Back to Journals » Research and Reports in Tropical Medicine » Volume 13
The Human Filaria Loa loa: Update on Diagnostics and Immune Response
Authors Dieki R, Nsi-Emvo E, Akue JP
Received 10 February 2022
Accepted for publication 3 June 2022
Published 1 August 2022 Volume 2022:13 Pages 41—54
DOI https://doi.org/10.2147/RRTM.S355104
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Mario Rodriguez-Perez
Roland Dieki,1,2 Edouard Nsi-Emvo,2 Jean Paul Akue1
1Department of Parasitology, International Centre of Medical Research of Franceville, Franceville, Gabon; 2Department of Chemistry, Université des Sciences et Techniques de Masuku (USTM), Franceville, Gabon
Correspondence: Jean Paul Akue, Department of Parasitology, International Centre of Medical Research of Franceville, Franceville, BP 769, Gabon, Email [email protected]
Abstract: Loa loa loiasis was considered an anecdotal disease 30 years ago. Its spread in Equatorial Africa and the side effects associated with mass drug administration programs against filariasis in co-endemic areas have drawn the attention of the international research community. Progress in research conducted to date has provided insight into the immunobiology of this parasite. An interesting finding reported in several studies is that 70% of individuals with loiasis do not carry microfilariae in their blood, and 30% are microfilaremic, suggesting the involvement of several immunological mechanisms, as shown by elevated specific IgG4 and IgE levels signifying a potential cross-linking mechanism between the two isotypes via L. loa antigen to prevent allergy. A mechanism of anergy in the appearance of microfilariae in the peripheral blood results in immunological unresponsiveness in individuals with microfilariae. There is an interaction between other pathogens (parasites, bacteria, viruses) in individuals co-infected with L. loa. The strong antigen cross-reactivity between L. loa and lymphatic filarial worms warrants a re-evaluation of the distribution of the latter in co-endemic regions. The mechanism of concomitant immunity observed in the elimination of microfilariae or infective larvae (third-stage larvae, L3) may be used for the conception of an immunoprophylactic strategy.
Keywords: Loa loa, diagnostics, immune anergy, immune evasion, cross-reactivity
Historical Perspective
The first adult Loa loa worm was extracted from the eye of a young slave in Maribou (Saint Domingue) by Mongin (1770), and 7 years later Bayon reported the parasite in a young girl from Cayenne. The first description of the parasite was made in Angola by Guyot (1778), who gave it the name “Loa” the word for “worm” used by the indigenous population. Later, the disease was reported by several other authors, namely, Lorenz (1890), Manson (1904), and Stiles (1905), who coined the definitive term “Loa loa.” Manson discovered the microfilariae of L. loa in the blood (1891) and provided the name Filaria diurna due to the periodicity of the parasite. Broden and Rodhain (1908) reported microfilariae in cerebrospinal fluid, while Kulz (1908) described the clinical symptoms of the disease, which resembled those of sleeping sickness in patients with a heavy load of L. loa microfilariae in the blood (Figure 1). Van Campenhoot (1900) established the relationship between edema and L. loa in several patients. These observations were also made by Mouchet in Leopoldville, while Robert Thomson Leiper (1912) determined that the larval cycle of the worm developed in Chrysops silacea and Chrysops dimidiata (1912) (Figure 2).
Overview of Epidemiology, Clinical Symptoms, Treatment, and Impact on Public Health
Between 3 and 13 million individuals are infected in the forest areas of central and west Africa, and approximately 30 million are exposed and at risk of infection in regions with intermediate transmission.1,2 The endemic countries are Angola, Cameroon, Congo Brazzaville, Gabon, Equatorial Guinea, Nigeria, Democratic Republic of Congo (DRC), South Soudan, and Chad.1 However, cases of transmission have been reported in non-endemic zones, too, such as Benin, Mali, the Ivory Coast, and Rwanda.1–6 Besides, imported cases have been cited around the world from expatriates who have visited endemic zones.7–11 A study by the GeoSentinel Network between 1995 and 2004 reported that loiasis represents 25% of cases of imported filarial disease globally.12
It has been reported that L. loa is the cause of all major consultations in endemic areas,13 and the burden of L. loa has been evaluated in terms of adjusted life expectancy (disability-adjusted life years [DALY]) in some areas. These data enable the classification of public health priorities.14 To date, there are no data on the general DALY of L. loa. However, in a recent study in Gabon, the most common symptoms of loiasis were used to estimate L. loa-specific DALY, including: ocular migration of adult worm, transient edema on articulation, arthralgia, and violent headache.15 From this study it appears that the economically active population aged 15–44 years and 45–59 years are the groups most affected, with 400 DALY per 100,000 inhabitants in rural areas and 82.2 DALY per 100,000 inhabitants in urban areas. These DALY values are similar to those described for urinary schistosomiasis, which are 103.5, and reflect the heavy burden that loiasis places on public health systems. Although the majority of infected people are asymptomatic, Calabar swelling, pruritus, and subconjunctival migration of adult worms are the most common signs reported, with possible complications such as endocarditis also occurring. Today, there is no reason to consider L. loa a benign disease, as recent studies have shown an increased incidence of fibrosis, nephropathy, neuropsychiatric complications, and spontaneous encephalitis;16–18 moreover, loiasis was associated with increased mortality in a recent study.19 The highest prevalence rates are observed in forest areas20–22 and in some savannahs20,22,23 where the prevalence can reach 54%.22 L. loa has been neglected because the impact on mortality was considered to be low.24 However, in the past 20 years, L. loa was found to be a major obstacle in the implementation of mass drug administration programs for Onchocerca volvulus and lymphatic filariasis in co-endemic areas, since hypermicrofilaremic L. loa may induce fatal side effects including death when DEC or ivermectin, the drug used for mass chemotherapy, is applied.25,26 Albeit in experimental conditions,27–29 endangered species of primates are receptive to L. loa. A recent sociological study in Cameroon showed a widespread lack of compliance to mass drug administration programs due to the side effects associated with the drug used against L. loa.30 Based on these contingencies, onchocerciasis is planned to be eliminated in 2045. This assertion was substantiated by other modeling studies, which suggest that elimination could be achieved before 2045.31,32 In this context, control strategies can be more effective by taking into account new reports showing the diverse distribution of the parasite within endemic areas.33
Progress in L. loa Diagnostics
