Back to Journals » Clinical Ophthalmology » Volume 17
The Injection Practice Patterns of Retina Specialists in Managing Exudative Age-Related Macular Degeneration: A Retrospective Study
Authors Karimaghaei C , Ali A, Safdar N, Tanwani A, Schmitz-Brown M, Banaee T, El-Annan J, Gupta PK
Received 26 September 2022
Accepted for publication 28 December 2022
Published 25 January 2023 Volume 2023:17 Pages 375—383
DOI https://doi.org/10.2147/OPTH.S391282
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Scott Fraser
Cina Karimaghaei,1,2,* Amir Ali,3,* Nida Safdar,4,5 Anika Tanwani,3 Mary Schmitz-Brown,2 Touka Banaee,2 Jaafar El-Annan,2 Praveena K Gupta2
1Department of Internal Medicine, University of Texas Medical Branch, Galveston, TX, USA; 2Department of Ophthalmology and Visual Sciences, University of Texas Medical Branch, Galveston, TX, USA; 3School of Medicine, University of Texas Medical Branch, Galveston, TX, USA; 4Department of Ophthalmology, Nazareth Hospital, Trinity Health Mid-Atlantic, Philadelphia, PA, USA; 5IC Laser Eye Care, Bensalem, PA, USA
*These authors contributed equally to this work
Correspondence: Praveena K Gupta Professor, Department of Ophthalmology and Visual Sciences, University of Texas Medical Branch, 301 University Blvd, Galveston, TX, 77555, USA, Tel +1 409-747-5823, Fax +1 409 747-5824, Email [email protected]
Purpose: To compare the PRN anti-VEGF injection patterns of four retina specialists with respect to the visual and anatomic outcomes in the management of wet age-related macular degeneration (AMD).
Methods: Medical records of patients who received bevacizumab, ranibizumab, and aflibercept anti-VEGF injections (years 2010– 2020) by four retina specialists were reviewed for frequency, injection intervals, best corrected visual acuity (BCVA), and central macular thickness, center involved (CMT) for statistical analysis. Outcomes measured were change in logMAR BCVA and CMT from the first to last injection visit.
Results: Out of 137 AMD patients, 172 eyes were injected by four retina specialists in PRN fashion. Although all four specialists started the injection at similar baseline BCVA and CMT (p > 0.1), significant differences in mean injection number (9.0, p = 0.0001), injection intervals (5.06 weeks, p = 0.001), and total length of treatments (53.3 weeks, p = 0.0001) were observed. The mean change in logMAR BCVA between the first and last injection was − 0.05, − 0.22, 0.07, and 0.06 for the four specialists, respectively (p = 0.031), and the mean change in CMT was – 53.3, − 41.4, − 72.7, and − 21.9 μm (p = 0.41), respectively.
Conclusion: Despite similar baseline criteria for injections by the retina specialists, different anti-VEGF injection regimens were practiced resulting in variations in BCVA and CMT outcomes. This suggests a need in establishing a universally adoptable injection regimen with possible integration of the confounding factors to reduce burden on both patients and retina specialists.
Keywords: retina specialists, practice patterns, anti-VEGF, central macular thickness, best corrected visual acuity, exudative age-related macular degeneration
Introduction
Anti-vascular endothelial growth factor (anti-VEGF) injections are the first-line therapy in the treatment of wet age-related macular degeneration (AMD). AMD is a chronic and progressive degenerative disorder of the macula that leads to a loss of central vision. AMD is recognized as the third-most common cause of blindness worldwide, with most affected individuals living in developed countries.1,2 There are two subtypes, “dry” and “wet” AMD. While dry AMD causes 85% to 90% of AMD cases, wet AMD explains 90% of severe vision loss from the disease.3 In wet AMD, upregulation of VEGF causes pathological choroidal neovascularization, resulting in loss of central vision.4 Therefore, targeting VEGF with intravitreal anti-VEGF injections has revolutionized management and helped decrease visual impairment from wet AMD.5–7
The anti-VEGF drugs commonly used for managing wet AMD include bevacizumab, ranibizumab, and aflibercept. Several studies comparing the different anti-VEGF drugs against one another have demonstrated similar efficacy in improving visual acuity and retinal thickness.8–10 With ample evidence demonstrating efficacy of individual anti-VEGF drugs and comparable effects between different drug choices, optimizing intravitreal injection treatment regimens has now become an area of research and of clinical interest.
