genome having been sequenced. Lastly, there is also the logistical element to consider: NMRs are poikilothermic, eusocial mammals, which means that to house this species a research facility must have sufficient space to accommodate a large caging system (interconnected by tunnels), that does not fit in standard cage racking systems; it is also necessary to be able to control both temperature and humidity within this space.
In summary, we believe that studying NMRs and their extreme biology will further our understanding of normal biology, which in turn will aid our investigation of mechanisms underlying various biological diseases which are having an increasing impact on modern society, such as neurodegenerative diseases, aging, cancer, and stroke.
Disclosure
The authors report no conflicts of interest in this work.
References
Jarvis JU. Eusociality in a mammal: cooperative breeding in naked mole-rat colonies. Science. 1981;212(4494):571–573. | |
Bennett NC, Faulkes CG. African Mole-Rats: Ecology and Eusociality. Cambridge University Press; 2000. | |
Faulkes CG, Bennett NC. Plasticity and constraints on social evolution in African mole-rats: ultimate and proximate factors. Philos Trans R Soc B Lond B Biol Sci. 2013;368(1618):20120347. | |
Jarvis JUM, Bennett NC. Ecology and behavior of the family Bathyergidae. In: Sherman P, Jarvis J, Alexander R, editors. The Biology of the Naked Mole-Rat. Princeton University Press; 1991:66–96. | |
Brett RA. The population structure of naked mole rat colonies. In: Sherman P, Jarvis J, Alexander R, editors. The Biology of the Naked Mole-Rat. Princeton University Press; 1991:97–136. | |
Lacey EA, Sherman PW. Social organization of naked mole-rat colonies: Evidence for divisions of Labor. In: Sherman P, Jarvis J, Alexander R, editors. The Biology of the Naked Mole-Rat. Princeton University Press; 1991:275–336. | |
Jarvis JUM. Reproduction of naked mole-rats. In: Sherman P, Jarvis J, Alexander M, editors. The Biology of the Naked Mole-Rat. Princeton University Press; 1991:384–425. | |
O’Riain MJ, Jarvis JU, Alexander R, Buffenstein R, Peeters C. Morphological castes in a vertebrate. Proc Natl Acad Sci U S A. 2000; 97(24):13194–13197. | |
Faulkes CG, Abbott DH, Liddell CE, George LM, Jarvis JUM. Hormonal and behavioral aspects of reproductive suppression in female naked mole-rats. In: Sherman P, Jarvis J, Alexander M, editors. The Biology of the Naked Mole-Rat. Princeton University Press; 1991:426–445. | |
Faulkes CG, Abbott DH, Jarvis JU. Social suppression of ovarian cyclicity in captive and wild colonies of naked mole-rats, Heterocephalus glaber. J Reprod Fertil. 1990;88(2):559–568. | |
Brett RA. The ecology of naked mole-rat colonies: burrowing, food and limiting factors. In: Sherman P, Jarvis J, Alexander R, editors. The Biology of the Naked Mole-Rat. Princeton University Press; 1991:137–184. | |
McNab BK. The metabolism of fossorial rodents: a study of convergence. Ecology. 1966;47(5):712–733. | |
McNab BK. The influence of body size on the energetics and distibution of fossorial and burrowing mammals. Ecology. 1979;60:1010–1021. | |
Buffenstein R, Yahav S. Is the naked mole-rat Heterocephalus glaber an endothermic yet poikilothermic? J therm Biol. 1991;16(4):227–232. | |
Daly TJ, Buffenstein R. Skin morphology and its role in thermoregulation in mole-rats, Heterocephalus glaber and Cryptomys hottentotus. J Anat. 1998;193(Pt 4):495–502. | |
