Back to Journals » Clinical Interventions in Aging » Volume 16
The Gut Microbial-Derived Metabolite Trimethylamine N-Oxide and Atrial Fibrillation: Relationships, Mechanisms, and Therapeutic Strategies
Received 16 September 2021
Accepted for publication 3 November 2021
Published 30 November 2021 Volume 2021:16 Pages 1975—1986
DOI https://doi.org/10.2147/CIA.S339590
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Zhi-Ying Wu
Rui Huang,1,* Li Yan,2,* Yuhua Lei1
1Cardiovascular Disease Center, Central Hospital of Enshi Tujia and Miao Autonomous Prefecture, Enshi Clinical College of Wuhan University, Enshi Prefecture, 445000, Hubei Province, People’s Republic of China; 2Pediatrics Department, Central Hospital of Enshi Tujia and Miao Autonomous Prefecture, Enshi Clinical College of Wuhan University, Enshi Prefecture, 445000, Hubei Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yuhua Lei No. 158 Wuyang Avenue, Enshi Prefecture, Hubei Province, People’s Republic of China
Email [email protected]
Abstract: Accumulating evidence has demonstrated that gut microbial-derived metabolite trimethylamine N-oxide (TMAO) plays a crucial role in the pathogenesis of many diseases and can be served as a prognostic biomarker for several cardiovascular disorders, including arrhythmia. Recently, some studies have documented that TMAO was associated with the occurrence, progression, recurrence, and embolism risk of atrial fibrillation (AF). The activation of related inflammatory signal pathways and the cardiac sympathetic nervous system (CSNS) caused by elevated TAMO may be the underlying mechanism. It is worth noting that intervention in the metabolic pathway of TMAO may be an underlying therapeutic target of AF. In addition, standardized and individualized treatment strategies in clinical practice may be of great significance for AF patients, particularly those with high serum TMAO concentrations. However, there are also contradictions in the current research on TMAO and AF. Moreover, notwithstanding the positive preclinical and clinical findings, data supporting a direct association between TMAO and AF is a paucity. Thus, conclusive evidence from preclinical studies and multi-center randomized controlled trials to reveal the essential relationship between TMAO and AF is needy. In this review, we have attempted to summarize recent studies on TMAO and AF, highlighted the potential therapeutic strategies for AF patients, followed by a discussion on directions for future research in this field.
Keywords: trimethylamine N-oxide, TMAO, atrial fibrillation
Introduction
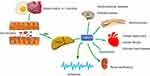
Growing evidence indicated that gut microbiota-associated mechanisms play a pivotal role in the pathogenesis of many different complex human diseases.1–4 The most well-studied agent exhibiting direct effects on arrhythmogenesis and other disorders is trimethylamine N-oxide (TMAO). This gut microbial-derived metabolite is derived from dietary choline or L-carnitine through gut microbiota, transforming choline to trimethylamine (TMA), a gas absorbed into the circulation and further converted into TMAO by flavin-containing monooxygenases (FMOs) in the liver.2,5 Previous studies reported that TMAO was related to many human diseases, including cerebrovascular diseases,6 renal insufficiency,7 and cardiovascular diseases2,4,8 such as hypertension,9 coronary heart disease,8,10 and heart failure.11 The metabolic pathway of TMAO and its adverse effects are shown in Figure 1. TMAO may also be associated with cardiovascular events and mortality, which has also been confirmed in a recent meta-analysis12 and hemodialysis sufferings.13
In addition, recent studies have begun to focus on the substantial role and pathogenetic mechanisms of intestinal microbiota and its metabolites, especially TMAO, in the field of arrhythmia and AF.14,15 Despite the positive preclinical and clinical findings, few data supported a direct link between TMAO and AF with conflicting findings. Here, we reviewed the latest research on TMAO-AF and discussed the value of TMAO as a potential therapeutic target for patients with AF.
TMAO and Risk Factors of AF
It is noteworthy that the intestinal microbiota is a dynamic ecosystem composed of symbiotic and pathogenic microorganisms, affecting the health of the host through the production of bioactive metabolites.15–18 TMAO is currently the most commonly studied intestinal microbial metabolite involved in human diseases.
Surprisingly, at present, more and more studies have found that TMAO is inextricably linked to hypertension,9 chronic obstructive pulmonary disease,19 heart failure,11,17,20 obesity,21 metabolic dysfunction,22 atherosclerosis and coronary heart diseases,9,10,23 which are known as the common risk factors for AF and motivates a renewed interest in examining the relation between TMAO and AF. However, these results have not been confirmed in AF models and prospective clinical studies, and the degree and mechanism of modifying these risk factors on AF by regulating intestinal microbes and their metabolites such as TMAO are still needed to be explored.
TMAO and AF
AF, the most common clinical cardiac rhythm disorder encountered in practice, is expected to afflict over 34 million people worldwide, is becoming markedly prevalent, and is independently associated with at least a two-fold increase in the risk of cardiovascular death.24–26
It is no exaggeration to state that AF is still a tremendously challenging problem in modern medical practice. Although there are established risk factors and scoring systems for AF, it is still a formidable hindrance to probe and elucidate the etiology of AF and seek corresponding therapeutic strategies and interventions to reduce the incidence, disability, and mortality rate. Lifestyle modification (weight loss, exercise, and change dietary habits), been reported to be correlated with alterations in gut microbiota, contributes to a rhythm control strategy and alleviates symptoms in AF sufferings.27,28
At present, more and more studies, including preclinical and clinical research, have found that TMAO was associated with the occurrence, progression, recurrence after ablation, embolism events and may serve as a potential therapeutic target of AF.15,29,30 Many observations have provided positive evidence for the relationship between TMAO and AF and explored the underlying mechanisms. However, some findings on the relationship between TMAO and AF come from small clinical observational studies with conflicting outcomes, which is unable to reveal the cause-and-effect and interactive relationship between TMAO and AF.