In a Medicare database study of the use of anti-VEGF drugs, there was a high variation in injection frequency identified across the United States despite the availability of established anti-VEGF guidelines. While variability in anti-VEGF regimens exists among retina specialists, there have been multiple studies comparing the efficacy of these regimens for each anti-VEGF drug option.11 Studies comparing PRN and fixed interval dosing of bevacizumab and ranibizumab have demonstrated improved visual and anatomic outcomes with both regimens but reduced treatment burden with a PRN strategy.12–14 Additional studies on ranibizumab comparing treat-and-extend (T&E) versus fixed interval monthly dosing and wait-and-extend (W&E) versus treat-and-observe (T&O) found similar efficacy between regimens but reduced treatment burden with T&E and W&E.15,16 Since intravitreal injections are associated with multiple financial and medical risks, treatment strategies that both minimize treatment burden and maintain efficacy are desirable in order to limit reported risks of ocular inflammation, elevated intraocular pressure, endophthalmitis,17 nephrotoxicity,18 cardiovascular compromise,19 discomfort and fear,20 anxiety and depression,20,21 and pain.22 While many studies have suggested utilizing a PRN injection strategy in order to reduce treatment burden without compromising clinical improvement, there still exists variability in the PRN protocols used by different retina specialists. Despite a consensus on monthly injections for the first three months of therapy, there is no standardized pattern of injections following treatment initiation.
This retrospective study provides whether a consistency exists on the injection patterns between retina specialists who practiced PRN strategies. Change in BCVA and optical coherence tomography (OCT) central macular thickness (CMT) were the outcome measures of interest. This is the first study to the best of our knowledge analyzing the injection patterns of individual retina specialists in the treatment of wet AMD.
Methods
Ethics
The study adheres to the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board at the University of Texas Medical Branch at Galveston, USA. Due to the retrospective nature of this study, the UTMB Institutional Review Board exempted from requiring written informed consent. All data collected were anonymized and de-identified to maintain confidentiality.
Study Design
In this retrospective study, electronic medical records (EMR) from current practicing retina specialists were reviewed from 01 July 2010 to 30 July 2020. Based on IRB, patients needed to be ages 18–100, with a diagnosis of exudative age-related macular degeneration (ICD H35.32), received intravitreal anti-VEGF injections of bevacizumab (Avastin; Genentech/Roche), ranibizumab (Lucentis; Genentech/Roche), and aflibercept (Eylea; Regeneron Pharmaceuticals, Inc.), either individualized or in combinations, in a PRN pattern after the first three monthly injections. Patients were excluded if they had macular edema due to other conditions like diabetes, retinal vascular occlusions, high myopia, trauma, or other surgical complications. Patients were also excluded if they were treated by more than one retina specialist and if they were seen by a specialist with less than five cases. All included retina specialists received Accreditation Council for Graduate Medical Education (ACGME) fellowship training in the United States.
Data Collection
Demographic data (age, gender, race, ethnicity) were collected along with ophthalmic information including diagnosis, other co-morbidities, OCT scans, number and frequency of injection treatment, BCVA at each visit, and CMT at each visit. Patients’ visual acuities were converted to Logarithm of the Minimum Angle of Resolution (LogMAR) for comparison.
Eight retina specialists were identified treating a total of 250 wet AMD patients during the studied period. Ninety-nine patients were excluded due to being treated by multiple providers. Eight patients were excluded due to a co-existence of retinal occlusive disease. Another six patients were excluded due to seeing a specialist with less than five cases. Ultimately, this study included patients from four retina specialists (>5 cases) and 172 eyes from 137 eligible patients. For relevance, we called the retina specialists A, B, C, and D throughout the text.
Statistical Analysis
Data were deidentified for analysis; descriptive statistics was used to summarize the demographics, and information is reported using percentage, mean, and standard deviation. Chi-square and Kruskal–Wallis tests were conducted for categorical and continuous data, respectively. Multivariable analysis of covariance was used to determine independent factors associated with visual acuity and macular thickness post-treatment. In the multivariate analysis conducted for the Specialist, Retina Specialist B was chosen as the reference provider due to their most significant improvement in BCVA over the time span of treatment. All analyses were conducted with SS 9.4 (Cary, NC). A p-value of less than 0.05 was used to indicate significance.