Daly TJ, Williams LA, Buffenstein R. Catecholaminergic innervation of interscapular brown adipose tissue in the naked mole-rat (Heterocephalus glaber). J Anat. 1997;190(Pt 3):321–326. | |
Yahav S, Buffenstein R. Huddling behavior facilitates homeothermy in the naked mole rat Heterocephalus-Glaber. Physiol Zool. 1991;64(3):871–884. | |
Buffenstein R, Woodley R, Thomadakis C, Daly TJ, Gray D a. Cold-induced changes in thyroid function in a poikilothermic mammal, the naked mole-rat. Am J Physiol Regul Integr Comp Physiol. 2001;280(1):R149–R155. | |
Healy K, Guillerme T, Finlay S, et al. Ecology and mode-of-life explain lifespan variation in birds and mammals. Proc Biol Sci. 2014;281(1784):20140298. | |
Buffenstein R. The naked mole-rat: a new long-living model for human aging research. J Gerontol A Biol Sci Med Sci. 2005;60(11):1369–1377. | |
Williams SA, Shattuck MR. Ecology, longevity and naked mole-rats: confounding effects of sociality? Proc Biol Sci. 2015;282:20141664. | |
Buffenstein R. Negligible senescence in the longest living rodent, the naked mole-rat: Insights from a successfully aging species. J Comp Physiol B. 2008;178(4):439–445. | |
O’Connor TP, Lee A, Jarvis JU, Buffenstein R. Prolonged longevity in naked mole-rats: Age-related changes in metabolism, body composition and gastrointestinal function. Comp Biochem Physiol A Mol Integr Physiol. 2002;133(3):835–842. | |
Grimes KM, Reddy AK, Lindsey ML, Buffenstein R. And the beat goes on: maintained cardiovascular function during aging in the longest-lived rodent, the naked mole-rat. Am J Physiol Heart Circ Physiol. 2014; 307(3):H284–H291. | |
Pérez VI, Buffenstein R, Masamsetti V, et al. Protein stability and resistance to oxidative stress are determinants of longevity in the longest-living rodent, the naked mole-rat. Proc Natl Acad Sci U S A. 2009;106(9):3059–3064. | |
MacRae SL, Zhang Q, Lemetre C, et al. Comparative analysis of genome maintenance genes in naked mole rat, mouse, and human. Aging Cell. 2015;14(2):288–291. | |
Fang X, Seim I, Huang Z, et al. Adaptations to a subterranean environment and longevity revealed by the analysis of mole rat genomes. Cell Rep. 2014;8(5):1354–1364. | |
Schmidt CM, Blount JD, Bennett NC. Reproduction is associated with a tissue-dependent reduction of oxidative stress in eusocial female Damaraland mole-rats (Fukomys damarensis). PLoS One. 2014; 9(7):e103286. | |
Schmidt CM, Jarvis JUM, Bennett NC. The long-lived queen: reproduction and longevity in female eusocial Damaraland mole-rats (Fukomys damarensis). African Zool. 2013;48(1):193–196. | |
Squier TC. Oxidative stress and protein aggregation during biological aging. Exp Gerontol. 2001;36(9):1539–1550. | |
Yang Z, Zhang Y, Chen L. Investigation of anti-cancer mechanisms by comparative analysis of naked mole rat and rat. BMC Syst Biol. 2013; 7 Suppl 2:S5. | |
Kim EB, Fang X, Fushan AA, et al. Genome sequencing reveals insights into physiology and longevity of the naked mole rat. Nature. 2011;479(7372):223–227. | |
Rodriguez KA, Edrey YH, Osmulski P, Gaczynska M, Buffenstein R. Altered composition of liver proteasome assemblies contributes to enhanced proteasome activity in the exceptionally long-lived naked mole-rat. PLoS One. 2012;7(5):e35890. | |
Rodriguez KA, Osmulski PA, Pierce A, Weintraub ST, Gaczynska M, Buffenstein R. A cytosolic protein factor from the naked mole-rat activates proteasomes of other species and protects these from inhibition. Biochim Biophys Acta. 2014;1842(11):2060–2072. | |