TMAO May Serve as a Potential Biomarker in AF
Currently, several studies suggested that TMAO was related to the occurrence, progression, and recurrence of AF after ablation (Table 1). However, some studies have reached contradictory conclusions.
 |
Table 1 Clinical Investigations into TMAO and AF |
In 2018, a study concluded that elevated serum TMAO was positively related to long-term AF in patients with or without suspected stable angina. After adjusting for latent confounding covariates, including dietary intake of TMAO precursor choline and betaine, the prospective association was independent of conventional AF risk factors.15 Thus, the circulating levels of TMAO may be a stand-alone predictor for AF incidence. These findings were further validated in a metagenomic data-mining analysis, which depicted the TMAO synthetic profiles based upon the genomic sequence analysis and clarified the vital intestinal bacteria that formed TMAO in AF patients.18
Similarly, in another exploratory analysis, researchers compared TMAO and its precursor in 56 subjects with paroxysmal AF and 22 individuals suffering from persistent AF and detected a significant relationship between TMAO levels and AF progression phenotypes without difference in precursors, which was the first time to confirmed the relationship between TMAO and the progression of AF. However, this study has some defects that need consideration when interpreting the results. Firstly, the author did not study the normal sinus rhythm subjects as a control group which may enable a causal interpretation about elevated TMAO in AF patients, and additional studies are required to corroborate the phenomenon. Secondly, this study has a relatively small research sample, and this conclusion may not necessarily apply to the general population. Lastly, patients with persistent AF have more underlying diseases, such as coronary heart disease, hypertension, and cardiac insufficiency, which will affect the results of TMAO.29
Strikingly, a study also revealed that intestinal microbiota disorder was a predictor of recurrence after AF ablation. The study recommended that intestinal microbiota disorder be included in the model for predicting AF recurrence.31 It is well established that inflammation is implicated in various physiological and pathological processes covering oxidative stress, myocardial fibrosis, and apoptosis that form AF substrate generation and predict the recurrence of AF.31–35 TMAO has been confirmed by many studies to participate in the inflammatory response in the body and activates multiple proinflammatory signal pathways.28,36–39 As a metabolite derived from intestinal flora, TAMO may also be involved in the recurrence of AF, but there is still a lack of relevant clinical studies, and scholars need to further confirm in follow-up studies.
Contrary to prior findings, in a research conducted by Büttner et al,40 they analyzed 45 AF patients and 20 matched controls and found no difference in baseline TMAO between the groups, and TMAO was not related to the progression phenotype of AF. Additionally, TMAO was measured 12–18 months after AF catheter ablation in 35 patients. Noteworthy, the TMAO levels at baseline and follow-up (3.9 ± 1.5 vs 4. 8 ± 2.8 μM, p = 0.036) were correlated (r=0.481, p=0.003).Significantly, the increase in TMAO was not related to the success of the ablation procedure (restoration of sinus rhythm). This negative correlation was inconsistent with previous studies, likely because this was a small-sample single-center study that might potentially lead to under-detection of a positive correlation between AF and TMAO levels. What’s more, additional factors affecting TMAO concentrations, such as high carnitine dietary, have not been excluded. This study has certain limitations and needs further confirmation by scholars. A bi-directional Mendelian randomization analysis also revealed that gut microbiota-dependent metabolites were not associated with cardiometabolic diseases such as AF.30 In addition, a follow-up study using two prospective case-control studies nested within the PREDIMED study cohort revealed that TMAO was not associated with AF. However, they found baseline plasma choline, betaine, and dimethylglycine levels were independently related to increased risk of both incident AF and heart failure.41
In a standard scenario, TMAO levels are affected by numerous factors such as diet, underlying diseases, lifestyles, and basic studies have found that TMAO may also be the causative agent of certain other cardiovascular diseases and neurological diseases, and these covariates may mask the authentic relationship between TMAO and AF as well as arrhythmia.42–45 In addition, most of the current studies did not consider the complexity and diversity of the structure of the intestinal microbes, such as other bacteria, viruses, and fungi, which may interfere with this association. Overall, despite an extensive body of evidence, whether TMAO is a risk factor, mediator, or bystander in the arrhythmia process remains largely unknown. More studies are called for further delineation in this subject to reach an accurate conclusion.45,46
TMAO is Related to Thrombosis and Embolism in AF Patients
Several observational studies have confirmed the association between TMAO and cerebrovascular disease.47–49 Studies have found that compared with the control group (3.9; IQR, 2.6–6.1 μM), stroke patients (5.8; IQR, 3.3–10.0μM) have higher plasma levels of TMAO.50 As such, some cardiovascular specialists have also begun to investigate the relationship between TMAO and stroke in AF patients.6 Gong et al51 studied 117 patients with rheumatic heart disease and AF and divided them into the thrombotic group (n=25) and non-thrombotic group (n=92). They found that elevated serum TMAO concentrations in patients with valvular AF were associated with platelet hyperreactivity and thrombosis risk. However, there may be a potential bias in patients selection in this small sample cohort study because rheumatic heart disease is a high-risk disease independent of AF.
Consistent with prior findings, another study revealed that high TMAO was associated with ischemic stroke in patients with AF for the first time.52 In this clinical study, compared with those with ischemic stroke, those without had remarkably lower serum TMAO concentrations (8.25 ± 1.58 µM vs 2.22 ± 0.09 µM, P< 0.01). Further analysis indicated that the best cutoff value of TMAO to distinguish between AF with or without ischemic stroke was 3.53μM. This phenomenon might be responsible for TMAO promoting atherosclerosis through alternative pathways such as activating macrophages and endothelial cells or potentiating platelet aggregation. Apart from the above, this study also showed that TMAO was closely related to the CHA2DS2-VASc score, a traditional embolization scoring system that is widely applied to assess the risk of subsequent embolism and to guide anticoagulant therapy in AF,53 and pointed out that elevated TMAO played a vital role in the identification of embolism risk for those with low-risk scores.52
Therefore, according to the current research reports, we believe that for AF patients, especially those with high TMAO levels, early intervention may be required to prevent possible thromboembolic events. One step further, perhaps it is plausible to add TMAO as a factor in the CHA2DS2-VASc scoring system.