Results
Demographics
Table 1 summarizes the demographics of the patients. Included patients were mostly females (66.4%), and Caucasian (92.7%). Of others, 5.11% were Black/African American, 1.46% were Asian, and 0.73% were American Indian/Alaskan Native. Additionally, 36.03% of patients were smokers, 19.71% of patients had chronic kidney disease, 53.28% of patients had hyperlipidemia, 11.68% of patients had hypertensive retinopathy, and 29.93% had glaucoma.
 |
Table 1 Patient Demographics and Medical History. Data are Presented as Mean (SD) for Continuous Data and N (%) for Categorical Data |
Anti-VEGF Drug Use
Table 2 demonstrates how many eyes were treated with bevacizumab, aflibercept, and ranibizumab, as well as single or multiple anti-VEGF drugs for each retina specialist. The most used drug was bevacizumab, followed by aflibercept, then ranibizumab. Based on the number of patients treated by each provider, Bevacizumab use was significantly varied (p = 0.045). Also, most eyes were only treated with single rather than multiple anti-VEGF drug therapy.
 |
Table 2 Anti-VEGF Drug Use Among Retina Specialists |
Practice Patterns of the Retina Specialists
The anti-VEGF practice patterns for each of the four retina specialists in this study were described by mean number and frequency of injections per eye during the treatment interval (Table 3). The mean number of injections for Specialist A, B, C, and D were 6.9, 8.3, 15.1, and 5.6 injections, respectively (p = 0.0001). The mean interval between injections for Specialist A, B, C, and D was 3.8, 4.4, 6.6, and 5.5 weeks, respectively (p = 0.001) during a total treatment duration of 28.4 weeks for Specialist A, 42.3 weeks for B, 104.9 weeks for C, and 37.7 weeks for D (p = 0.0001).
 |
Table 3 Anti-VEGF Injection Practice Pattern of Retina Specialists, Expressed in Mean Number of Injections per Eye, Mean Time Interval Between Injections, and Total Treatment Duration |
Primary Outcome
The primary outcome measured was change in logMAR BCVA from the first to last injection visit. The mean logMAR BCVA at the first injection visit for Specialist A, B, C, and D was 0.85, 1.11, 0.94, and 0.78, respectively (p = 0.167). The mean change in logMAR BCVA between the first and last injection visit (calculated as last – first) for Specialist A, B, C, and D was −0.05, −0.22, 0.07, and 0.06, respectively (p = 0.031). These are presented in Table 3.
Secondary Outcome
The change in CMT during the treatment duration is presented in Table 3. The mean CMT at the first injection visit for Specialist A, B, C, and D was 306, 302, 362, and 302 µm (p = 0.177), respectively, and the mean change from the first to last injection visit was –53.3, −41.4, −72.7, and −21.9 µm (p = 0.409), respectively.
Best Corrected Visual Acuity Linear Regression
Multivariate analysis of covariance was used to determine factors associated with change in BCVA. The effects of these factors on BCVA are displayed in Table 4. When accounting for all variables including initial BCVA, retina specialists, injection numbers, injection intervals, age, sex, race, and smoking status; number of injections and interval between injections did not have a significant impact on visual outcomes. Instead, there was a significant association between initial BCVA and age with change in BCVA. Initial BCVA was negatively correlated with change in BCVA, while age was positively correlated with change in BCVA. With reference to Specialist B, treatment by Specialist C was significantly associated with greater changes in BCVA.