Staining of blood smears and concentration techniques are still commonly used in the field. However, numerous approaches to improve diagnostics have been initiated using biomarkers, such as immunoglobulin 4 (IgG4), polymerase chain reaction (PCR), reverse transcriptase PCR (RT-PCR), loop-mediated isothermal amplification, and lateral flow assays (LFAs). In a recent evaluation of diagnostic tools for L. loa, the role of biomarkers in defining a clear pattern of infection was highlighted. The advantages and disadvantages of these tests have been shown.34 For antibody detection, the most promising tests are based on the detection of specific IgG4. This subclass has been reported to be elevated in both microfilaremic and amicrofilaremic individuals.35 IgG4 has been used with recombinant antigen designed as Ll-SXP-1.36 To standardize this approach, the method was improved by using successively a luciferase immunoprecipitation system (LIPS)37 and an LFA, which achieved 94% sensitivity and 100% specificity compared to 82–88% specificity for other filarial species.38 This test is currently undergoing point-of-care evaluation.39 Circulating antigen tests have also been developed; however, these tests are limited by cross-reactivity with other filarial species.40–43 Because of the fatal side effects related to microfilariae, quantification of this larval stage in the blood is necessary, and therefore a test using LOAG-16297 and LOAG-17808 based on a competitive LIPS assay was developed. This test was reported to have a significant correlation with circulating microfilariae.44 Nucleic acid detection has also been used in diagnostic tests developed for L. loa in the form of amplification testing, ranging from classic PCR, nested PCR, qPCR,45–47 and RFLP-PCR. Although these techniques are very sensitive, because of logistics and the technical procedure involved, they can be used only in specific laboratories. However, an approach based on loop isothermal amplification48 seems to be promising as it offers the sensitivity of amplification and the simplicity of isothermal amplification, allowing it to be applied anywhere at room temperature with the end result being visible with the naked eye. Noninvasive methods with urine-derived filarial antigens have been explored;44 reverse LIPS methods with urine-derived filarial antigen can detect elevated IgG4 in both microfilaremic and amicrofilaremic L. loa patients.49
Immunological Mechanisms in L. loa Infection
Protective Immunity in Loiasis
Immuno-epidemiology studies of human loiasis have indicated the possible existence of protective immunity among populations exposed to L. loa. First, it was observed that 70% of individuals with loiasis are amicrofilaremic and 30% are microfilaremic.50 This observation was confirmed by a longitudinal follow-up study of a rural population over 1 year.51 A similar observation was made in Cameroon after a 23-year follow-up.52 Recent reports from urban areas state that despite the low prevalence, the distribution of microfilaremic versus amicrofilaremic individuals remains the same.33 These reports show that despite the spatial variability in prevalence, the distribution of parasitological status remains the same. The non-cumulative disease aspect of loiasis is confirmed by the fact that in Cameroon, after a 23-year follow-up, only the prevalence of L. loa increased but not the intensity of blood microfilariae. Genetic factors have been suggested to influence the susceptibility or resistance to parasitic infections. In loiasis, an association was found between the mother’s microfilaremic status and her offspring, but none with the father. This study suggests the existence of a dominant major gene predisposing individuals to a microfilaremic status.53 However, whether this potential gene or locus has a link to the major histocompatibility complex (MHC) remains unknown. The possible prenatal sensitization of offspring does not seem to be a common phenomenon, since there is no obvious transplacental passage of L. loa antigen, based on observations that there is no specific synthesis of IgE by newborns.54 Second, a role of the immune response is suggested by the fact that Ig levels are generally elevated in amicrofilaremic individuals compared to microfilaremic individuals, both from non-endemic and endemic areas.6 A striking observation was that elevated levels of specific IgG1 were inversely correlated with the density of microfilariae when antigen from infective larvae was used,55 while specific IgG1 and IgG3 contributed to the effector mechanism for killing L. loa larvae.56 This assertion is substantiated by a study showing that the serum of amicrofilaremic individuals kills microfilariae via a mechanism involving complement and antibodies called “antibody-dependent cell-mediated cytotoxicity (ADCC)”.56 Furthermore, a new report based on MALDI-TOF mass spectrometry of microfilaria protein led to the hypothesis of the existence of clonal diversity in L. loa microfilariae.57 Its relationship to the fatal side effects observed in individuals with heavy microfilaremic load and its association with clinical symptoms need to be investigated.
Cross-Linking Mechanism to Combat Allergy in Loiasis
Allergic diseases are increasing worldwide, particularly in developed countries where parasites are scarce; by contrast, these allergic diseases are less prevalent in under-developed regions, despite the abundance of parasites in the latter. It has been suggested that hygiene conditions may limit allergic manifestations in individuals exposed to parasites.58 In L. loa endemic countries, allergic diseases do not seem to be prevalent. Although no specific study has been conducted on the topic,59 a noteworthy observation was made in this area: The levels of specific IgG4 – the less distributed subclass of IgG in the human body – are elevated and total IgE is also elevated in loiasis.60 This elevation in IgG4 levels has been documented in several cases of chronic exposure to different stimuli, eg, in bee-keepers and for different parasites, including other filarial disease. Competition between IgG4 and IgE for the same epitope has been reported in filarial infection.60 Among other properties of IgG4, they can compete for fixation on the same site as IgE on mast cells and eosinophils.61,62 Furthermore, IgG4 can inhibit complement activation by other antibodies,63 but IgG4 cannot induce ADCC. Results from histamine release assays using lymphatic filarial-infected sera show that depletion of IgG4 increases the level of histamines.64 In parallel, studies have shown a strong allergic reaction and elevated level of eosinophils in temporary residents compared with permanent residents of L. loa endemic areas.65 Elevated levels of IgG4 as well as IgE isotypes in L. loa have been reported.60 However, the significance of this phenomenon is not well understood, and we hypothesized that the elevation of IgG4 and IgE may contribute toward decreasing allergic reactions through a mechanism of cross-linkage between IgE and IgG4 via L. loa antigens (Figure 3).59 It is known that an immunoreceptor tyrosine-based activation motif/immunoreceptor tyrosine-based inhibitory motif (ITAM/ITIM) exists in immune cells, including eosinophils, neutrophils, natural killer (NK) cells, macrophages, and dendritic cells. We posited that inhibition of cell activation by ITIM followed the cross-linkage between IgE/IgG4 (via L. loa antigens) and fixation on FcεRI by IgE and on FcγRII by IgG4, resulting in the absence of allergic reaction.59 The process involves the phosphorylation of tyrosine residues in protein or inositol lipids followed by activation of the signaling cascade by recruiting and phosphorylating additional signal molecules. The process may start when cells are activated, and the ITAM undergoes tyrosine phosphorylation and recruits signaling kinase. Inhibition of cell activation starts when the ITIM undergoes tyrosine phosphorylation and recruits cellular phosphatases such as those containing inositol phosphatase (SHP-1) and SHP-2; these phosphatases dephosphorylate the ITAM and suppress cellular activation.
