Back to Journals » Cancer Management and Research » Volume 12
The Influence of Venous Characteristics on Peripherally Inserted Central Catheter-Related Symptomatic Venous Thrombosis in Cancer Patients
Authors Wang GD, Wang HZ , Shen YF, Dong J, Wang XP, Wang II XZ, Zheng YY, Chen J, Guo SS
Received 16 September 2020
Accepted for publication 5 November 2020
Published 20 November 2020 Volume 2020:12 Pages 11909—11920
DOI https://doi.org/10.2147/CMAR.S282370
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
Guo-Dong Wang, Hong-Zhi Wang, Yan-Fen Shen, Jing Dong, Xin-Peng Wang, Xiao-Zheng Wang II, Yuan-Yuan Zheng, Jie Chen, Shuang-Shuang Guo
Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), ICU, Peking University Cancer Hospital & Institute, Beijing 100142, People’s Republic of China
Correspondence: Hong-Zhi Wang; Yan-Fen Shen
Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), ICU, Peking University Cancer Hospital & Institute, No. 52 Fucheng Road, Haidian District, Beijing 100142, People’s Republic of China
Tel/Fax +86 10-88196112
Email [email protected]; [email protected]
Background: With increasing use, peripherally inserted central catheters (PICCs) are associated with the risk of venous thrombosis. Few studies have focused on the relationships between venous thrombosis and venous characteristics. This study aimed to identify effects of venous characteristics on symptomatic PICC-related venous thrombosis in cancer patients and explore the relationship between venous characteristics and blood flow velocity.
Methods: The data of patients who underwent placement of PICC were retrospectively studied between January 2015 and September 2017. Symptomatic PICC-related venous thrombosis was confirmed by ultrasound. Univariable, multivariable logistic regression analyses were performed to identify the risk factors associated with PICC-related venous thrombosis. In October 2017, 169 patients with PICCs were enrolled prospectively, and the relationships between blood flow velocity and venous characteristics were recorded and analyzed.
Results: A total of 2933 cancer patients were enrolled in this study; of these patients, 68 experienced symptomatic venous thrombosis. In the bivariate analysis, body mass index (BMI), history of venous thrombosis, triglycerides, tumor category, vessel diameter, vessel depth and arm circumference were associated with thrombosis. The multivariable analyses showed that arm circumference, vascular diameter, triglyceride level and tumor category were independent risk factors for thrombosis. Blood flow velocity was positively correlated with vessel depth and arm circumference but not with vessel diameter.
Conclusion: Different venous characteristics can lead to different blood flow rates, which can affect the incidence of thrombosis. A vein depth of greater than 1.07cm or less than 0.57cm was associated with a higher incidence of PICC-related venous thrombosis, and the greater the arm circumference and vessel diameter, the greater the risk of venous thrombosis.
Keywords: peripherally inserted central catheters, cancer, venous thrombosis, risk factors, venous characteristics
Introduction
Peripherally inserted central venous catheters (PICCs) are often the primary approach for providing venous access for administration of chemotherapy in cancer patients. PICCs reduce the risk of drug infiltration and alleviate the inflammation of vessels by drugs. Moreover, cancer patients can leave the hospital with PICCs during chemotherapy intervals, which avoids pain caused by repeated punctures and significantly improves their quality of life. However, both patients and doctors are concerned with PICC-related venous thrombosis. The incidence of PICC-related venous thrombosis is 5%~20%, among which symptomatic PICC-related venous thrombosis accounts for 1%~4%.1,2,4–7 Symptomatic venous thrombosis might result in a series of adverse consequences. ① Venous thrombosis may result in the following clinical manifestations: an enlarged arm circumference; reddening, flushing and swelling of the skin on the upper extremity; and tenderness at the catheter site or neighboring sites.8 ② Although extremely rare, detachment of a venous thrombus from an upper extremity may cause pulmonary embolism, which is life threatening.9 ③ Post-thrombosis syndrome (PTS) in the late stage after thrombus formation could hamper the functions of venous valves, leading to pain, swelling and dysfunction of the affected extremity and reducing patient quality of life.10 ④ Thrombosis is also the primary reason for the non-planned removal of PICCs.1,2 As PICC-related venous thrombosis could result in a series of adverse events, which interrupt clinical treatment and reduce patient quality of life, identifying the risk factors for PICC-related venous thrombosis and taking specific prevention measures are of great clinical significance. Early studies have demonstrated that a number of modifiable and unmodifiable factors are associated with PICC-related venous thrombosis. Nonetheless, there remains a paucity of data regarding the relationships between PICC-related venous thrombosis and venous characteristics such as vein diameter, vein depth, arm circumference. In addition, the effects of different venous conditions on blood flow velocity are also unknown.