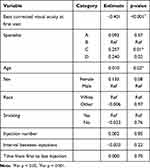 |
Table 4 Factors Associated with Change in Best Corrected Visual Acuity Using Multivariate Analysis of Covariance |
Central Macular Thickness Linear Regression
Multivariate analysis of covariance was also used to determine factors associated with change in central macular thickness. The same factors studied in the multivariate analysis of BCVA were analyzed, and the effects of these factors on central macular thickness are displayed in Table 5. There was a significant negative correlation between initial central macular thickness and change in central macular thickness over treatment, and females were significantly more likely to have a greater change in central macular thickness. Again, when accounting for all of other variables, retina specialist’s practice patterns (number of injections, interval of injections) did not have a statistically significant association with change in central macular thickness.
 |
Table 5 Factors Associated with Change in Central Macular Thickness Over Course of Treatment Using Multivariate Analysis of Covariance |
Discussion
In this study, we show the anti-VEGF dosing regimen of four retina specialists in treating wet AMD using the PRN strategy. Though all four retina specialists started the first injection at similar baseline BCVA and CMT (p > 0.1), they deviated significantly in mean number of injections, time intervals between injections, and total duration of treatment. While this indicates a consensus on when to initiate therapy, it did not correlate to mean change in BCVA outcomes. For instance, Specialists A and B demonstrated 2.5 and 11 letters improvement, respectively, while Specialists C and D both ended up with worsening of BCVA by 3.5 and 3 letters, respectively. With respect to CMT, all four retina specialists displayed similar baseline CMT and mean change in CMT ranging from 21.9 to 72.7 µm during the treatment period.
On further analysis to find the variables that may be associated with mean change in BCVA, the mean number and frequency of injections were not significantly associated with change in visual outcomes between the retina specialists. Similarly, the number of injections, frequency of injections, and total treatment window were also not associated with mean change in CMT between the four retina specialists in the multivariate analysis. Moreover, female sex was associated with greater changes in CMT compared to male sex. Therefore, the significantly different visual outcomes of specialists in treating wet AMD are likely due to other variables such as effectiveness of anti-VEGF drug, environmental and genetic dispositions of the patient, and severity of the disease. In addition, as mentioned by Gurung et al, 2021, BCVA is a subjective measure and is influenced by patient’s refractive status or cataracts, thus making it a soft metric for analysis in such efficacy studies.23
It has been previously reported that worse baseline vision is associated with better visual gains but worse final vision.24 This is seen in our results, as Specialists B achieved better visual gains than Specialists C and D. Specialists C and D both had a larger interval of treatment of 6.6 and 5.5 weeks when compared to the 3.8- and 4.4-week mean injection intervals of Specialists A and B, respectively. Also, Specialist C had the highest number of injections when compared to A, B, and D and greatest outcomes in CMT. However, the dosing regimens of injections did not associate with the change in BCVA in the multivariate analysis between the retina specialists. Thus, a clinically meaningful conclusion remains elusive until large-scale controlled clinical trials are conducted.
Although imaging evaluation may indicate fluid and leaky vessels, which can be the driving force of administrating more anti-VEGF treatment over time, the results of utilizing more anti-VEGF in these circumstances may be uncertain. For example, VIEW 1 and VIEW 2 studies have shown that aflibercept dosed monthly or 2 monthly produced similar efficacy and safety outcomes as monthly dosing with Ranibizumab.10 Given the complexity and variability in clinical presentation of wet AMD, a strict practice regimen of dosing anti-VEGF is perhaps unrealistic. Nonetheless, it is worthwhile to streamline anti-VEGF treatment of wet AMD to help reduce the burden on both patients and retina specialists. This is more so important now than ever, due to the dilemma faced by retina specialists in choosing the best option of dosing the anti-VEGFs and calculating the treatment window. In addition, having a unified treatment pattern by retina specialists may reduce the financial and emotional burden on the wet AMD patients that receive injections. The American Academy of Ophthalmology (AAO) advocates on establishing protocols for preferred practice guidelines. While the AAO does not have specific guidelines in place other than recommending anti-VEGF as first-line therapy, this will likely be informed by ongoing research in the field.
In the multivariate analysis of our present study, initial logMAR BCVA and initial central macular thickness were inversely correlated with visual and anatomical improvement, respectively. While greater initial logMAR BCVA (worse vision) and macular edema would be expected to have more room for improvement and, therefore, be expected to be associated with greater improvement, our contrary findings are not necessarily unexpected. For example, in a recent large real-life US cohort, visual acuity remained stable after one year of treatment rather than demonstrating the larger visual acuity gains typically observed in randomized controlled trials.25 This is likely due to the highly selective inclusion criteria and strict follow-up of RCTs, whereas a certain degree of variability in response to treatment is present in real-life populations due to differences in baseline characteristics and heterogeneity of underlying disease. Therefore, the response to treatment observed in our real-world study could indeed be expected.