However, the current indirect evidence on TMAO and the embolism risk of AF predominantly derived from clinical studies, and there was no multi-center prospective randomized controlled trial to confirm. In addition, due to the complex structure of the intestinal microbiota, the role of metabolites derived from microorganisms other than TAMO during thrombogenic formation remains unclear. In the future, more preclinical studies involving a variety of gut microbiota are needed to explore the mechanisms of increased thrombosis risk in-depth.
Mechanism of TMAO Leading to AF
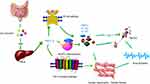
Although there are many relevant studies, the specific mechanisms of AF induced by TMAO are still indistinct.54 Up to date, five main mechanisms are believed to be the dominant causes that are involved in the pathophysiological process of TMAO-induced arrhythmia:1) activated cardiac autonomic nervous system,35,55 2) inflammation,28 3) cardiac remodeling and myocardial fibrosis,56,57 4) oxidative stress,38,39,58 5) endothelial dysfunction58 and vascular aging39 (Table 2). However, the current evidence of the mechanisms of TMAO leading to AF is primarily indirect, and additional models of AF are required to validate it. The underlying mechanisms of AF caused by TMAO are shown in Figure 2.
 |
Table 2 Main Pathophysiological Mechanisms of TMAO in AF: Directly and Indirectly |
 |
Table 3 Knowledge Gaps and Studies Needed to Be Performed |
TMAO Acts on the CSNS
Animal models demonstrated that stimulating the atrial autonomic nervous system can lead to electrical remodeling and promote the development of paroxysmal AF and arrhythmia.59–61 Some scholars have confirmed that a canine AF model induced by rapid atrial pacing in ganglionated plexi (GP) can increase the expression of nerve growth factor (NGF) and lead to atrial electrical remodeling, thereby creating a matrix for AF.62 Inspired by the study of intestinal microbes and cardiovascular diseases, scholars began to explore the relationship between TMAO and arrhythmia.
Interestingly, a study conducted by Meng et al14 showed that TMAO could further activate CSNS and initiate arrhythmia via direct way through the left stellate ganglion and indirect mechanisms by the central nervous system. Consistent with the above findings, in a study performed by Yu et al.35 TMAO was locally injected into four major anterior right GP and increased neural activity and the susceptibility of atrial to AF through changing the atrial electrophysiological properties by activating p65 NF-κB signaling pathway resulting in the expression of inflammatory cytokines, which may induce atrial rhythm disorders.
However, whether the increase in plasma TMAO levels caused by changes in dietary structure or imbalance of the intestinal microbiota is sufficient to regulate the CSNS and induce arrhythmias directly is currently unclear. In addition, the role of TMAO in the causation of this phenomenon remains largely unknown.
TMAO Leads to Cardiac Hypertrophy, Myocardial Fibrosis, and Myocardial Remodeling
Myocardial fibrosis and remodeling are the end stage of various heart diseases.11,63 Numerous studies have demonstrated that TMAO is closely correlated with heart failure. One animal research found that TMAO directly induced myocardial hypertrophy and fibrosis, and Abs treatment can reduce TMAO and attenuate myocardial remodeling.57 The study further indicated that the TGF-β1/Smad3 signaling pathway was activated in TMAO-induced cardiac hypertrophy and that SIS3, the inhibition of Smad3, could alleviate TMAO-induced cardiac remodeling.57 Other studies have led to a similar conclusion. High choline or gut microbial-dependent metabolite TMAO diet will increase the susceptibility of mice to heart failure.64 Moreover, another study revealed that 3,3-dimethyl-1-Butanol (DMB) reduced the plasma TMAO levels and attenuated the pressure overload-induced cardiac hypertrophy, cardiac fibrosis, and ventricular arrhythmia by inhibiting the p65 NF-κB and TGF-β1/Smad3 signaling pathway, which may further confirm the role of TMAO in cardiac remodeling.56
It is well known that arrhythmias, including AF and various ventricular arrhythmias, usually occur in cardiac hypertrophy and fibrosis patients. The studies of myocardial remodeling caused by TMAO suggest that it may contribute to arrhythmia in this way. Nevertheless, added preclinical studies, as well as prospective clinical studies, are warranted to confirm the present findings.
Inflammation, Oxidative Stress, TMAO and AF
Inflammation plays a vital role in the occurrence and progression of AF, demonstrated in a series of studies.32,33,65 Some studies have indicated that increased serum TMAO concentrations contributed significantly to inflammation via various pathways, which may indirectly bond TMAO with the occurrence of arrhythmias such as AF.28,38
One recent study suggested that age-associated microbiota dysbiosis played a significant role in the genesis of AF through a microbiota-gut-atria axis partly. They found in their research that the circulating lipopolysaccharide (LPS) and glucose levels were dramatically elevated in the fecal microbiota transplantation (FMT) rat model, leading to the up-regulated expression of NOD-like receptor protein (NLRP)-3 inflammasome, which contributed to AF, thus promoting the occurrence of AF. These results were also corroborated in elderly clinical subjects.28 In addition, the researchers revealed that the microbiota-intestinal barrier-atrial NLRP3 inflammatory body axis might be a potential therapeutic target for senile arrhythmia diseases.28
Another study exposed neonatal cardiac fibroblasts to different concentrations of TMAO for 24 hours and found that NOD-, LRR- and NLRP3 inflammasome activities also increased correspondingly, which significantly increased the body’s oxidative stress response.38
Best of our knowledge, these findings indicated that multiple inflammatory-activated signaling pathways might play an essential role in the occurrence of AF. However, which pathways are the leading causes of AF have not yet been fully elucidated.