Zhao S, Lin L, Kan G, et al. High autophagy in the naked mole rat may play a significant role in maintaining good health. Cell Physiol Biochem. 2014;33(2):321–332. | |
Azpurua J, Ke Z, Chen IX, et al. Naked mole-rat has increased translational fidelity compared with the mouse, as well as a unique 28S ribosomal RNA cleavage. Proc Natl Acad Sci U S A. 2013;110(43):17350–17355. | |
Edrey YH, Medina DX, Gaczynska M, et al. Amyloid beta and the longest-lived rodent: The naked mole-rat as a model for natural protection from alzheimer’s disease. Neurobiol Aging. 2013;34(10):2352–2360. | |
Orr ME, Garbarino VR, Salinas A, Buffenstein R. Sustained high levels of neuroprotective, high molecular weight, phosphorylated tau in the longest-lived rodent. Neurobiol Aging. 2015;36(3):1496–1504. | |
Edrey YH, Casper D, Huchon D, et al. Sustained high levels of neuregulin-1 in the longest-lived rodents; A key determinant of rodent longevity. Aging Cell. 2012;11(2):213–222. | |
Hedhli N, Huang Q, Kalinowski A, et al. Endothelium-derived neuregulin protects the heart against ischemic injury. Circulation. 2011;123(20):2254–2262. | |
Li J, Ichikawa T, Janicki JS, Cui T. Targeting the Nrf2 pathway against cardiovascular disease. Expert Opin Ther Targets. 2009;13(7):785–794. | |
Mann GE, Niehueser-Saran J, Watson A, et al. Nrf2/ARE regulated antioxidant gene expression in endothelial and smooth muscle cells in oxidative stress: implications for atherosclerosis and preeclampsia. Sheng Li Xue Bao. 2007;59(2):117–127. | |
Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284(20):13291–13295. | |
Kobayashi A, Kang MI, Okawa H, et al. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol. 2004;24(16):7130–7139. | |
Rada P, Rojo AI, Chowdhry S, McMahon M, Hayes JD, Cuadrado A. SCF/{beta}-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner. Mol Cell Biol. 2011;31(6):1121–1133. | |
Lewis KN, Wason E, Edrey YH, Kristan DM, Nevo E, Buffenstein R. Regulation of Nrf2 signaling and longevity in naturally long-lived rodents. Proc Natl Acad Sci U S A. 2015;112(12):3722–3727. | |
Shimokawa I, Higami Y, Tsuchiya T, et al. Life span extension by reduction of the growth hormone-insulin-like growth factor-1 axis: relation to caloric restriction. FASEB J. 2003;17(9):1108–1109. | |
Masoro EJ. Potential role of the modulation of fuel use in the antiaging action of dietary restriction. Ann N Y Acad Sci. 1992;663:403–411. | |
Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126(9):913–922. | |
Azpurua J, Yang JN, Van Meter M, et al. IGF1R levels in the brain negatively correlate with longevity in 16 rodent species. Aging (Albany NY). 2013;5(4):304–314. | |
Fang X, Nevo E, Han L, et al. Genome-wide adaptive complexes to underground stresses in blind mole rats Spalax. Nat Commun. 2014;5:3966. | |
Cancer Research UK [webpage on the Internet]. Cancer statistics for the UK. Available from: http://www.cancerresearchuk.org/health-professional/cancer-statistics#heading-Two. Accessed June 2015. | |
Manov I, Hirsh M, Iancu TC, et al. Pronounced cancer resistance in a subterranean rodent, the blind mole-rat, Spalax: in vivo and in vitro evidence. BMC Biol. 2013;11:91. | |
Seluanov A, Hine C, Azpurua J, et al. Hypersensitivity to contact inhibition provides a clue to cancer resistance of naked mole-rat. Proc Natl Acad Sci U S A. 2009;106(46):19352–19357. | |