This being the first study that compares the injection patterns of retina specialists in a real-world hospital setting means that it needs to be validated with large-scale studies. It is reasonable to integrate an anti-VEGF treatment algorithm for retina specialists in order to streamline the treatment approach. Limitations of this study include retrospective chart analysis and variable sample size between retina specialists. Indeed, a larger sample size would increase the power of a future study. We also did not account for the complexity of the wet AMD disease and the type of anti-VEGF drug used in controlling the disease. Nonetheless, this interesting observational study highlights the consensus between retina specialists despite a lack of a fixed dosing regimen when using a PRN protocol.
Conclusion
We present the different practice patterns adopted by four retina specialists when treating wet AMD. While all the specialists showed improvement in CMT, despite choosing different practice pattern approaches in terms of number, frequency, and total duration of injections, only two of the four retina specialists demonstrated improvement in BCVA. This suggests a need in establishing a universally adoptable injection regimen, specific to each anti-VEGF agent, integrating the confounding factors in the treatment algorithm to reduce burden on both patients and retina specialists.
Data Sharing Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Statement of Ethics
This study protocol was reviewed and approved by the UTMB Institutional Review Board (IRB), IRB #: 17-0065.
Consent to Participate Statement
Due to the retrospective nature of this study, exemption from requiring written informed consent was granted by the UTMB Institutional Review Board. All data collected were anonymized and de-identified to maintain confidentiality.
Acknowledgments
We express our sincerest gratitude to Efstathia Polychronopoulou for her invaluable contributions to the statistical analysis of our study. Cina Karimaghaei and Amir Ali are co-first authors for this study. This study was presented at the Association for Research in Vision and Ophthalmology Annual Meeting (May 3, 2021) and the 62nd Annual National Student Research Forum (May 15, 2021).
Author Contributions
All authors including Cina Karimaghaei, Amir Ali, Nida Safdar, Anika Tanwani, Mary Schmitz-Brown, Touka Banaee, Jaafar El-Annan, and Praveena Gupta made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The research was supported by the Robertson-Poth Distinguished Chair in Ophthalmology Endowment Grant.
Disclosure
All authors report no conflicts of interest in this work. The authors have no affiliation with the companies manufacturing the intravitreal agents used in this study.
References
1. Gheorghe A, Mahdi L, Musat O. Age-related macular degeneration. Rom J Ophthalmol. 2015;59(2):74–77.
2. Mitchell P, Liew G, Gopinath B, Wong TY. Age-related macular degeneration. Lancet. 2018;392(10153):1147–1159. doi:10.1016/S0140-6736(18)31550-2
3. Seddon JM. Epidemiology of age-related macular degeneration. In: Schachat AP, Ryan S, editors. Retina.
4. Van Lookeren Campagne M, LeCouter J, Yaspan BL, Ye W. Mechanisms of age-related macular degeneration and therapeutic opportunities. J Pathol. 2014;232(2):151–164.
5. Coleman HR, Chan CC, Ferris FL, Chew EY. Age-related macular degeneration. Lancet. 2008;372(9652):1835–1845. doi:10.1016/S0140-6736(08)61759-6
6. Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. Lancet. 2012;379(9827):1728–1738. doi:10.1016/S0140-6736(12)60282-7
7. Bourne RRA, Stevens GA, White RA, et al. Causes of vision loss worldwide, 1990–2010: a systematic analysis. Lancet Glob Heal. 2013;1(6):339–349.
8. Berg K, Pedersen TR, Sandvik L, Bragadóttir R. Comparison of ranibizumab and bevacizumab for neovascular age-related macular degeneration according to LUCAS treat-and-extend protocol. Ophthalmology. 2015;122(1):146–152. doi:10.1016/j.ophtha.2014.07.041
9. Gillies MC, Hunyor AP, Arnold JJ, et al. Effect of ranibizumab and aflibercept on best-corrected visual acuity in treat-and-extend for neovascular age-related macular degeneration: a randomized clinical trial. JAMA Ophthalmol. 2019;137(4):372–379.
10. Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537–2548. doi:10.1016/j.ophtha.2012.09.006
11. Erie JC, Barkmeier AJ, Hodge DO, Mahr MA. High variation of intravitreal injection rates and medicare anti-vascular endothelial growth factor payments per injection in the United States. Ophthalmology. 2016;123(6):1257–1262. doi:10.1016/j.ophtha.2016.02.015
12. El-Mollayess GM, Mahfoud Z, Schakal AR, Salti HI, Jaafar D, Bashshur ZF. Fixed-interval versus OCT-guided variable dosing of intravitreal bevacizumab in the management of neovascular age-related macular degeneration: a 12-month randomized prospective study. Am J Ophthalmol. 2012;153(3):481–489.e1. doi:10.1016/j.ajo.2011.08.018
13. Ho AC, Busbee BG, Regillo CD, et al. Twenty-four-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology. 2014;121(11):2181–2192. doi:10.1016/j.ophtha.2014.05.009
14. Feltgen N, Bertelmann T, Bretag M, et al. Efficacy and safety of a fixed bimonthly ranibizumab treatment regimen in eyes with neovascular age-related macular degeneration: results from the RABIMO trial. Graefes Arch Clin Exp Ophthalmol. 2017;255(5):923–934. doi:10.1007/s00417-017-3589-x
15. Kertes PJ, Galic IJ, Greve M, et al. Canadian treat-and-extend analysis trial with ranibizumab in patients with neovascular age-related macular disease: one-year results of the randomized Canadian treat-and-extend analysis trial with ranibizumab study. Ophthalmology. 2019;126(6):841–848. doi:10.1016/j.ophtha.2019.01.013
16. Eldem BM, Muftuoglu G, Topbaş S, et al. A randomized trial to compare the safety and efficacy of two ranibizumab dosing regimens in a Turkish cohort of patients with choroidal neovascularization secondary to AMD. Acta Ophthalmol. 2015;93(6):e458–64. doi:10.1111/aos.12540
17. Solomon SD, Lindsley K, Vedula SS, Krzystolik MG, Hawkins BS. Anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2019;3(3):1.
18. Hanna RM, Barsoum M, Arman F, Selamet U, Hasnain H, Kurtz I. Nephrotoxicity induced by intravitreal vascular endothelial growth factor inhibitors: emerging evidence. Kidney Int. 2019;96(3):572–580. doi:10.1016/j.kint.2019.02.042
19. Porta M, Striglia E. Intravitreal anti-VEGF agents and cardiovascular risk. Intern Emerg Med. 2020;15(2):199–210. doi:10.1007/s11739-019-02253-7
20. Boyle J, Vukicevic M, Koklanis K, Itsiopoulos C. Experiences of patients undergoing anti-VEGF treatment for neovascular age-related macular degeneration: a systematic review. Psychol Heal Med. 2015;20(3):296–310. doi:10.1080/13548506.2014.936886
21. Lee WJ, Cho HY, Kim DH, et al. Depression of late age-related macular degeneration patients in Korea. Asia Pacific J Ophthalmol. 2013;2(1):23–27. doi:10.1097/APO.0b013e31827be8b1
22. Moisseiev E, Regenbogen M, Bartfeld Y, Barak A. Evaluation of pain in intravitreal bevacizumab injections. Curr Eye Res. 2012;37(9):813–817. doi:10.3109/02713683.2012.681335
23. Gurung RL, Fitzgerald LM, McComish BJ, Hewitt AW, Verma N, Burdon KP. Comparing vision and macular thickness in neovascular age-related macular degeneration, diabetic macular oedema and retinal vein occlusion patients treated with intravitreal antivascular endothelial growth factor injections in clinical practice. BMJ Open Ophthalmol. 2021;6(1):1–9.
24. Ying G, Maguire MG, Pan W, et al. Baseline predictors for five-year visual acuity outcomes in the comparison of AMD treatment trials. Ophthalmol Retin. 2018;2(6):525–530. doi:10.1016/j.oret.2017.10.003
25. Lotery A, Griner R, Ferreira A, Milnes F, Dugel P. Real-world visual acuity outcomes between ranibizumab and aflibercept in treatment of neovascular AMD in a large US data set. Eye. 2017;31(12):1697–1706. doi:10.1038/eye.2017.143
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