Endothelial Dysfunction and Vascular Aging Caused by TMAO
Studies have confirmed that endothelial dysfunction and vascular aging may be correlated to AF through various routes. Prior findings have demonstrated that vascular and atrial endothelial dysfunction was associated with AF via a number of different mechanisms, including the alterations in hemodynamics parameters and endothelial cell shear stress, aggravating oxidative stress, exacerbating proinflammatory agents, and down-regulation of NOS expression and NO* production in atrial endocardium.66–68 In addition, studies have also found that restoration and maintenance of sinus rhythm through radiofrequency ablation may ameliorate endothelial function.69 Furthermore, previous studies have also linked vascular aging to various diseases such as acute myocardial infarction, peripheral arterial disease, stroke, and heart failure, all known as the common risk factors for AF.70,71
At present, studies have detected that TMAO can cause endothelial dysfunction and vascular aging, which has aroused great interest because it may offer a new direction for further investigating the pathogenesis, diagnosis, and treatment of AF.39,58 For example, in a study, researchers indicated that TMAO mediated the inhibition of sirtuin 1 (SIRT1) expression, then activated the p53/p21/Rb pathway, leading to increased p53, p53 acetylation, and p21 acetylation, and reduces CDK2, cyclinE1, and Rb phosphorylation, thus resulting in endothelial cell senescence and arterial aging, which may induce cardiovascular diseases such as arrhythmia.39
Nevertheless, there is still a little doubt in this regard, and future studies are needed to obtain a definitive sight of the association between TMAO-induced endothelial dysfunction and vascular aging and AF.
Therapeutic Strategies for TMAO-Related AF
To the best of our knowledge, TMAO has been discussed as a prognostic predictor and an underlying pathophysiological mechanism in several cardiovascular conditions. Current studies also believe that targeted TMAO and its metabolic pathway may potentially possess therapeutic value in various diseases. In addition, standardized and individualized treatment strategies in clinical practice may be of great significance for AF patients, particularly those with high circulating TMAO concentrations.
Standardized Antithrombotic Treatment Protocols
Anticoagulant therapy in AF patients is a necessary treatment to reduce thrombosis and improve prognosis. At present, the CHA2DS2-VASc score is commonly used to estimate the risk of embolism so as to implement appropriate treatment. However, the scoring system has some limitations in clinical application. Therefore, more research is needed to improve the predictive and diagnostic value of the scoring system. Because elevated TMAO has a strong effect of promoting atherosclerosis and platelet aggregation, the risk of thrombosis and the probability of embolic events in AF will significantly increase.2,3
Therefore, in a recent literature report, scholars indicated that for AF patients, especially those with elevated levels of intestinal microbial metabolites, it might be reasonable to communicate antiplatelet and anticoagulant therapy orally.51 Alignment with this concept, Liang et al52 considered that elevated TMAO in AF patients could guide antithrombotic therapy for patients with low and intermediate risk of embolism.
However, the availability of data on guiding anticoagulants in AF patients with elevated TMAO is still limited. Therefore, the implementation of the antithrombotic therapy process should necessarily consider both the stroke risk and bleeding risk as well as other features of the patient. In other words, individualized management is still required in AF patients with elevated TMAO.
Dietary Interventions
The human gut microbiota is an intricate dynamic ecosystem composed of millions of microorganisms.72–74 Increasing evidence indicates that interventions targeting the flora may exert potential benefits in preventing several cardiovascular disorders, including hypertension, coronary heart disease, and heart failure.75 Targeted regulation of the intestinal microbiota may help superior control AF via modifying diverse risk factors for AF. It has been indicated in various studies that diet contributes to the daily fluctuations of the intestinal microbiota structure, which will affect the concentrations of TMAO and may exert considerable effects on the body.76,77
Studies have shown that elevated circulating TMAO concentrations were associated with a high dietary intake of lipid phosphatidylcholine such as milk, liver, fish, egg yolk.78,79 In a mouse model, supplementation with choline for 8 weeks increased the serum TMAO concentration by 1.6 times and increased by two times after 16 weeks.80 Consequently, scholars also provide recommendations that it is advisable to reduce the intake of phosphatidylcholine to reduce TMAO in AF patients, especially those with other cardiovascular diseases.51,52,81
However, this evidence is still speculative and is merely hypothesis-generating to a large extent and needs to be confirmed by well-designed multi-center randomized clinical trials, including prospective and standardized analysis of the flora. In addition, because Asians and other races have utterly different dietary practices, regional differences should be taken into consideration in the future study of dietary regulation of gut microbiota.
Restore the Diversity of Intestinal Microflora and Target the Metabolic Pathway of TMAO
The gut microbial flora and humans coexist under normal circumstances, and many gut microbes are essential components of human metabolism. However, in some specific cases, the imbalance of the microbiota may lead to severe diseases. Of course, as is known, the imbalance of the intestinal microflora may affect the metabolic process. It has been found that the higher the proportion of Firmicutes and Bacteroides, the greater the response of TMAO to dietary precursor intake, suggesting the existence of individual differences in TMAO’s response to dietary precursors, which may be related to the intestinal microbiome.82 Therefore, scholars believe that relevant therapeutic strategies are urgently required to increase intestinal microbial diversity and restore the symbiotic relationship between intestinal microorganisms and patients.51
Moreover, researchers have also suggested that targeted TMA synthesis pathways and intestinal microbial dysregulation may have therapeutic potential as an intervention strategy for AF.18 Not surprisingly, TMA formation inhibitors targeting microbial TMA lyase have been developed, reducing TMAO levels in mouse models and reversing atherosclerosis, which may also become a means of treatment for AF.83 Current research believes that prebiotics and probiotics, antibiotics, and FMT are potential candidates for adjusting and restoring the intestinal structure.84 Targeted regulation of TMAO may be a possible therapeutic target for myocardial hypertrophy and myocardial fibrosis, which may exert anti-arrhythmic effects.57 Further researches should be conducted to substantiate or qualify the present findings.