In this study, we retrospectively analyzed PICC-related venous thrombosis and its risk factors in cancer patients and prospectively analyzed the relationship between blood flow velocity and the catheterized vein to explore the effects of venous conditions on thrombosis and to explain this phenomenon.
Methods
Subjects
This study used a retro-prospective study design, and the data consisted of two components: retrospective data collected from our hospital between January 2015 and September 2017 and prospective data collected 1 month later from the same hospital. All the patients signed the informed consent before PICC insertion and the study was approved by the ethics committee of our hospital.
Inclusion Criteria
a. Aged≥18 years old;
b. Pathologically or cytologically confirmed malignant tumor;
c. Underwent PICC in our hospital and received systematic treatment;
d. Received a venous duplex ultrasound.
e. Catheter/venous diameter ratio is equal to 45% or less.3
Exclusion Criteria
a. Communication disorders;
b. Leukemia, pregnant or lactating, or had an active infection requiring treatment;
c. Predicted survival time less than 1 month.
d. Patients were excluded if they were already receiving either prophylactic or therapeutic anticoagulant therapy at the time of the PICC line insertion.
PICC Insertion Method
Personnel Qualifications
All the nurses who inserted PICCs were specialized venous treatment nurses who had performed the procedure for more than 5 years; each nurse inserted more than 300 PICCs per year on average.
Catheter Types
The front end of a type A catheter was open, and the tip had a flat design. The tip of the catheter was trimmed before delivery. The catheter was made of a polyurethane material, and the pipe diameter was 4Fr (circumference 4.19 mm, outer diameter 1.333 mm). The front end of a type B had a three-way valve, which prevents blood from flowing into the catheter. The catheter was made of silicone material, and the pipe diameter was 4Fr. Since the lumen diameter of 4Fr can minimize the incidence of PICC-venous thrombosis2 and meet the clinical needs, all patients were treated with 4Fr catheters.
Insertion Site and Vein
The left or right upper extremity was chosen according to the patients’ willingness and vessel characteristics. Usually, the right upper extremity was preferred. Regarding the puncture vein, the basilic vein was the first choice, followed by the brachial vein and cephalic vein.
Insertion Procedure
Before insertion, the patients’ vein conditions were accessed by nurses in the Venous Access Center using the LOGIQ Book Ultrasound System. The inner diameter of the target vein and depth of the vessel were measured and recorded, arm circumference at the 10cm above the elbow joint were measured and recorded together. It has been reported that the modified Seldinger technique (MST) can reduce the incidence of PICC-related thrombosis,11 so in this study, all patients underwent PICC placement according to the MST under ultrasound guidance.
It has been reported that placing the catheter tip in the lower 1/3 of the superior vena cava (SVC) leads to the lowest risk of thrombosis.12 Therefore, during the insertion process, we used ultrasound to explore the internal jugular vein and the subclavian vein to ensure that the catheter was in the right vein. Catheter placement was determined by right atrial electrocardiogram to ensure that the catheter tip was located at the junction of the SVC and right atrium or the lower segment of the SVC.13 The position of the tip of the catheter was determined by chest radiography after surgery. If an abnormality was present, it was addressed in a timely manner. After catheterization, the patients were encouraged to remain physically active and avoid excessive bedrest to prevent thrombosis. Routine device and site checks are performed weekly by the venous access team or earlier if malfunction occurs. All PICC lumens are flushed with 10 mL normal saline and 5 mL heparin daily according to a defined maintenance protocol. In the event of luminal occlusion (failure to flush or withdraw from the PICC), 2 mg/mL of tissue plasminogen-activator is instilled in each lumen to “declot” the device.
Examination of Venous Thrombosis
During PICC insertion, doctors or nurses observed the arm for swelling (by measuring the arm circumference) if there was a color change in the skin and pain. If a patient had at least one of the above symptoms or if a patient was suspected to have developed thrombosis, color Doppler ultrasound was applied to make a definite diagnosis. All the patients were followed-up until catheter removal or venous thrombosis occurred.
Data Collection
The following data were collected: the vein used for PICC insertion, the type of catheter, the length of the catheter inserted into the body, the location of the catheter tip, the patient’s age, sex, height and weight, cancer type, and co-morbidities. Additionally, the results of laboratory tests performed within one week of PICC insertion, including routine blood tests, blood biochemical examinations, and blood coagulation tests, were also recorded.