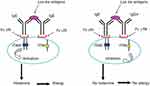 |
Figure 3 Potential mechanism of blocking allergic reactions in loiasis. Cross-linkage between IgE/IgG4 (via L. loa antigens) and fixation on FcεRI (IgE) and FcγRII by IgG4, resulting in the absence of allergic reaction.54 |
Mechanism of Anergy in Loiasis
The immune response between individuals with different status of L. loa infection was compared and a diminished immune response was observed in those harboring microfilariae. Knowing that the synthesis of immunoglobulin is under the control of T helper (Th) subsets and some cytokines, further studies were conducted to compare the cellular immune response of microfilaremic and amicrofilaremic individuals. It was found that proliferation was high in the amicrofilaremic group compared with the microfilaremic group when using microfilarial and adult antigens. A subset of cytokines was elevated in the amicrofilaremic group compared with the microfilaremic group, dominated by both Th1 cytokines (interleukin [IL]-2, interferon [IFN]-gamma) and Th2 cytokines (IL-4, IL-5).66 This diminished proliferation of cells and cytokines in the L. loa microfilaremic group seems to be due to the mechanism of anergy, which is characterized by a lack of appropriate co-stimulation signals. The fact that co-stimulator molecule B7 is produced may signify that the presentation of filarial antigens was made by nonprofessional antigen-presenting cells (APC) or via an interaction between CTLA-4 and B7 (Figure 4).
Regulation of Immune Response to L. loa
All the aforementioned mechanisms could not work without an ongoing regulatory process. Although this aspect has not been studied in loiasis as was done for other filariae or nematodes, the assertion is backed by several findings from human and animal models of L. loa; for example, human L. loa as the most chronic helminth infection is characterized by elevated levels of specific IgG4 subclass.35 There is a hyporesponsiveness of cellular immune response in individuals harboring microfilariae.66 This hyporesponse is demonstrated by the low proliferative level of peripheral blood mononuclear cells (PBMC) against adult and microfilarial antigens in microfilaremic individuals. Another striking finding is the synthesis of cytokines. In loiasis, cytokine synthesis is dominated by the secretion of IFN-gamma, IL-2, IL-4, IL-5, and IL-10.66 This profile suggests an imbalance toward the Th2 subset of lymphocytes in amicrofilaremic individuals. It is known that IL-10 and IgG4 are produced at a high concentration in L. loa infection. IL-10 and regulatory T cells promote the production of IgG4 by B cells,67 and the induction of IgG4 by regulatory T cells CD4(+) CD25(+) FOXP3(+) has been reported.68 Based on these observations, it can be hypothesized that a similar phenomenon may occur in loiasis. Thus, microfilaremic loiasis induces tolerogenic APC, which in turn produce IL-10, TGF-beta, regulatory T cell (Treg), CD4(+) CD25(+) FOXP3(+) Treg, and regulatory type 1 (Tr1) cells, which interact with B cells to enhance the production of IgG4. On the other hand, in individuals infected with adult worms but not with microfilariae in the peripheral blood (amicrofilaremic), antigen may be presented by immunocompetent APC with the production of IL-12, IFN-gamma, and IL-4 characteristic of Th1 Th2, Th17. These cytokines act on B cells to produce IgG1, IgG2, IgG3, and IgE and therefore induce effector mechanisms responsible for the killing of adult, microfilaria or infective larvae (third-stage larvae, L3). These hypotheses are based on the study of other filarial infections and need to be confirmed or disproved for loaisis.69,70
Mechanisms of Immune Evasion by L. loa
Several mechanisms of immune evasion by the parasite have been described. These range from antigenic disguise, stage-specific antigens, molecular mimicry modification or suppression of host immunological factors, complement evasion, modulation of immune complex formation, or suppression of host immune responses.71 The coating of human protein on the surface of the parasite is one of the most well-known mechanisms of escaping the human immune system by helminths, especially in the larval stage of the filariae called “microfilariae.” In their study of loiasis, Egwang et al (1988) discovered human albumin on the filarial sheath.72 The mechanism, which has been clearly described, is related to complement. Complement comprises a group of 35 proteins circulating or attached to the cell membrane. There are secreted by the liver and activated as a cascade through the cleavage of major molecules. Complement plays a role in innate and adaptive immunity. There are three ways to activate complement: the classic pathway, an alternative pathway, and the lectin pathway; the activation of complement is controlled by regulatory proteins, among them factor H. It has been demonstrated in vivo that L. loa microfilariae can evade complement binding in vivo by factor H acquired on their sheath. The microfilariae can block complement activation using the complement regulatory factor H and c4b-binding protein acquired in the circulating blood.73
Cross-Reactivity with Antigens of Other Filariae
Cross-reactive antigens are widespread among nematodes, even with other organisms. The major disadvantage of this is the lack of specificity of diagnostic tests used to identify these infections, especially in the case of mixed infections in endemic zones. Most cross-reactivity is due to conformational epitopes or common epitopes.42,74–77 However, some cross-reactive antigens may be useful for protective immunity. In this regard, studies have been conducted to show that there are similarities between L. loa and lymphatic filariasis, but this similarity is linked to the peptide backbone and not to the post-translational structure.78 The implications of this observation are very important for public health, because in areas of co-endemicity between L. loa and lymphatic filariasis, the distribution of the latter has to be re-evaluated before any implementation of control programs. This observation may also be useful for immunoprophylaxis.