Therapeutic Strategies for TMAO-Mediated Inflammation
An increasing amount of evidence has indicated that TMAO mediates the activation of a variety of inflammatory signal pathways in the body, thereby stimulating the CSNS and increasing inflammatory markers in the body, leading to myocardial fibrosis, which may be the mechanism of AF.28,38,65 Therefore, some experts believe that the regulation of TMAO-mediated inflammatory response is also one of the highly effective methods for AF.
For instance, Zhang et al28 indicated in their study that the microbiota-intestinal barrier-atrial NLRP3 inflammatory body axis may be a reasonable molecular target for the treatment of senile arrhythmia diseases. The targeting of excessive TMAO intake-associated inflammation in the heart–gut axis can also prevent cardiac arrhythmia.65
Conclusions and Future Perspectives
Studies have demonstrated that gut microbiota and its metabolites, in particular, the TMAO, play a pivotal role in the pathogenesis of AF. The pathophysiological mechanisms and therapeutic strategies have been discussed in extensive studies. However, there is still a big step called for to figure out further the molecular and genetic mechanisms of TMAO in the occurrence, progression of AF, as summarized in table 3. Besides, direct evidence of TMAO and AF susceptibility is still insufficient, which needs to be verified in a well-designed prospective cohort study with targeted regulation of TMAO without AF. In addition, an animal model with high TMAO serum levels created by supplementing choline, TMAO, or fecal transplantation is required to verify whether TMAO affects the AF matrix in the rapid atrial pacing AF model.
Although modern research has fully cognized the importance of intestinal microbiota, current research is usually restricted to specific bacteria, missing the remaining microorganisms such as fungi and viruses. Therefore, in future models, the influence of a variety of microorganisms and their metabolites on AF will be considered.
Moreover, TMAO increased the risk of thrombosis in AF patients and was associated with recurrence after ablation, mostly from observational clinical studies with small sample sizes, which needs to be further validated in animal experiments or multi-center clinical randomized controlled trials.
In addition, because most of the clinical evidence between TMAO and AF came from Asia, it is currently impossible to extrapolate the changes in TMAO observed in this population to Western countries. More evidence from Western countries or worldwide needs to be provided to fully reveal the underlying mechanism and genuine relationship between TMAO and AF due to regional differences and variation in dietary habits. More extensive preclinical and clinical studies, as well as randomized controlled trials, are also required to verify further the beneficial effect of targeting TMAO and its metabolic process, such as prebiotics and probiotics in AF patients.
We conducted a systematic and comprehensive search of articles using MEDLINE (from inception to 1 September 2021), EMBASE (from 1980), and Cochrane.
Funding
This work was funded by the National Natural Science Foundation of China (No.82160072) and the Science and Technology Support Project of Enshi Prefecture Science and Technology Bureau (No.E20180003).
Disclosure
The authors declare no conflict of interest.
References
1. Xu F, Fu Y, Sun TY, et al. The interplay between host genetics and the gut microbiome reveals common and distinct microbiome features for complex human diseases. Microbiome. 2020;8(1):145. doi:10.1186/s40168-020-00923-9
2. Peng J, Xiao X, Hu M, Zhang X. Interaction between gut microbiome and cardiovascular disease. Life Sci. 2018;214:153–157. doi:10.1016/j.lfs.2018.10.063
3. Tang WH, Kitai T, Hazen SL. Gut microbiota in cardiovascular health and disease. Circ Res. 2017;120(7):1183–1196. doi:10.1161/CIRCRESAHA.117.309715
4. Witkowski M, Weeks TL, Hazen SL. Gut microbiota and cardiovascular disease. Circ Res. 2020;127(4):553–570. doi:10.1161/CIRCRESAHA.120.316242
5. Yang S, Li X, Yang F, et al. Gut microbiota-dependent marker TMAO in promoting cardiovascular disease: inflammation mechanism, clinical prognostic, and potential as a therapeutic target. Front Pharmacol. 2019;10:1360. doi:10.3389/fphar.2019.01360
6. Zhu W, Gregory JC, Org E, et al. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;165(1):111–124. doi:10.1016/j.cell.2016.02.011
7. Tang WH, Wang Z, Kennedy DJ, et al. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2015;116(3):448–455. doi:10.1161/CIRCRESAHA.116.305360
8. Li XS, Obeid S, Wang Z, et al. Trimethyllysine, a trimethylamine N-oxide precursor, provides near- and long-term prognostic value in patients presenting with acute coronary syndromes. Eur Heart J. 2019;40(32):2700–2709. doi:10.1093/eurheartj/ehz259
9. Verhaar BJH, Prodan A, Nieuwdorp M, Muller M. Gut microbiota in hypertension and atherosclerosis: a review. Nutrients. 2020;12(10):2982. doi:10.3390/nu12102982
10. Stubbs JR, House JA, Ocque AJ, et al. Serum trimethylamine-N-Oxide is elevated in CKD and correlates with coronary atherosclerosis burden. J Am Soc Nephrol. 2016;27(1):305–313. doi:10.1681/ASN.2014111063