Statistical Analysis
Descriptive data are presented as the median and interquartile range (IQR, ie, 25–75th percentiles). Proportions of events were compared using Fisher’s exact test or Pearson’s χ2 test. Continuous variables were compared by Student’s t-test or Mann–Whitney rank sum test according to the normality of their distribution. Univariate and multivariate logistic regression models were employed to estimate the risk factors for PICC-related venous thrombosis. The risk assessments are presented as odds ratios (ORs) with 95% confidence intervals (95% CIs). All statistical analyses were performed using SPSS version 16.0 (SPSS Inc., Chicago, IL, USA) for Windows, with a P-value <0.05 considered statistically significant.
Results
The retrospective study included a selection of 2933 samples obtained in 2833 patients, of which 78 patients underwent PICC placement twice or more. The patient characteristics are described in Table 1. Missing data were excluded from the statistical analysis. The median PICC use duration was 125 days (IQR 70–173). The success rate of PICC insertion after a single attempt was 93.8%, and the success rate of PICC insertion after two attempts was 98.7%. The median diameter of the vein chosen for insertion was 0.40 cm (IQR 0.35 cm-0.46 cm); the median Catheter/venous diameter ratio was 0.33 (IQR 0.29–0.38). The median vascular depth was 0.85 cm (IQR 0.57 cm-1.07 cm), and the arm circumference was 26.57cm (IQR 24.5 cm-28.5 cm).
 |
Table 1 Demographic, Clinical and Procedural Data of the Study Population |
Currently, no common and standardized definitions of vascular depth, arm circumference and vessel diameter system exist. We first calculated the distribution of the venous characteristics interquartile range; then, values greater than the upper threshold or less than the lower threshold values were defined as outliers.
PICC-Related Thrombus
A total of 68 patients (2.3%) developed symptomatic venous thrombosis. The median time to thrombosis was 34 days (range 1~230). Of the 68 patients, 73.53% (50/68) exhibited swelling of the limbs, of which 60.29% (41/68) affected the upper limbs, and 13.24% (9/68) exhibited swelling of the cervical region. A total of 26.47% (18/68) of the patients complained of distending limb pain. Two patients exhibited PTS.
Univariate Analysis (Table 2)
There were no relationships between the incidence of thrombosis and age, sex, history of diabetes or hypertension, hemoglobin, blood platelet count, activated partial thromboplastin time, insertion site and vein, PICC type or number of punctures. Patients with a high body mass index (BMI), history of venous thrombosis and high triglycerides had an increased incidence of thrombosis. Patients who suffered from lung cancer had a higher risk for thrombosis than those suffering from other cancer types. A large vessel diameter, a large upper extremity circumference, and unsuitable catheter insertion vein depth (too deep or too shallow) were also risk factors for thrombosis.
 |
Table 2 Bivariable (Unadjusted) Analysis of Risk Factors Associated with PICC-Related VTE |
Multivariate Analysis (Table 3)
To further analyze the risk factors for PICC-related upper extremity venous thrombosis, a multivariate analysis was performed. The statistical results showed that arm circumference, vascular diameter, triglyceride level and tumor type were independent influencing factors for thrombosis. The larger the arm circumference and vessel diameter, the higher the incidence of thrombus.
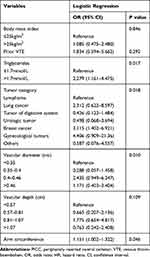 |
Table 3 Multivariate Logistic Analyses for PICC-Related VTE |
Venous Hemodynamic Analysis
We found that the incidence of thrombosis was lowest when the vessel depth was approximately average (P=0.040), and it increased with the increase of vessel diameter (P=0.001) and arm circumference (P<0.001) (Figure 1). This is strange because the traditional view is that the shallower or bigger the vessel is, the easier it is to puncture, resulting in a low incidence of thrombosis.
To conduct better research on the relationship between blood flow velocity and vessel, we prospectively enrolled 169 patients with PICCs in October 2017 and recorded blood flow velocity and venous depth, diameter and arm circumference. The median flow velocity was 13.53 cm/s (IQR 11.20–17.08). It was found that the blood flow velocity was slow when the vessel depth was shallow and had a tendency to increase with increasing vessel depth (P=0.096), and the similar result was found of arm circumference (P=0.005). However, there is no significant trend in venous diameter (P=0.829). (Figure 2)
We also found that arm circumference was positively correlated with venous diameter (P=0.042) and depth (P<0.001), yet there was no significant correlation between venous diameter and venous depth (P=0.846). (Figure 3)
Discussion
Infusion Nursing Standards of Practice (INS) has pointed out that for adults, a catheter/venous diameter ratio of 45% or less is recommended to reduce the incidence of thrombosis.3 However, there is still no international study on small catheter/vein diameter ratios, our study is the first to analyze the relationships among catheter/vein diameter ratio, venous depth, arm circumference and incidence of thrombosis. For the first time, the relationship between upper arm vessel and blood flow velocity was explored to explain the relationship.