Delaney MA, Nagy L, Kinsel MJ, Treuting PM. Spontaneous histologic lesions of the adult naked mole rat (Heterocephalus glaber): a retrospective survey of lesions in a zoo population. Vet Pathol. 2013;50(4):607–621. | |
Greenberg RA. Telomeres, crisis and cancer. Curr Mol Med. 2005;5(2):213–218. | |
Liang S, Mele J, Wu Y, Buffenstein R, Hornsby PJ. Resistance to experimental tumorigenesis in cells of a long-lived mammal, the naked mole-rat (Heterocephalus glaber). Aging Cell. 2010;9(4):626–635. | |
Finch CE. Update on slow aging and negligible senescence – A mini-review. Gerontology. 2009;55(3):307–313. | |
Seluanov A, Chen Z, Hine C, et al. Telomerase activity coevolves with body mass not lifespan. Aging Cell. 2007;6(1):45–52. | |
Gorbunova V, Seluanov A. Coevolution of telomerase activity and body mass in mammals: From mice to beavers. Mech Ageing Dev. 2009;130(1–2):3–9. | |
Seluanov A, Hine C, Bozzella M, et al. Distinct tumor suppressor mechanisms evolve in rodent species that differ in size and lifespan. Aging Cell. 2008;7(6):813–823. | |
Sherr CJ, McCormick F. The RB and p53 pathways in cancer. Cancer Cell. 2002;2(2):103–112. | |
Polyak K, Kato JY, Solomon MJ, et al. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 1994;8(1):9–22. | |
Zhang HS, Postigo AA, Dean DC. Active transcriptional repression by the Rb–E2F complex mediates G1 arrest triggered by p16INK4a, TGFβ, and contact inhibition. Cell. 1999;97(1):53–61. | |
Dulić V, Kaufmann WK, Wilson SJ, et al. p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell. 1994;76(6):1013–1023. | |
Kohda D, Morton CJ, Parkar AA, et al. Solution structure of the link module: a hyaluronan-binding domain involved in extracellular matrix stability and cell migration. Cell. 1996;86(5):767–775. | |
Tian X, Azpurua J, Hine C, et al. High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat. Nature. 2013; 499(7458):346–349. | |
Keane M, Craig T, Alföldi J, et al. The Naked Mole Rat Genome Resource: facilitating analyses of cancer and longevity-related adaptations. Bioinformatics. 2014;30(24):3558–3560. | |
Gorbunova V, Hine C, Tian X, et al. Cancer resistance in the blind mole rat is mediated by concerted necrotic cell death mechanism. Proc Natl Acad Sci U S A. 2012;109(47):19392–19396. | |
Faulkes CG, Davies KT, Rossiter SJ, Bennett NC. Molecular evolution of the hyaluronan synthase 2 gene in mammals: implications for adaptations to the subterranean niche and cancer resistance. Biol Lett. 2015;11(5):20150185. | |
Avivi A, Ashur-Fabian O, Joel A, et al. P53 in blind subterranean mole rats – loss-of-function versus gain-of-function activities on newly cloned Spalax target genes. Oncogene. 2007;26(17):2507–2512. | |
Tian X, Azpurua J, Ke Z, et al. INK4 locus of the tumor-resistant rodent, the naked mole rat, expresses a functional p15/p16 hybrid isoform. Proc Natl Acad Sci U S A. 2015;112(4):1053–1058. | |
Michiels C. Physiological and pathological responses to hypoxia. Am J Pathol. 2004;164(6):1875–1882. | |
Gao L, Tian S, Gao H, Xu Y. Hypoxia increases Aβ-induced tau phosphorylation by calpain and promotes behavioral consequences in AD transgenic mice. J Mol Neurosci. 2013;51(1):138–147. | |
Peers C, Dallas ML, Boycott HE, Scragg JL, Pearson HA, Boyle JP. Hypoxia and neurodegeneration. Ann N Y Acad Sci. 2009; 1177:169–177. | |
Harris JJ, Jolivet R, Attwell D. Synaptic energy use and supply. Neuron. 2012;75(5):762–777. | |
Howarth C, Gleeson P, Attwell D. Updated energy budgets for neural computation in the neocortex and cerebellum. J Cereb Blood Flow Metab. 2012;32(7):1222–1232. | |
Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev. 1997;77(3):731–758. | |
Haddad GG, Jiang C. O2 deprivation in the central nervous system: on mechanisms of neuronal response, differential sensitivity and injury. Prog Neurobiol. 1993;40(3):277–318. | |
Martin RL, Lloyd HG, Cowan AI. The early events of oxygen and glucose deprivation: setting the scene for neuronal death? Trends Neurosci. 1994;17(6):251–257. | |
Kristián T, Siesjö BK. Calcium in ischemic cell death. Stroke. 1998; 29(3):705–718. | |
Xiong ZG, Zhu XM, Chu XP, et al. Neuroprotection in Ischemia: blocking calcium-permeable acid-sensing ion channels. Cell. 2004; 118(6):687–698. | |
Bickler PE, Buck LT. Hypoxia tolerance in reptiles, amphibians, and fishes: life with variable oxygen availability. Annu Rev Physiol. 2007;69:145–170. | |
Larson J, Drew KL, Folkow LP, Milton SL, Park TJ. No oxygen? No problem! Intrinsic brain tolerance to hypoxia in vertebrates. J Exp Biol. 2014;217(Pt 7):1024–1039. | |
Nathaniel TI, Otukonyong E, Abdellatif A, Soyinka JO. Effect of hypoxia on metabolic rate, core body temperature, and c-fos expression in the naked mole rat. Int J Dev Neurosci. 2012;30(6):539–544. | |
Shams I, Avivi A, Nevo E. Hypoxic stress tolerance of the blind subterranean mole rat: expression of erythropoietin and hypoxia-inducible factor 1 alpha. Proc Natl Acad Sci U S A. 2004;101(26):9698–9703. | |
Shams I, Avivi A, Nevo E. Oxygen and carbon dioxide fluctuations in burrows of subterranean blind mole rats indicate tolerance to hypoxic-hypercapnic stresses. Comp Biochem Physiol A Mol Integr Physiol. 2005;142(3):376–382. | |
Johansen K, Lykkeboe G, Weber RE, Maloiy GM. Blood respiratory properties in the naked mole rat Heterocephalus glaber, a mammal of low body temperature. Respir Physiol. 1976;28(3):303–314. | |
Goldman BD, Goldman SL, Lanz T, Magaurin A, Maurice A. Factors Influencing Metabolic Rate in Naked Mole-Rats (Heterocephalus glaber). Physiol Behav. 1999;66(3):447–459. | |
Mink JW, Blumenschine RJ, Adams DB. Ratio of central nervous system to body metabolism in vertebrates: its constancy and functional basis. Am J Physiol. 1981;241(3):R203–R212. | |
Selman C, Lumsden S, Bünger L, Hill WG, Speakman JR. Resting metabolic rate and morphology in mice (Mus musculus) selected for high and low food intake. J Exp Biol. 2001;204(Pt 4):777–784. | |
Buck LT, Pamenter ME. Adaptive responses of vertebrate neurons to anoxia-Matching supply to demand. Respir Physiol Neurobiol. 2006;154(1–2):226–240. | |
Hochachka PW, Buck LT, Doll CJ, Land SC. Unifying theory of hypoxia tolerance: molecular/metabolic defense and rescue mechanisms for surviving oxygen lack. Proc Natl Acad Sci U S A. 1996;93(18):9493–9498. | |
Wood SC. Interactions between hypoxia and hypothermia. Annu Rev Physiol. 1991;53:71–85. | |
Sakoh M, Gjedde A. Neuroprotection in hypothermia linked to redistribution of oxygen in brain. Am J Physiol Hear Circ Physiol. 2003;285(1):H17–H25. | |
Gordon CJ. The therapeutic potential of regulated hypothermia. Emerg Med J. 2001;18(2):81–89. | |
Nathaniel TI, Otukonyong EE, Okon M, Chaves J, Cochran T, Nathaniel AI. Metabolic regulatory clues from the naked mole rat: toward brain regulatory functions during stroke. Brain Res Bull. 2013;98:44–52. | |
Larson J, Park TJ. Extreme hypoxia tolerance of naked mole-rat brain. Neuroreport. 2009;20(18):1634–1637. | |