11. Zhang Y, Wang Y, Ke B, Du J. TMAO: how gut microbiota contributes to heart failure. Transl Res. 2021;228:109–125. doi:10.1016/j.trsl.2020.08.007
12. Schiattarella GG, Sannino A, Toscano E, et al. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: a systematic review and dose-response meta-analysis. Eur Heart J. 2017;38(39):2948–2956. doi:10.1093/eurheartj/ehx342
13. Zhang P, Zou JZ, Chen J, et al. Association of trimethylamine N-oxide with cardiovascular and all-cause mortality in hemodialysis patients. Ren Fail. 2020;42(1):1004–1014. doi:10.1080/0886022X.2020.1822868
14. Meng G, Zhou X, Wang M, et al. Gut microbe-derived metabolite trimethylamine N-oxide activates the cardiac autonomic nervous system and facilitates ischemia-induced ventricular arrhythmia via two different pathways. EBioMedicine. 2019;44:656–664. doi:10.1016/j.ebiom.2019.03.066
15. Svingen GFT, Zuo H, Ueland PM, et al. Increased plasma trimethylamine-N-oxide is associated with incident atrial fibrillation. Int J Cardiol. 2018;267:100–106. doi:10.1016/j.ijcard.2018.04.128
16. Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar Reddy D. Role of the normal gut microbiota. World J Gastroenterol. 2015;21(29):8787–8803. doi:10.3748/wjg.v21.i29.8787
17. Zhou X, Jin M, Liu L, Yu Z, Lu X, Zhang H. Trimethylamine N-oxide and cardiovascular outcomes in patients with chronic heart failure after myocardial infarction. ESC Heart Fail. 2020;7(1):188–193. doi:10.1002/ehf2.12552
18. Zuo K, Liu X, Wang P, et al. Metagenomic data-mining reveals enrichment of trimethylamine-N-oxide synthesis in gut microbiome in atrial fibrillation patients. BMC Genomics. 2020;21(1):526. doi:10.1186/s12864-020-06944-w
19. Ottiger M, Nickler M, Steuer C, et al. Gut, microbiota-dependent trimethylamine-N-oxide is associated with long-term all-cause mortality in patients with exacerbated chronic obstructive pulmonary disease. Nutrition (Burbank, Los Angeles County, Calif). 2018;45:135–41.e1. doi:10.1016/j.nut.2017.07.001
20. Drapala A, Szudzik M, Chabowski D, et al. Heart failure disturbs gut-blood barrier and increases plasma trimethylamine, a toxic bacterial metabolite. Int J Mol Sci. 2020;21(17):6161. doi:10.3390/ijms21176161
21. Schugar RC, Shih DM, Warrier M, et al. The TMAO-producing enzyme flavin-containing monooxygenase 3 regulates obesity and the beiging of white adipose tissue. Cell Rep. 2017;19(12):2451–2461. doi:10.1016/j.celrep.2017.05.077
22. Chen S, Henderson A, Petriello MC, et al. Trimethylamine N-oxide binds and activates PERK to promote metabolic dysfunction. Cell Metab. 2019;30(6):1141–51.e5. doi:10.1016/j.cmet.2019.08.021
23. Din AU, Hassan A, Zhu Y, Yin T, Gregersen H, Wang G. Amelioration of TMAO through probiotics and its potential role in atherosclerosis. Appl Microbiol Biotechnol. 2019;103(23–24):9217–9228. doi:10.1007/s00253-019-10142-4
24. van Ouwerkerk AF, Bosada FM, van Duijvenboden K, et al. Identification of atrial fibrillation associated genes and functional non-coding variants. Nat Commun. 2019;10(1):4755. doi:10.1038/s41467-019-12721-5
25. Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129(8):837–847. doi:10.1161/CIRCULATIONAHA.113.005119
26. Kolek MJ, Graves AJ, Xu M, et al. Evaluation of a prediction model for the development of atrial fibrillation in a repository of electronic medical records. JAMA Cardiol. 2016;1(9):1007–1013. doi:10.1001/jamacardio.2016.3366
27. Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373–498. doi:10.1093/eurheartj/ehaa612
28. Zhang Y, Zhang S, Li B, et al. Gut microbiota dysbiosis promotes age-related atrial fibrillation by lipopolysaccharide and glucose-induced activation of NLRP3-inflammasome. Cardiovasc Res. 2021. doi:10.1093/cvr/cvab114
29. Nguyen BO, Meems LMG, van Faassen M, et al. Gut-microbe derived TMAO and its association with more progressed forms of AF: results from the AF-RISK study. Int J Cardiol Heart Vasc. 2021;34:100798. doi:10.1016/j.ijcha.2021.100798
30. Jia J, Dou P, Gao M, et al. Assessment of causal direction between gut microbiota-dependent metabolites and cardiometabolic health: a bidirectional Mendelian randomization analysis. Diabetes. 2019;68(9):1747–1755. doi:10.2337/db19-0153
31. Li J, Zuo K, Zhang J, et al. Shifts in gut microbiome and metabolome are associated with risk of recurrent atrial fibrillation. J Cell Mol Med. 2020;24(22):13356–13369. doi:10.1111/jcmm.15959
32. Hu YF, Chen YJ, Lin YJ, Chen SA. Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol. 2015;12(4):230–243. doi:10.1038/nrcardio.2015.2
33. Boos CJ. Infection and atrial fibrillation: inflammation begets AF. Eur Heart J. 2020;41(10):1120–1122. doi:10.1093/eurheartj/ehz953
34. Guo Y, Lip GY, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol. 2012;60(22):2263–2270. doi:10.1016/j.jacc.2012.04.063
35. Yu L, Meng G, Huang B, et al. A potential relationship between gut microbes and atrial fibrillation: trimethylamine N-oxide, a gut microbe-derived metabolite, facilitates the progression of atrial fibrillation. Int J Cardiol. 2018;255:92–98. doi:10.1016/j.ijcard.2017.11.071