Possible Negative Effect of Loiasis on Immune Response of Co-Infected Human Host
L. loa infection occurs in human hosts susceptible to others parasites, bacteria, and viruses. This means that the immune system faces several challenges simultaneously, and therefore its response may be influenced by the environment created by the poly-infection. For L. loa, it was shown that transmission also affects this process, such that in areas of high transmission intensity of L. loa there is a low proliferation of PBMC as opposed to low-transmission areas where cell proliferation is high but cytokines IL-4, IL-5, IL-10, and IFN-gamma do not seem to vary significantly. This trend in cell reactions was also seen when cells were stimulated by purified protein derivative under different transmission intensity.79 This result suggests that immune responses against L. loa antigens have an effect on nonspecific antigens as well; therefore, it can be hypothesized that some vaccines administered in L. loa-intensive transmission zones may not be effective.80 One of the most striking cases of co-infection reported was that between L. loa and human T lymphotropic virus type 1 (HTLV1). In this case, the results suggest that L. loa microfilaria carriage may affect the carriage of HTLV1 and probably the outcome of leukemia or of the neurological symptoms and encephalitis seen in loiasis. Whether the hypermicrofilarial or proviral load of HTLV1 serves as trigger or co-factor to induce leukemia or encephalitis has not been studied. The mechanisms underlying this relationship need to be elucidated.81
Contribution of Animal Models to the Study of Immune Mechanisms in Loiasis
Studies with animal models have helped to confirm or disprove some observations from sero-epidemiological research. Animal models have the advantage of offering conditions of a controlled infection, and the course of infection can be examined step by step. In this context, most of the studies conducted recently have been carried out with nonhuman primates. One aspect studied was the elucidation of the mechanisms underlying encephalitis, a fatal side effect of loiasis that remains unexplained to date, with some believing it is an allergic reaction. In a baboon model of hypermicrofilaremia,29 administration of ivermectin resulted in a drop in hemoglobin, and a post-mortem examination showed hemorrhages in the brain, lungs, and heart of the animal, as well as degeneration of microfilariae in small vessels, with an increased presence of eosinophils and inflammatory response in many organs.82 An experimental model was developed with Mandrillus sphinx and similarities to human infection were found with cases of amicrofilaremic individuals. Serum samples from these animals showed the same reaction as their human counterparts by the killing of microfilariae, suggesting that the effector mechanism involved in killing microfilariae requires Ig and eosinophils or others immune cells.83 It has also been suggested that the regimen and dose of L3 inoculated may have an impact on the microfilaria density.84 An attempt was made to evaluate protective immunity using this model with irradiated larvae;85 an elevated level of antibodies was found,86 correlating with the delay in appearance of microfilarial peak. Moreover, 21-kDa antigens were identified in microfilaria extract and 20-kDa antigens for L3.85 Cell-mediated immune responses were evaluated with the same model and the lack of a response in individuals with microfilariae was confirmed. The study suggests that the appearance of microfilariae is characterized by decreasing levels of IL-12, known to act synergistically with the B7–CD28 superfamily and to promote cytokine and cell proliferation. By contrast, IL-10, an inhibitor of IL-12, was found to be increased during the appearance of microfilariae.87 It was hypothesized that a massive release of microfilariae promotes non-active B cells, which are non-professional APC. As a consequence, a state of anergy occurs in microfilaremic individuals (Figure 4). A clear involvement of cytokines in response to L. loa infection was shown in a mouse model.88 This model demonstrated that the lack of IL-4R and IL-5 plays a critical role in host resistance in Balb/c mice, since larval development continued up to 70 days in these mice, constituting the longest period compared with other genetically modified mice. Furthermore, a recent study of Balb/c mice has demonstrated that different developmental stages of L. loa can induce protection against reinfection.89
Molecular Targets in L. loa Parasites
The identification of antigens reacting with host immunoglobulin (Ig) and host cells was not well established in adult worms, microfilariae, or infective larvae. Studies have been conducted for the identification and localization of somatic, surface, or excreted/secreted antigens. Researchers have found molecules with sizes varying from 200 kDa to 10 kDa in adult worms, microfilariae, and L3, and some of these antigens were preferentially recognized by defined groups such as amicrofilaremic groups in areas of endemic control, while this recognition was weak in microfilaremic groups. Diversity in Ig target antigens has also been reported.90 In addition, immunodominant surface antigens from adult worms and microfilariae were identified;13,91 while the glycosylated nature of the adult 30–31-kDa surface antigens was shown, it appears that the microfilarial 23-kDa major surface antigen was not glycosylated. At this stage, it is difficult to further characterize these antigens due to a lack of abundant parasite material, and therefore cloning represents a good alternative for overcoming this problem. A genomic bank derived from adult genomic DNA was made with vector λgt 11 and screened with amicrofilaremic sera from Gabon.92 One major clone from this genomic bank appears to be a long repeat gene of 1700 bp without introns. Structural analysis of this gene revealed that it is composed of five repeat sequences designated R1, R2, R3, R4, R5 with 30–100% homology to the 15-kDa gene of Ascaris.46 This 15-kDa gene is claimed to be the major allergen in this species; the sub-cloning and expression of the L. loa R3 repeat generated a recombinant protein, which shows that it was antigenic and immunogenic.93 Another approach was used to clone the major sheath protein of L. loa microfilariae.78 This approach generated a molecule with a theoretical molecular weight of 18 kDa, rich in valine (Figure 5). Generating genes or L. loa molecules is now facilitated by the availability of the L. loa genome.94 This genome comprises six chromosomes, among them five autosomes and one sex chromosome. There are 14,907 genes, half of which have a defined function. It is interesting to note that according to this genome, L. loa is able to synthesize some metabolites by itself, while other filarial parasites require the endosymbiont Wolbachia, which does not occur in loiasis.95
 |
Figure 5 Three-dimensional Loa loa microfilaria sheath protein generated with the Swiss-Prot bioinformatics program. The molecular weight is 17.225 kDa, with a PI of 5.80 containing a valine-rich region, leucine slipper, phosphorylation site, myristoylation site, and glycosylation site, with alternating hydrophobic and hydrophilic regions.57 |
Outlook: Concomitant Immunity as Key in the Development of a Potential Protective Strategy
There is ample evidence in the field showing that protective immune responses are active in L. loa-exposed populations. First, a follow-up study of exposed individuals in Cameroon over 23 years reported that the prevalence but not the density of L. loa microfilariae in the blood increases over time.52 This suggests that the new larvae did not succeed in establishing new adult worms. However, in onchocerciasis no evidence was found that more adult worms would translate into increased microfilarial density. Therefore, the corollary is also not supported by any evidence. Density-dependent processes are ongoing to regulate the microfilarial density, and the most notable one results in the infected but amicrofilaremic status.96
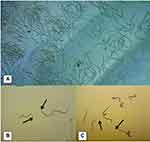
Second, it was shown that microfilariae increase with age up to adulthood and then start to decrease in older age, while simultaneously specific IgG4 continues to increase at an older age, suggesting that there is a continuous transmission of larvae and the presence of old resident adult worms with decreased production of microfilariae97 (Figure 6). Third, serum from amicrofilaremic individuals can kill microfilariae via a mechanism involving antibodies and eosinophils cells.56 Fourth, several molecules seem to be the preferential target of amicrofilaremic antibodies but not antibodies from microfilaremic individuals.91 All these observations mean that any strategy aiming to prevent L. loa infection should target either defined molecules or larvae (microfilariae or L3). Most studies have been conducted with adult worms or microfilariae, but few have focused on L3-stage individuals. Although obtaining biological material from L. loa is very difficult, we have developed methods that facilitate the collection of pure microfilariae using Percoll from hypermicrofilaremic individuals (Figure 7), and infective L3 (Figure 8) from naturally infected Chrysops using the Baerman technique (Figure 9). The availability of the L. loa genome today94 combined with the availability of larvae paves the way for a vaccine strategy. All these arguments are boosted by advances in other filarial infections where a strategy toward the development of immunoprophylactic tools is being developed using either multiple antigens or larval antigens.98
 |
Figure 6 Indices of concomitant immunity. Microfilariae and specific IgG4 prevalence plotted against age. As shown, both increase up to a certain age, after which microfilariae start decreasing while specific IgG4 remains high, suggesting a continuous stimulation either by adult resident worms or new infective L3 without establishment of a new infection. Note: Reproduced from Djikeussi ED, Akue JP. Age-dependent prevalence of Loa loa amicrofilaremia and microfilaremia status as defined by two markers: microfilaria and specific IgG4. Afr J Biotechnol. 2014;13(4):593–597.97 |
 |
Figure 7 Isolation and purification of microfilariae by Percoll gradients. Iso-osmotic Percoll was prepared by adding 9 parts (v/v) Percoll to RPMI-1640 MEDIUM 10x (SIP), and adding 2 mL gradient solution of 40%, 50%, 65% SIP in a 15-mL polystyrene tube, respectively, over the layer of these gradient solutions. Subsequently, 2 mL whole blood from an infected individual was added and centrifuged at 1000 g for 20 min. Layers 3 and 4 containing microfilariae were isolated and passed through a 5-µm filter. The motile microfilariae were then released in RPMI-1640 MEDIUM 1x (Van Hoegaerden and Ivanoff, 1986).57 These microfilariae are a source of crude antigens and genomic DNA. |
Conclusion
Much progress has been made in the field of L. loa immunobiology. The data gathered to date have led to the development of sensitive and specific diagnostic methods, some of them under evaluation to be used as point-of-care techniques. Moreover, a better definition of the distribution of the parasite and the risk factors for fatal side effects has led to improved control strategies in co-endemic regions for L. loa and O. volvulus. However, additional approaches based on immunoprophylaxis, which can impede L3 developing into adult worms, will be an asset for the prevention of new infections.