Nathaniel TI, Saras A, Umesiri FE, Olajuyigbe F. Tolerance to oxygen nutrient deprivation in hippocampal of naked mole-rats. J Integr Neurosci. 2009;8(2):123–136. | |
Peterson BL, Larson J, Buffenstein R, Park TJ, Fall CP. Blunted neuronal calcium response to hypoxia in naked mole-rat hippocampus. PLoS One. 2012;7(2):e31568. | |
Bickler PE, Donohoe PH, Buck LT. Hypoxia-induced silencing of NMDA receptors in turtle neurons. J Neurosci. 2000;20(10):3522–3528. | |
Zhao HW, Ross AP, Christian SL, Buchholz JN, Dre. Decreased NR1 phosphorylation and decreased NMDAR function in hibernating Arctic ground squirrels. J Neurosci Res. 2006;84(2):291–298. | |
Band M, Malik A, Joel A, Avivi A. Hypoxia associated NMDA receptor 2 subunit composition: Developmental comparison between the hypoxia-tolerant subterranean mole-rat, Spalax, and the hypoxia-sensitive rat. J Comp Physiol B. 2012;182(7):961–969. | |
Wilkie MP, Pamenter ME, Alkabie S, Carapic D, Shin DS, Buck LT. Evidence of anoxia-induced channel arrest in the brain of the goldfish (Carassius auratus). Comp Biochem Physiol C Toxicol Pharmacol. 2008;148(4):355–362. | |
Ellefsen S, Sandvik GK, Larsen HK, et al. Expression of genes involved in excitatory neurotransmission in anoxic crucian carp (Carassius carassius) brain. Physiol Genomics. 2008;35(1):5–17. | |
Bickler PE, Fahlman CS, Taylor DM. Oxygen sensitivity of NMDA receptors: Relationship to NR2 subunit composition and hypoxia tolerance of neonatal neurons. Neuroscience. 2003;118(1):25–35. | |
Peterson BL, Park TJ, Larson J. Adult naked mole-rat brain retains the NMDA receptor subunit GluN2D associated with hypoxia tolerance in neonatal mammals. Neurosci Lett. 2012;506(2):342–345. | |
Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139(2):267–284. | |
Smith ES, Lewin GR. Nociceptors: a phylogenetic view. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2009;195(12):1089–1106. | |
Dubin AE, Patapoutian A. Nociceptors: The sensors of the pain pathway. J Clin Invest. 2010;120(11):3760–3772. | |
Sneddon LU. Pain in aquatic animals. J Exp Biol. 2015;218(Pt 7):967–976. | |
Woolf CJ, Walters ET. Common patterns of plasticity contributing to nociceptive sensitization in mammals and Aplysia. Trends Neurosci. 1991;14(2):74–78. | |
Kavaliers M. Evolutionary and comparative aspects of nociception. Brain Res Bull. 1988;21(6):923–931. | |
Sneddon LU. Evolution of nociception in vertebrates: comparative analysis of lower vertebrates. Brain Res Brain Res Rev. 2004;46(2):123–130. | |
Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: Prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10(4):287–333. | |
Lewin GR, Moshourab R. Mechanosensation and pain. J Neurobiol. 2004;61(1):30–44. | |
Molliver DC, Radeke MJ, Feinstein SC, Snider WD. Presence or absence of TrkA protein distinguishes subsets of small sensory neurons with unique cytochemical characteristics and dorsal horn projections. J Comp Neurol. 1995;361(3):404–416. | |
St John Smith E, Purfürst B, Grigoryan T, Park TJ, Bennett NC, Lewin GR. Specific paucity of unmyelinated C-fibers in cutaneous peripheral nerves of the African naked-mole rat: Comparative analysis using six species of bathyergidae. J Comp Neurol. 2012;520(12):2785–2803. | |
Park TJ, Comer C, Carol A, Lu Y, Hong HS, Rice FL. Somatosensory organization and behavior in naked mole-rats: II. Peripheral structures, innervation, and selective lack of neuropeptides associated with thermoregulation and pain. J Comp Neurol. 2003;465(1):104–120. | |