36. Janeiro MH, Ramírez MJ, Milagro FI, Martínez JA, Solas M. Implication of trimethylamine N-oxide (TMAO) in disease: potential biomarker or new therapeutic target. Nutrients. 2018;10(10):1398. doi:10.3390/nu10101398
37. Chen ML, Zhu XH, Ran L, Lang HD, Yi L, Mi MT. Trimethylamine-N-Oxide induces vascular inflammation by activating the NLRP3 inflammasome through the SIRT3-SOD2-mtROS signaling pathway. J Am Heart Assoc. 2017;6(9). doi:10.1161/JAHA.117.006347
38. Li X, Geng J, Zhao J, et al. Trimethylamine N-oxide exacerbates cardiac fibrosis via activating the NLRP3 Inflammasome. Front Physiol. 2019;10:866. doi:10.3389/fphys.2019.00866
39. Ke Y, Li D, Zhao M, et al. Gut flora-dependent metabolite Trimethylamine-N-oxide accelerates endothelial cell senescence and vascular aging through oxidative stress. Free Radic Biol Med. 2018;116:88–100. doi:10.1016/j.freeradbiomed.2018.01.007
40. Büttner P, Okun JG, Hauke J, et al. Trimethylamine N-oxide in atrial fibrillation progression. Int J Cardiol Heart Vasc. 2020;29:100554. doi:10.1016/j.ijcha.2020.100554
41. Papandreou C, Bulló M, Hernández-Alonso P, et al. Choline metabolism and risk of atrial fibrillation and heart failure in the PREDIMED study. Clin Chem. 2021;67(1):288–297. doi:10.1093/clinchem/hvaa224
42. Gawałko M, Jespersen T, Dobrev D, Linz D. The gut microbial-derived metabolite trimethylamine N-oxide: a missing link between lifestyle-components and atrial fibrillation? Int J Cardiol Heart Vasc. 2020;29:100581. doi:10.1016/j.ijcha.2020.100581
43. van den Munckhof ICL, Kurilshikov A, Ter Horst R, et al. Role of gut microbiota in chronic low-grade inflammation as potential driver for atherosclerotic cardiovascular disease: a systematic review of human studies. Obes Rev. 2018;19(12):1719–1734. doi:10.1111/obr.12750
44. Ge X, Zheng L, Zhuang R, et al. The gut microbial metabolite trimethylamine N-oxide and hypertension risk: a systematic review and dose-response meta-analysis. Adv Nutr. 2020;11(1):66–76. doi:10.1093/advances/nmz064
45. Gawałko M, Linz D, Dobrev D. Gut-microbiota derived TMAO: a risk factor, a mediator or a bystander in the pathogenesis of atrial fibrillation? Int J Cardiol Heart Vasc. 2021;34:100818. doi:10.1016/j.ijcha.2021.100818
46. Schotten U, Verheule S, Kirchhof P, Goette A. Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev. 2011;91(1):265–325. doi:10.1152/physrev.00031.2009
47. Yin J, Liao SX, He Y, et al. Dysbiosis of gut microbiota with reduced Trimethylamine-N-Oxide level in patients with large-artery atherosclerotic stroke or transient ischemic attack. J Am Heart Assoc. 2015;4(11). doi:10.1161/JAHA.115.002699
48. Wu C, Xue F, Lian Y, et al. Relationship between elevated plasma trimethylamine N-oxide levels and increased stroke injury. Neurology. 2020;94(7):e667–e77. doi:10.1212/WNL.0000000000008862
49. Schneider C, Okun JG, Schwarz KV, et al. Trimethylamine-N-oxide is elevated in the acute phase after ischaemic stroke and decreases within the first days. Eur J Neurol. 2020;27(8):1596–1603. doi:10.1111/ene.14253
50. Rexidamu M, Li H, Jin H, Huang J. Serum levels of Trimethylamine-N-oxide in patients with ischemic stroke. Biosci Rep. 2019;39(6). doi:10.1042/BSR20190515
51. Gong D, Zhang L, Zhang Y, Wang F, Zhao Z, Zhou X. Gut microbial metabolite trimethylamine N-oxide is related to thrombus formation in atrial fibrillation patients. Am J Med Sci. 2019;358(6):422–428. doi:10.1016/j.amjms.2019.09.002
52. Liang Z, Dong Z, Guo M, et al. Trimethylamine N-oxide as a risk marker for ischemic stroke in patients with atrial fibrillation. J Biochem Mol Toxicol. 2019;33(2):e22246. doi:10.1002/jbt.22246
53. Melgaard L, Gorst-Rasmussen A, Lane DA, Rasmussen LH, Larsen TB, Lip GY. Assessment of the CHA2DS2-VASc score in predicting ischemic stroke, thromboembolism, and death in patients with heart failure with and without atrial fibrillation. JAMA. 2015;314(10):1030–1038. doi:10.1001/jama.2015.10725
54. Chelu MG, Li N. Biomarkers (plasma trimethylamine-N-oxide) to predict atrial fibrillation: are we there yet? Int J Cardiol. 2018;267:116–117. doi:10.1016/j.ijcard.2018.05.106
55. Hou Y, Scherlag BJ, Lin J, et al. Ganglionated plexi modulate extrinsic cardiac autonomic nerve input: effects on sinus rate, atrioventricular conduction, refractoriness, and inducibility of atrial fibrillation. J Am Coll Cardiol. 2007;50(1):61–68. doi:10.1016/j.jacc.2007.02.066
56. Wang G, Kong B, Shuai W, Fu H, Jiang X, Huang H. 3,3-Dimethyl-1-butanol attenuates cardiac remodeling in pressure-overload-induced heart failure mice. J Nutr Biochem. 2020;78:108341. doi:10.1016/j.jnutbio.2020.108341
57. Li Z, Wu Z, Yan J, et al. Gut microbe-derived metabolite trimethylamine N-oxide induces cardiac hypertrophy and fibrosis. Lab Invest. 2019;99(3):346–357. doi:10.1038/s41374-018-0091-y
58. Brunt VE, Gioscia-Ryan RA, Casso AG, et al. Trimethylamine-N-Oxide promotes age-related vascular oxidative stress and endothelial dysfunction in mice and healthy humans. Hypertension (Dallas, Tex: 1979). 2020;76(1):101–112. doi:10.1161/HYPERTENSIONAHA.120.14759
59. Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92(7):1954–1968.