The overall thrombus incidence in our study was lower (2.3%) than that in other studies.1,2,4 Compared with other studies, we adopted the following methods to minimize the incidence of PICC thrombosis. In this study, PICCs of the same size (4F) were used, and the catheters were placed by experienced specialist nurses under ultrasonic guidance to ensure that the success rate of the first puncture was as high as possible and reduce the risk of PICC-related thrombosis caused by multiple puncture injuries to the venous wall. The location of the catheter tip is also a key factor in venous thrombosis. Currently, all guidelines and standards recommend that the head of the PICC should be located in the SVC, and the optimal position is in the lower 1/3 of the middle and lower segments of the SVC. The junction between the SVC and the right atrium should be 3–4 cm so that the catheter cannot enter the right atrium or the right ventricle.12 During catheterization, catheter orientation was detected by ultrasound, and the catheterization technique was assessed by a right atrial electrocardiogram.13 The position of the catheter tip was confirmed by chest radiography after the operation to ensure that the position of the catheter tip was satisfactory.
Many of the risk factors identified in this analysis agree with earlier studies. Our findings of a strong association of venous thrombosis with lung cancer and gynecologic tumors are consistent with prior reports.14 Our study did not include sufficient numbers of patients with brain or pancreas cancers, which have also been strongly associated with venous thrombosis. We also found that patients with a previous history of venous thrombosis have a higher risk of re-thrombosis, especially in combination with other risk factors, which are consistent with prior study.15
Some studies have found that a BMI > 25 kg/m2 is an independent risk factor for catheter-related thrombosis.1,16 Our study also found that a BMI > 25 kg/m2 was one of the major risk factors for PICC-related venous thrombosis. Obesity increases the difficulty of catheterization, and repeated punctures may damage the venous wall, resulting in an increased incidence of PICC-related venous thrombosis. It has also been shown that adipocytokines, such as leptin and adiponectin, can enhance the activity of the coagulation system and reduce fibrinolytic activity by interfering with the metabolism of fat and sugar in the blood.17,18 Our study found that triglycerides were significantly elevated in obese patients, and we also found that patients with high triglycerides had an increased incidence of PICC-related venous thrombosis. Triglyceride deposits in the venous wall gradually formed small plaques. In contrast, large deposits in the venous wall gradually expand in area and thickness, decreasing the inner diameter of the vessels and slowing blood flow, thus accelerating the process of developing a venous blockage; blood flow will be interrupted in severe cases. Studies have shown that the concentration of triglycerides is positively correlated with the response intensity of venous endothelial cells to inflammatory cytokines, and triglycerides can promote the activation of inflammatory cytokines, such as tumor necrosis factor, on the intima of vessels.19 When the concentration of triglycerides is increased, free fatty acids released from the triglyceride nucleus provide a contact surface for coagulation factor activation and increase the charge density on the surface of large granular lipoproteins, activating coagulation factor VII. The tissue factor inhibitor associated with thrombosis is a lipoprotein-related coagulation inhibitor, which may play an important role in venous thrombosis. Vascular endothelial cell injury causes in vivo anticoagulation dysfunction, increased blood coagulation, and thrombosis.
Interestingly, we found that both deep and shallow vessels were associated with an increased incidence of thrombosis. Patients with vessels that were too deep were generally obese, which was related to high triglyceride levels, and leading to difficult venous punctures. However, a shallow vessel depth was related to relatively a slow blood flow velocity. Virchow’s triad is traditionally invoked to explain pathophysiologic mechanisms leading to thrombosis, alleging concerted roles for abnormalities in blood composition, vessel wall components, and blood flow in the development of arterial and venous thrombosis.20 We analyzed the relationship between vessel depth and the incidence of thrombosis and found that when the vessel depth was too shallow, the blood flow velocity was relatively slow, which explains why we observed an increased incidence of thrombosis when the vessel depth was shallow. Studies have shown that the blood pumping index of muscle is better in obese patients than in nonobese patients;19 therefore, we speculated that the vessel depth was too shallow in thin patients and the blood pumping function of the muscle was poor, resulting in a relatively slow blood flow velocity. Studies have shown that the velocity of venous blood flow in the lower limbs of obese pregnant women is significantly higher than that in nonobese pregnant women;21 yet, the incidence of venous thrombosis of the lower limbs is still high. The high incidence of thrombosis is not only due to sluggish blood flow but also due to other causes of obesity. We have concluded that deep vessels, even without a sluggish blood flow velocity, are caused by factors such as obesity and lead to a high incidence of thrombosis.