Kanui TI, Hole K. Morphine induces aggression but not analgesia in the naked mole-rat (Heterocephalus glaber). Comp Biochem Physiol C. 1990;96(1):131–133. | |
Park TJ, Lu Y, Jüttner R, et al. Selective inflammatory pain insensitivity in the African naked mole-rat (Heterocephalus glaber). PLoS Biol. 2008;6(1):e13. | |
LaVinka PC, Brand A, Landau VJ, Wirtshafter D, Park TJ. Extreme tolerance to ammonia fumes in African naked mole-rats: Animals that naturally lack neuropeptides from trigeminal chemosensory nerve fibers. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2009;195(5):419–427. | |
Smith ES, Blass GR, Lewin GR, Park TJ. Absence of histamine-induced itch in the African naked mole-rat and “rescue” by Substance P. Mol Pain. 2010;6:29. | |
Brand A, Smith ES, Lewin GR, Park TJ. Functional neurokinin and NMDA receptor activity in an animal naturally lacking substance P: the naked mole-rat. PLoS One. 2010;5(12):e15162. | |
LaVinka PC, Park TJ. Blunted behavioral and c Fos responses to acidic fumes in the african naked mole-rat. PLoS One. 2012; 7(9):e45060. | |
Smith ES, Omerbašić D, Lechner SG, Anirudhan G, Lapatsina L, Lewin GR. The molecular basis of acid insensitivity in the African naked mole-rat. Science. 2011;334(6062):1557–1560. | |
Liu Z, Wang W, Zhang T, et al. Repeated functional convergent effects of NaV1.7 on acid insensitivity in hibernating mammals. Proc Biol Sci. 2013;281(1776):20132950. | |
Christiansen J, Douglas CG, Haldane JS. The absorption and dissociation of carbon dioxide by human blood. J Physiol. 1914;48(4):244–271. | |
Wilson SG, Mogil JS. Measuring pain in the (knockout) mouse: big challenges in a small mammal. Behav Brain Res. 2001;125(1–2):65–73. | |
Towett PK, Kanui TI, Juma FD. Stimulation of mu and delta opioid receptors induces hyperalgesia while stimulation of kappa receptors induces antinociception in the hot plate test in the naked mole-rat (Heterocephalus glaber). Brain Res Bull. 2006;71(1–3):60–68. | |
Kanui TI, Karim F, Towett PK. The formalin test in the naked mole-rat (Heterocephalus glaber): Analgesic effects of morphine, nefopam and paracetamol. Brain Res. 1993;600(1):123–126. | |
Towett PK, Kanui TI, Maloiy GMO, Juma F, Olongida Ole Miaron J. Activation of mu, delta or kappa opioid receptors by DAMGO, DPDPE, U-50488 or U-69593 respectively causes antinociception in the formalin test in the naked mole-rat (Heterocephalus glaber). Pharmacol Biochem Behav. 2009;91(4):566–572. | |
Tang CM, Yu J. Hypoxia-inducible factor-1 as a therapeutic target in cancer. J Gastroenterol Hepatol. 2013;28(3):401–405. | |
Liu H, Le W. Epigenetic modifications of chronic hypoxia-mediated neurodegeneration in Alzheimer’s disease. Transl Neurodegener. 2014;3(1):7. | |
Rodriguez KA, Wywial E, Perez VI, et al. Walking the oxidative stress tightrope: a perspective from the naked mole-rat, the longest-living rodent. Curr Pharm Des. 2011;17(22):2290–2307. | |
Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation and cancer: How are they linked? Free Radic Biol Med. 2011;49(11):1603–1616. | |
Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 2009;7(1):65–74. | |
Roellig K, Drews B, Goeritz F, Hildebrandt TB. The long gestation of the small naked mole-rat (Heterocephalus glaber) studied with ultrasound biomicroscopy and 3d-ultrasonography. PLoS One. 2011;6(3):e17744. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.