Acknowledgments
This work was supported by the International Center for Medical Research, Franceville (CIRMF), Gabon.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Zouré HG, Wanji S, Noma M, et al. The geographic distribution of Loa loa in Africa: results of large-scale implementation of the Rapid Assessment Procedure for Loiasis (RAPLOA). PLoS Negl Trop Dis. 2011;5(6):e1210. doi:10.1371/journal.pntd.0001210
2. UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases. Guidelines for Rapid Assessment of Loa Loa. World Health Organization; 2002.
3. Bouchaud O, Matheron S, Loarec A, et al. Imported loiasis in France: a retrospective analysis of 167 cases with comparison between sub-Saharan and non sub-Saharan African patients. BMC Infect Dis. 2020;20(1):63. doi:10.1186/s12879-019-4740-6
4. Boussinesq M. Loiasis. Ann Trop Med Parasitol. 2006;100(8):715–731. doi:10.1179/136485906X112194
5. Peters W. Manson’s Tropical Diseases. Trans R Soc Trop Med Hyg. 1996;90(2):207–208.
6. Klion AD, Massougbodji A, Sadeler BC, Ottesen EA, Nutman TB. Loiasis in endemic and nonendemic populations: immunologically mediated differences in clinical presentation. J Infect Dis. 1991;163(6):1318–1325. doi:10.1093/infdis/163.6.1318
7. Carme B, Danis M, Gentilini M. Traitement de la filariose à Loa loa; complications, résultats. A propos de 100 observations. Med Mal Infect. 1982;13:184–188. doi:10.1016/S0399-077X(82)80063-2
8. Gantois N, Rapp C, Gautret P, et al. Imported loiasis in France: a retrospective analysis of 47 cases. Travel Med Infect Dis. 2013;11(6):366–373. doi:10.1016/j.tmaid.2013.08.005
9. Churchill DR, Morris C, Fakoya A, Wright SG, Davidson RN. Clinical and laboratory features of patients with loiasis (Loa loa filariasis) in the U.K. J Infect. 1996;33(2):103–109. doi:10.1016/s0163-4453(96)93005-4
10. Gobbi F, Postiglione C, Angheben A, et al. Imported loiasis in Italy: an analysis of 100 cases. Travel Med Infect Dis. 2014;12(6 Pt B):713–717. doi:10.1016/j.tmaid.2014.07.004
11. Herrick JA, Metenou S, Makiya MA, et al. Eosinophil-associated processes underlie differences in clinical presentation of loiasis between temporary residents and those indigenous to Loa-endemic areas. Clin Infect Dis. 2015;60(1):55–63. doi:10.1093/cid/ciu723
12. Lipner EM, Law MA, Barnett E, et al. Filariasis in travelers presenting to the GeoSentinel Surveillance Network. PLoS Negl Trop Dis. 2007;1(3):e88. doi:10.1371/journal.pntd.0000088
13. Pinder M, Dupont A, Egwang TG. Identification of a surface antigen on Loa loa microfilariae the recognition of which correlates with the amicrofilaremic state in man. J Immunol. 1988;141(7):2480–2486.
14. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. doi:10.1016/S0140-6736(18)32279-7
15. Veletzky L, Hergeth J, Stelzl DR, et al. Burden of disease in Gabon caused by loiasis: a cross-sectional survey. Lancet Infect Dis. 2020;20(11):1339–1346. doi:10.1016/S1473-3099(20)30256-5
16. Cruel T, Arborio M, Schill H, et al. Néphropathie et filariose à Loa loa. A propos d’un cas de réaction adverse à la prise d’ivermectine [Nephropathy and filariasis from Loa loa. Apropos of 1 case of adverse reaction to a dose of ivermectin]. Bull Soc Pathol Exot. 1997;90(3):179–181.
17. Mackenzie C, Geary T, Prichard R, Boussinesq M. Where next with Loa loa encephalopathy? Data are badly needed. Trends Parasitol. 2007;23(6):237–238. doi:10.1016/j.pt.2007.04.007
18. Buell KG, Whittaker C, Chesnais CB, et al. Atypical Clinical Manifestations of Loiasis and Their Relevance for Endemic Populations. Open Forum Infect Dis. 2019;6(11):ofz417. doi:10.1093/ofid/ofz417
19. Chesnais CB, Takougang I, Paguélé M, Pion SD, Boussinesq M. Excess mortality associated with loiasis: a retrospective population-based cohort study. Lancet Infect Dis. 2017;17(1):108–116. doi:10.1016/S1473-3099(16)30405-4
20. Thomson MC, Obsomer V, Kamgno J, et al. Mapping the distribution of Loa loa in Cameroon in support of the African Programme for Onchocerciasis Control. Filaria J. 2004;3(1):7. doi:10.1186/1475-2883-3-7
21. Ufomadu GO, Nwoke BE, Akoh JI, et al. The occurrence of loiasis, mansonellosis and wuchereriasis in the Jarawa River Valley, central Nigeria. Acta Trop. 1990;48(2):137–147. doi:10.1016/0001-706x(90)90053-3
22. Wanji S, Tendongfor N, Esum M, Atanga SN, Enyong P. Heterogeneity in the prevalence and intensity of loiasis in five contrasting bioecological zones in Cameroon. Trans R Soc Trop Med Hyg. 2003;97(2):183–187. doi:10.1016/s0035-9203(03)90114-3
23. Takougang I, Meli J, Lamlenn S, Tatah PN, Ntep M. Loiasis–a neglected and under-estimated affliction: endemicity, morbidity and perceptions in eastern Cameroon. Ann Trop Med Parasitol. 2007;101(2):151–160. doi:10.1179/136485907X154511
24. Metzger WG, Mordmüller B. Loa loa-does it deserve to be neglected? Lancet Infect Dis. 2014;14(4):353–357. doi:10.1016/S1473-3099(13)70263-9
25. Chippaux JP, Boussinesq M, Gardon J, Gardon-Wendel N, Ernould JC. Severe adverse reaction risks during mass treatment with ivermectin in loiasis-endemic areas. Parasitol Today. 1996;12(11):448–450. doi:10.1016/0169-4758(96)40006-0
26. Gardon J, Gardon-Wendel N, Demanga-Ngangue KJ, Chippaux JP, Boussinesq M. Serious reactions after mass treatment of onchocerciasis with ivermectin in an area endemic for Loa loa infection. Lancet. 1997;350(9070):18–22. doi:10.1016/S0140-6736(96)11094-1
27. Duke BO. Studies on loiasis in monkeys. II.–The population dynamics of the microfilariae of Loa in experimentally infected drills (Mandrillus leucophaeus). Ann Trop Med Parasitol. 1960;54:15–31.