Previous studies have shown that the thinner the diameter of vessels, the more prone they are to thrombosis.5 The reason for this is because a small vessel size can result in a difficult puncture, and multiple punctures or tube insertions cause damage to the inner walls of vessels, leading to thrombosis. Prior studies have not featured a dedicated central venous catheter insertion team. We hypothesize that the standardized insertion technique of our PICC line team may have diminished the impact of certain technical variables that had previously potentiated line-related thrombosis, allowing for the emergence of other predictive signals. It was found that patients with larger vessels had a higher risk of thrombosis because patients with larger vessels were more likely to be obese than those with smaller vessels, leading to a higher incidence of thrombosis. Of course, if vessel size was too small will slow down the blood flow and increase the difficulty of puncture, which will increase the incidence of thrombosis, so INS recommends a catheter/venous diameter ratio of 45% or less is necessary and helpful to decrease this complication.
Similarly, we found that patients with a large arm circumference had an increased incidence of thrombosis because patients with a large arm circumference were more obese than those with a small arm circumference. Obesity led to difficult venous punctures, and obese patients had relatively high triglycerides, which led to an increase in the incidence of thrombosis. Because arm circumference was positively correlated with venous diameter, so when we chose patients whose vessel diameter was not too small, we avoided the slow blood flow caused by too small arm circumference.
We acknowledge some limitations. First, this is a retrospective cohort study, and the results may be subject to bias or incomplete information. Second, although our study included a large number of patients, it was conducted at a single institution, and the results may not be generalizable to hospitals with different case mixes or practice patterns. Third, potential confounding variables, such as duration of PICC line insertion, or unrecognized embolism, could not be evaluated. Finally, despite performing a careful chart review, some episodes of PICC-related venous thrombosis may have been missed. In particular, venous thrombosis diagnosed at other institutions may have been overlooked.
Nevertheless, our study has important strengths, including a large sample size and a highly skilled insertion technique. Additionally, we further explained the relationship between blood flow velocity and venous characteristics, which was previously lacking.
Conclusion
It appears that a catheter-to-vein ratio less than 45% can reduce the risk of venous thrombosis. Additionally, our study has determined that when the venous depth is moderate, it is less likely to develop venous thrombosis, and the greater the arm circumference and venous diameter, the greater the risk of venous thrombosis. PICC-related venous thrombosis is a complex process with multiple influencing factors. Therefore, a prospective study should further explore the relationships among blood venous depth, venous diameter, arm circumference and venous thrombosis, as well as methods to select the most appropriate vessels to actively prevent early venous thrombosis in high-risk patients and reduce the incidence of PICC-related venous thrombosis.
Data Sharing Statement
The data sets used and/or analyzed during the current study are available from Hong-Zhi Wang on reasonable request (Email: [email protected]).
Ethics Approval and Consent to Participate
The study was in line with the Helsinki Declaration and approved by the Peking University Cancer Hospital Ethics Committee. Written informed consent was obtained from the patients for their anonymized information to be published in this study.
Acknowledgments
We thank the PICC team nurses who judiciously maintain the PICC database.
Funding
This study was supported by B.Braun Anaesthesia Science Research Foundation (BBFD-2015-16).
Disclosure
All the authors state that they have no conflict of interest.