60. Chen PS, Tan AY. Autonomic nerve activity and atrial fibrillation. Heart Rhythm. 2007;4(3 Suppl):S61–4. doi:10.1016/j.hrthm.2006.12.006
61. Chen PS, Chen LS, Fishbein MC, Lin SF, Nattel S. Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ Res. 2014;114(9):1500–1515. doi:10.1161/CIRCRESAHA.114.303772
62. Zhou Z, Li S, Sheng X, et al. Interactions between metabolism regulator adiponectin and intrinsic cardiac autonomic nervous system: a potential treatment target for atrial fibrillation. Int J Cardiol. 2020;302:59–66. doi:10.1016/j.ijcard.2019.12.031
63. Tang WHW, Li DY, Hazen SL. Dietary metabolism, the gut microbiome, and heart failure. Nat Rev Cardiol. 2019;16(3):137–154. doi:10.1038/s41569-018-0108-7
64. Organ CL, Otsuka H, Bhushan S, et al. Choline diet and its gut microbe-derived metabolite, Trimethylamine N-Oxide, exacerbate pressure overload-induced heart failure. Circ Heart Fail. 2016;9(1):e002314. doi:10.1161/CIRCHEARTFAILURE.115.002314
65. Cheng WL, Li SJ, Lee TI, et al. Sugar fructose triggers gut dysbiosis and metabolic inflammation with cardiac arrhythmogenesis. Biomedicines. 2021;9(7):728. doi:10.3390/biomedicines9070728
66. Guazzi M, Arena R. Endothelial dysfunction and pathophysiological correlates in atrial fibrillation. Heart (British Cardiac Society). 2009;95(2):102–106. doi:10.1136/hrt.2007.135277
67. Cai H, Li Z, Goette A, et al. Downregulation of endocardial nitric oxide synthase expression and nitric oxide production in atrial fibrillation: potential mechanisms for atrial thrombosis and stroke. Circulation. 2002;106(22):2854–2858. doi:10.1161/01.CIR.0000039327.11661.16
68. Corban MT, Godo S, Burczak DR, et al. Coronary endothelial dysfunction is associated with increased risk of incident atrial fibrillation. J Am Heart Assoc. 2020;9(8):e014850. doi:10.1161/JAHA.119.014850
69. Skalidis EI, Zacharis EA, Tsetis DK, et al. Endothelial cell function during atrial fibrillation and after restoration of sinus rhythm. Am J Cardiol. 2007;99(9):1258–1262. doi:10.1016/j.amjcard.2006.12.044
70. Nilsson PM, Boutouyrie P, Laurent S. Vascular aging: a tale of EVA and ADAM in cardiovascular risk assessment and prevention. Hypertension (Dallas, Tex: 1979). 2009;54(1):3–10. doi:10.1161/HYPERTENSIONAHA.109.129114
71. Ungvari Z, Tarantini S, Sorond F, Merkely B, Csiszar A. Mechanisms of vascular aging, A geroscience perspective: JACC focus seminar. J Am Coll Cardiol. 2020;75(8):931–941. doi:10.1016/j.jacc.2019.11.061
72. Wang B, Xu Y, Hou X, et al. Small intestinal bacterial overgrowth in subclinical hypothyroidism of pregnant women. Front Endocrinol (Lausanne). 2021;12:604070. doi:10.3389/fendo.2021.604070
73. Sittipo P, Lobionda S, Lee YK, Maynard CL. Intestinal microbiota and the immune system in metabolic diseases. J Microbiol. 2018;56(3):154–162. doi:10.1007/s12275-018-7548-y
74. Sommer F, Anderson JM, Bharti R, Raes J, Rosenstiel P. The resilience of the intestinal microbiota influences health and disease. Nat Rev Microbiol. 2017;15(10):630–638. doi:10.1038/nrmicro.2017.58
75. Tang WH, Hazen SL. The gut microbiome and its role in cardiovascular diseases. Circulation. 2017;135(11):1008–1010. doi:10.1161/CIRCULATIONAHA.116.024251
76. Bibbò S, Ianiro G, Giorgio V, et al. The role of diet on gut microbiota composition. Eur Rev Med Pharmacol Sci. 2016;20(22):4742–4749.
77. Kolodziejczyk AA, Zheng D, Elinav E. Diet-microbiota interactions and personalized nutrition. Nat Rev Microbiol. 2019;17(12):742–753. doi:10.1038/s41579-019-0256-8
78. Zeisel SH, Mar MH, Howe JC, Holden JM. Concentrations of choline-containing compounds and betaine in common foods. J Nutr. 2003;133(5):1302–1307. doi:10.1093/jn/133.5.1302
79. Zhang AQ, Mitchell SC, Smith RL. Dietary precursors of trimethylamine in man: a pilot study. Food Chem Toxicol. 1999;37(5):515–520. doi:10.1016/S0278-6915(99)00028-9
80. Aldana-Hernández P, Leonard KA, Zhao YY, Curtis JM, Field CJ, Jacobs RL. Dietary choline or trimethylamine N-oxide supplementation does not influence atherosclerosis development in Ldlr-/- and Apoe-/- male mice. J Nutr. 2020;150(2):249–255. doi:10.1093/jn/nxz214
81. Lin T, Li K, He W, Chen L, Wang T, Wang N. Trimethylamine N-oxide: a new therapeutic target for atrial fibrillation? Int J Cardiol. 2019;274:194. doi:10.1016/j.ijcard.2018.07.147
82. Cho CE, Taesuwan S, Malysheva OV, et al. Trimethylamine-N-oxide (TMAO) response to animal source foods varies among healthy young men and is influenced by their gut microbiota composition: a randomized controlled trial. Mol Nutr Food Res. 2017;61(1):1600324.
83. Wang Z, Roberts AB, Buffa JA, et al. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell. 2015;163(7):1585–1595. doi:10.1016/j.cell.2015.11.055
84. Xu J, Yang Y. Gut microbiome and its meta-omics perspectives: profound implications for cardiovascular diseases. Gut Microbes. 2021;13(1):1936379. doi:10.1080/19490976.2021.1936379
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.