28. Orihel TC, Moore PJ. Loa loa: experimental infection in two species of African primates. Am J Trop Med Hyg. 1975;24(4):606–609. doi:10.4269/ajtmh.1975.24.606
29. Wanji S, Eyong EE, Tendongfor N, et al. Parasitological, Hematological and Biochemical Characteristics of a Model of Hyper-microfilariaemic Loiasis (Loa loa) in the Baboon (Papio anubis). PLoS Negl Trop Dis. 2015;9(11):e0004202. doi:10.1371/journal.pntd.0004202
30. Senyonjo L, Oye J, Bakajika D, et al. Factors Associated with Ivermectin Non-Compliance and Its Potential Role in Sustaining Onchocerca volvulus Transmission in the West Region of Cameroon. PLoS Negl Trop Dis. 2016;10(8):e0004905. doi:10.1371/journal.pntd.0004905
31. Kim YE, Remme JH, Steinmann P, Stolk WA, Roungou JB, Tediosi F. Control, elimination, and eradication of river blindness: scenarios, timelines, and ivermectin treatment needs in Africa. PLoS Negl Trop Dis. 2015;9(4):e0003664. doi:10.1371/journal.pntd.0003664
32. Verver S, Walker M, Kim YE, et al. How Can Onchocerciasis Elimination in Africa Be Accelerated? Modeling the Impact of Increased Ivermectin Treatment Frequency and Complementary Vector Control. Clin Infect Dis. 2018;66(suppl_4):S267–S274. doi:10.1093/cid/cix1137
33. Roland D. Prevalence of Loa loa and Mansonella perstans detection in urban areas of Southeast Gabon. J Parasitol Vector Biol. 2020;12(2):44–51. doi:10.5897/jpvb2020.0390
34. Akue JP, Eyang-Assengone ER, Dieki R. Loa loa infection detection using biomarkers: current perspectives. Res Rep Trop Med. 2018;9:43–48. doi:10.2147/RRTM.S132380
35. Akue JP, Egwang TG, Devaney E. High levels of parasite-specific IgG4 in the absence of microfilaremia in Loa loa infection. Trop Med Parasitol. 1994;45(3):246–248.
36. Klion AD, Vijaykumar A, Oei T, Martin B, Nutman TB. Serum immunoglobulin G4 antibodies to the recombinant antigen, Ll-SXP-1, are highly specific for Loa loa infection. J Infect Dis. 2003;187(1):128–133. doi:10.1086/345873
37. Burbelo PD, Ramanathan R, Klion AD, Iadarola MJ, Nutman TB. Rapid, novel, specific, high-throughput assay for diagnosis of Loa loa infection. J Clin Microbiol. 2008;46(7):2298–2304. doi:10.1128/JCM.00490-08
38. Pedram B, Pasquetto V, Drame PM, et al. A novel rapid test for detecting antibody responses to Loa loa infections. PLoS Negl Trop Dis. 2017;11(7):e0005741. doi:10.1371/journal.pntd.0005741
39. Gobbi F, Buonfrate D, Boussinesq M, et al. Performance of two serodiagnostic tests for loiasis in a Non-Endemic area. PLoS Negl Trop Dis. 2020;14(5):e0008187. doi:10.1371/journal.pntd.0008187
40. Jaoko WG. Loa loa antigen detection by ELISA: a new approach to diagnosis. East Afr Med J. 1995;72(3):176–179.
41. Walker-Deemin A, Ferrer A, Gauthier F, Kombila M, Richard-Lenoble D. Identification and specificity of a 38 kDa Loa loa antigenic fraction in sera from high-microfilaraemic Gabonese patients. Parasitol Res. 2004;92(2):128–132. doi:10.1007/s00436-003-1024-1
42. Wanji S, Amvongo-Adjia N, Koudou B, et al. Cross-Reactivity of Filariais ICT Cards in Areas of Contrasting Endemicity of Loa loa and Mansonella perstans in Cameroon: implications for Shrinking of the Lymphatic Filariasis Map in the Central African Region. PLoS Negl Trop Dis. 2015;9(11):e0004184. doi:10.1371/journal.pntd.0004184
43. Wanji S, Amvongo-Adjia N, Njouendou AJ, et al. Further evidence of the cross-reactivity of the Binax NOW® Filariasis ICT cards to non-Wuchereria bancrofti filariae: experimental studies with Loa loa and Onchocerca ochengi. Parasit Vectors. 2016;9:267. doi:10.1186/s13071-016-1556-8
44. Drame PM, Meng Z, Bennuru S, Herrick JA, Veenstra TD, Nutman TB. Identification and Validation of Loa loa Microfilaria-Specific Biomarkers: a Rational Design Approach Using Proteomics and Novel Immunoassays. mBio. 2016;7(1):e02132–15. doi:10.1128/mBio.02132-15
45. Touré FS, Bain O, Nerrienet E, et al. Detection of Loa loa-specific DNA in blood from occult-infected individuals. Exp Parasitol. 1997;86(3):163–170. doi:10.1006/expr.1997.4168
46. Ajuh PM, Akue JP, Boutin P, Everaere S, Egwang TG. Loa loa: structural diversity of a 15-kDa repetitive antigen. Exp Parasitol. 1995;81(2):145–153. doi:10.1006/expr.1995.1103
47. Fink DL, Kamgno J, Nutman TB. Rapid molecular assays for specific detection and quantitation of Loa loa microfilaremia. PLoS Negl Trop Dis. 2011;5(8):e1299. doi:10.1371/journal.pntd.0001299
48. Fernández-Soto P, Mvoulouga PO, Akue JP, et al. Development of a highly sensitive loop-mediated isothermal amplification (LAMP) method for the detection of Loa loa. PLoS One. 2014;9(4):e94664. doi:10.1371/journal.pone.0094664
49. Rush EAE. Analyse des complexes immuns circulants et des protéines urinaires des personnes infectées par Loa loa à l’aide des sous-classes d’immunoglobuline G1 et G4. [Mémoire de Master]. Burkina Faso: Université de Ouagadougou; 2015.