References
1. Yi XL, Chen J, Li J, et al. Risk factors associated with PICC-related upper extremity venous thrombosis in cancer patients. J Clin Nurs. 2014;23(5–6):837–843. doi:10.1111/jocn.12227
2. Chopra V, Ratz D, Kuhn L, Lopus T, Lee A, Krein S. Peripherally inserted central catheter-related deep vein thrombosis: contemporary patterns and predictors. J Thromb Haemost. 2014;12(6):847–854. doi:10.1111/jth.12549
3. Gorski LA. The 2016 infusion therapy standards of practice. Home Healthc Now. 2017;35(1):10–18. doi:10.1097/NHH.0000000000000481
4. Bertoglio S, Faccini B, Lalli L, Cafiero F, Bruzzi P. Peripherally inserted central catheters (PICCs) in cancer patients under chemotherapy: a prospective study on the incidence of complications and overall failures. J Surg Oncol. 2016;113(6):708–714. doi:10.1002/jso.24220
5. Menéndez JJ, Verdú C, Calderón B, et al. Incidence and risk factors of superficial and deep vein thrombosis associated with peripherally inserted central catheters in children. J Thromb Haemost. 2016;14(11):2158–2168. doi:10.1111/jth.13478
6. Koo CM, Vissapragada R, Sharp R, et al. ABO blood group related venous thrombosis risk in patients with peripherally inserted central catheters. Br J Radiol. 2018;91(1082):20170560.
7. Nash EF, Helm EJ, Stephenson A, Tullis E. Incidence of deep vein thrombosis associated with peripherally inserted central catheters in adults with cystic fibrosis. J Vasc Interv Radiol. 2009;20(3):347–351. doi:10.1016/j.jvir.2008.11.018
8. Periard D, Monney P, Waeber G, et al. Randomized controlled trial of peripherally inserted central catheters vs. peripheral catheters for middle duration in-hospital intravenous therapy. J Thromb Haemost. 2008;6(8):1281–1288.
9. Moran J, Colbert CY, Song J, et al. Screening for novel risk factors related to peripherally inserted central catheter-associated complications. J Hosp Med. 2014;9(8):481–489. doi:10.1002/jhm.2207
10. Errichi BM, Belcaro G, Hosoi M, et al. Prevention of post thrombotic syndrome with Pycnogenol® in a twelve month study. Panminerva Med. 2011;53(3 Suppl 1):21–27.
11. Li J, Fan YY, Xin MZ, et al. A randomised, controlled trial comparing the long-term effects of peripherally inserted central catheter placement in chemotherapy patients using B-mode ultrasound with modified Seldinger technique versus blind puncture. Eur J Oncol Nurs. 2014;18(1):94–103. doi:10.1016/j.ejon.2013.08.003
12. DeChicco R, Seidner DL, Brun C, Steiger E, Stafford J, Lopez R. Tip position of long-term central venous access devices used for parenteral nutrition. JPEN J Parenter Enteral Nutr. 2007;31(5):382–387. doi:10.1177/0148607107031005382
13. Ortiz-Miluy G, Sánchez-Guerra C. [Intracavitary electrocardiogram during the insertion of peripherally inserted central catheters]. Enferm Clin. 2013;23(4):148–153. Spanish. doi:10.1016/j.enfcli.2013.05.002
14. Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111(10):4902–4907. doi:10.1182/blood-2007-10-116327
15. Chemaly RF, de Parres JB, Rehm SJ, et al. Venous thrombosis associated with peripherally inserted central catheters: a retrospective analysis of the Cleveland Clinic experience. Clin Infect Dis. 2002;34(9):1179–1183. doi:10.1086/339808
16. Al-Asadi O, Almusarhed M, Eldeeb H. Predictive risk factors of venous thrombosis (venous thrombosis) associated with peripherally inserted central catheters (PICC) in ambulant solid cancer patients: retrospective single centre cohort study. Thromb J. 2019;17:2. doi:10.1186/s12959-019-0191-y
17. Abdollahi M, Cushman M, Rosendaal FR. Obesity: risk of venous thrombosis and the interaction with coagulation factor levels and oral contraceptive use. Thromb Haemost. 2003;89(3):493–498. doi:10.1055/s-0037-1613379
18. Darvall KA, Sam RC, Silverman SH, Bradbury AW, Adam DJ. Obesity and thrombosis. Eur J Vasc Endovasc Surg. 2007;33(2):223–233. doi:10.1016/j.ejvs.2006.10.006
19. van Rij AM, De Alwis CS, Jiang P, et al. Obesity and impaired venous function. Eur J Vasc Endovasc Surg. 2008;35(6):739–744. doi:10.1016/j.ejvs.2008.01.006
20. Wolberg AS, Aleman MM, Leiderman K, Machlus KR. Procoagulant activity in hemostasis and thrombosis: Virchow’s triad revisited. Anesth Analg. 2012;114(2):275–285. doi:10.1213/ANE.0b013e31823a088c
21. Dutta EH, Burns RN, Pacheco LD, Marrs CC, Koutrouvelis A, GLO K. Lower extremity blood flow velocity in obese versus nonobese pregnant women. Am J Perinatol. 2019.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