50. Dupont A, Zue-N’dong J, Pinder M. Common occurrence of amicrofilaraemic Loa loa filariasis within the endemic region. Trans R Soc Trop Med Hyg. 1988;82(5):730. doi:10.1016/0035-9203(88
51. Van Hoegaerden M, Chabaud B, Akue JP, Ivanoff B. Filariasis due to Loa loa and Mansonella perstans: distribution in the region of Okondja, Haut-Ogooué Province, Gabon, with parasitological and serological follow-up over one year. Trans R Soc Trop Med Hyg. 1987;81(3):441–446. doi:10.1016/0035-9203(87)90163-5
52. Mogoung-Wafo AE, Nana-Djeunga HC, Domche A, et al. Prevalence and intensity of Loa loa infection over twenty-three years in three communities of the Mbalmayo health district (Central Cameroon). BMC Infect Dis. 2019;19(1):146. doi:10.1186/s12879-019-3776-y
53. Eyebe S, Sabbagh A, Pion SD, et al. Familial Aggregation and Heritability of Loa loa Microfilaremia. Clin Infect Dis. 2018;66(5):751–757. doi:10.1093/cid/cix877
54. Van Hoegaerden M, Akué JP. Lack of evidence for transplacental transfer of microfilarial antigens in filariasis due to Loa loa and Mansonella perstans. Trop Med Parasitol. 1986;37(2):121–123.
55. Akue JP, Hommel M, Devaney E. High levels of parasite-specific IgG1 correlate with the amicrofilaremic state in Loa loa infection. J Infect Dis. 1997;175(1):158–163. doi:10.1093/infdis/175.1.158
56. Pinder M, Leclerc A, Everaere S. Antibody-dependent cell-mediated immune reactions to Loa loa microfilariae in amicrofilaraemic subjects. Parasite Immunol. 1992;14(5):541–556. doi:10.1111/j.1365-3024.1992.tb00027.x
57. Elsa Rush Eyang Assengone. Caractérisation moléculaire et immunologique des antigènes de la microfilaire de Loa loa [Thèse de Doctorat]. Gabon: Ecole Doctorale Régionale en Infectiologie Tropicale de Franceville (EDR); 2021.
58. Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296(5567):490–494. doi:10.1126/science.296.5567.490
59. Paul AJ. Loa loa Pathogenesis in Humans. In: Singh SK editor. Human Emerging and Re-Emerging Infections: Viral & Parasitic Infections (Pp.12). John Wiley & Sons, Inc; 2015:441–452. doi:10.1002/9781118644843
60. Akue JP, Hommel M, Devaney E. Markers of Loa loa infection in permanent residents of a loiasis endemic area of Gabon. Trans R Soc Trop Med Hyg. 1996;90(2):115–118. doi:10.1016/s0035-9203(96)90105-4
61. Zola H, Garland LG, Cox HC, Adcock JJ. Separation of IgE from IgG subclasses using staphylococcal protein A. Int Arch Allergy Appl Immunol. 1978;56(2):123–127. doi:10.1159/000232014
62. Aalberse RC, Stapel SO, Schuurman J, Rispens T. Immunoglobulin G4: an odd antibody. Clin Exp Allergy. 2009;39(4):469–477. doi:10.1111/j.1365-2222.2009.03207.x
63. Van der Zee JS, van Swieten P, Aalberse RC. Inhibition of complement activation by IgG4 antibodies. Clin Exp Immunol. 1986;64(2):415–422.
64. Hussain R, Poindexter RW, Ottesen EA. Control of allergic reactivity in human filariasis. Predominant localization of blocking antibody to the IgG4 subclass. J Immunol. 1992;148(9):2731–2737.
65. Nutman TB, Miller KD, Mulligan M, Ottesen EA. Loa loa infection in temporary residents of endemic regions: recognition of a hyperresponsive syndrome with characteristic clinical manifestations. J Infect Dis. 1986;154(1):10–18. doi:10.1093/infdis/154.1.10
66. Baize S, Wahl G, Soboslay PT, Egwang TG, Georges AJ. T helper responsiveness in human Loa loa infection; defective specific proliferation and cytokine production by CD4+ T cells from microfilaraemic subjects compared with amicrofilaraemics. Clin Exp Immunol. 1997;108(2):272–278. doi:10.1046/j.1365-2249.1997.d01-1010.x
67. Satoguina JS, Weyand E, Larbi J, Hoerauf A. T regulatory-1 cells induce IgG4 production by B cells: role of IL-10. J Immunol. 2005;174(8):4718–4726. doi:10.4049/jimmunol.174.8.4718
68. Meiler F, Klunker S, Zimmermann M, Akdis CA, Akdis M. Distinct regulation of IgE, IgG4 and IgA by T regulatory cells and toll-like receptors. Allergy. 2008;63(11):1455–1463. doi:10.1111/j.1398-9995.2008.01774.x
69. Metenou S, Nutman TB. Regulatory T cell subsets in filarial infection and their function. Front Immunol. 2013;4:305. doi:10.3389/fimmu.2013.00305
70. Metenou S, Dembele B, Konate S, et al. At homeostasis filarial infections have expanded adaptive T regulatory but not classical Th2 cells. J Immunol. 2010;184(9):5375–5382. doi:10.4049/jimmunol.0904067
71. Chulanetra M, Chaicumpa W. Revisiting the Mechanisms of Immune Evasion Employed by Human Parasites. Front Cell Infect Microbiol. 2021;11:702125. doi:10.3389/fcimb.2021.702125
72. Egwang TG, Dupont A, Akué JP, Pinder M. Biochemical and immunochemical characterization of surface and excretory-secretory antigens of Loa loa microfilariae. Mol Biochem Parasitol. 1988;31(3):251–261. doi:10.1016/0166-6851(88
73. Haapasalo K, Meri T, Jokiranta TS. Loa loa Microfilariae evade complement attack in vivo by acquiring regulatory proteins from host plasma. Infect Immun. 2009;77(9):3886–3893. doi:10.1128/IAI.01583-08
74. Hertz MI, Nana-Djeunga H, Kamgno J, et al. Identification and characterization of Loa loa antigens responsible for cross-reactivity with rapid diagnostic tests for lymphatic filariasis. PLoS Negl Trop Dis. 2018;12(11):e0006963. doi:10.1371/journal.pntd.0006963
75. Bakajika DK, Nigo MM, Lotsima JP, et al. Filarial antigenemia and Loa loa night blood microfilaremia in an area without bancroftian filariasis in the Democratic Republic of Congo. Am J Trop Med Hyg. 2014;91(6):1142–1148. doi:10.4269/ajtmh.14-0358
76. Pion SD, Montavon C, Chesnais CB, et al. Positivity of Antigen Tests Used for Diagnosis of Lymphatic Filariasis in Individuals Without Wuchereria bancrofti Infection But with High Loa loa Microfilaremia. Am J Trop Med Hyg. 2016;95(6):1417–1423. doi:10.4269/ajtmh.16-0547
77. Lal RB, Ottesen EA. Phosphocholine epitopes on helminth and protozoal parasites and their presence in the circulation of infected human patients. Trans R Soc Trop Med Hyg. 1989;83(5):652–655. doi:10.1016/0035-9203(89)90387-8
78. Akue JP. Immunological and Molecular Similarities between Human Filarial Loa loa and Brugia pahangi Antigens. Microbiology. 2020;16(1):1–14.
79. Chatterjee S, Clark CE, Lugli E, Roederer M, Nutman TB. Filarial infection modulates the immune response to Mycobacterium tuberculosis through expansion of CD4+ IL-4 memory T cells. J Immunol. 2015;194(6):2706–2714. doi:10.4049/jimmunol.1402718
80. Akue JP, Devaney E. Transmission intensity affects both antigen-specific and nonspecific T-cell proliferative responses in Loa loa infection. Infect Immun. 2002;70(3):1475–1480. doi:10.1128/IAI.70.3.1475-1480.2002
81. Akue J-P. Coinfection of Human Filarial Loa loa, Mansonnella perstans in Human T Lymphotropic Virus Type 1 Infected Individuals in Gabon. Adv Clin Transl Res. 2020;3:100027.
82. Wanji S, Eyong EJ, Tendongfor N, et al. Ivermectin treatment of Loa loa hyper-microfilaraemic baboons (Papio anubis): assessment of microfilarial load reduction, haematological and biochemical parameters and histopathological changes following treatment. PLoS Negl Trop Dis. 2017;11(7):e0005576. doi:10.1371/journal.pntd.0005576
83. Pinder M, Everaere S, Roelants GE. Loa loa: immunological responses during experimental infections in mandrills (Mandrillus sphinx). Exp Parasitol. 1994;79(2):126–136. doi:10.1006/expr.1994.1072
84. Akue JP, Makouloutou P. Influence of infective stage (L3) dose on the outcome of microfilaremia, peripheral white blood cells and humoral immune response in Loa loa experimentally infected Mandrillus sphinx. J Parasitol Vector Biol. 2016;8(4):39–46.
85. Akue JP, Dubreuil G, Moukana H. The relationship between parasitological status and humoral responses to Loa loa antigens in the Mandrillus sphinx model after immunization with irradiated L3 and infection with normal L3. Parasitology. 2001;123(Pt 1):71–76. doi:10.1017/s0031182001007910
86. Akue JP, Morelli A, Moukagni R, Moukana H, Blampain AG. Parasitological and immunological effects induced by immunization of Mandrillus sphinx against the human filarial Loa loa using infective stage larvae irradiated at 40 Krad. Parasite. 2003;10(3):263–268. doi:10.1051/parasite/2003103263
87. Leroy E, Baize S, Wahl G, Egwang TG, Georges AJ. Experimental infection of a nonhuman primate with Loa loa induces transient strong immune activation followed by peripheral unresponsiveness of helper T cells. Infect Immun. 1997;65(5):1876–1882. doi:10.1128/iai.65.5.1876-1882.1997
88. Tendongfor N, Wanji S, Ngwa JC, et al. The human parasite Loa loa in cytokine and cytokine receptor gene knock out BALB/c mice: survival, development and localization. Parasit Vectors. 2012;5:43. doi:10.1186/1756-3305-5-43
89. Chunda VC, Ritter M, Bate A, et al. Comparison of immune responses to Loa loa stage-specific antigen extracts in Loa loa-exposed BALB/c mice upon clearance of infection. Parasit Vectors. 2020;13(1):51. doi:10.1186/s13071-020-3921-x
90. Akue JP, Hommel M, Devaney E. IgG subclass recognition of Loa loa antigens and their correlation with clinical status in individuals from Gabon. Parasite Immunol. 1998;20(8):387–393. doi:10.1046/j.1365-3024.1998.00172.x
91. Egwang TG, Dupont A, Leclerc A, Akué JP, Pinder M. Differential recognition of Loa loa antigens by sera of human subjects from a loiasis endemic zone. Am J Trop Med Hyg. 1989;41(6):664–673. doi:10.4269/ajtmh.1989.41.664
92. Egwang T, Pinder M, Akue JP. Loa loa: identification of genomic DNA clones expressing recombinant antigens. Exp Parasitol. 1990;70(4):490–493. doi:10.1016/0014-4894(90)90135-y
93. Azzibrouck GB, Akue JP, Lenoble DR. Production and immunological characterization of a recombinant subunit of a Loa loa polyprotein antigen. Parasitology. 2010;137(7):1119–1128. doi:10.1017/S0031182009991740
94. Desjardins CA, Cerqueira GC, Goldberg JM, et al. Genomics of Loa loa, a Wolbachia-free filarial parasite of humans. Nat Genet. 2013;45(5):495–500. doi:10.1038/ng.2585
95. McGarry HF, Pfarr K, Egerton G, et al. Evidence against Wolbachia symbiosis in Loa loa. Filaria J. 2003;2(1):9. doi:10.1186/1475-2883-2-9
96. Duerr HP, Dietz K, Schulz-Key H, Büttner DW, Eichner M. Density-dependent parasite establishment suggests infection-associated immunosuppression as an important mechanism for parasite density regulation in onchocerciasis. Trans R Soc Trop Med Hyg. 2003;97(2):242–250. doi:10.1016/s0035-9203(03
97. Djikeussi ED, Akue JP. Age-dependent prevalence of Loa loa amicrofilaremia and microfilaremia status as defined by two markers: microfilaria and specific IgG4. Afr J Biotechnol. 2014;13(4):593–597.
98. Hess JA, Zhan B, Bonne-Année S, et al. Vaccines to combat river blindness: expression, selection and formulation of vaccines against infection with Onchocerca volvulus in a mouse model. Int J Parasitol. 2014;44(9):637–646. doi:10.1016/j.ijpara.2014.04.006
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.