Back to Journals » Clinical and Experimental Gastroenterology » Volume 14
The Efficacy of Intraoperative Fluorescent Imaging Using Indocyanine Green for Cholangiography During Cholecystectomy and Hepatectomy
Authors Shibata H , Aoki T, Koizumi T, Kusano T , Yamazaki T, Saito K, Hirai T , Tomioka K, Wada Y , Hakozaki T, Tashiro Y, Nogaki K , Yamada K, Matsuda K, Fujimori A, Enami Y, Murakami M
Received 10 August 2020
Accepted for publication 6 April 2021
Published 30 April 2021 Volume 2021:14 Pages 145—154
DOI https://doi.org/10.2147/CEG.S275985
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Andreas M. Kaiser
Hideki Shibata, Takeshi Aoki, Tomotake Koizumi, Tomokazu Kusano, Tatsuya Yamazaki, Kazuhiko Saito, Takahito Hirai, Kodai Tomioka, Yusuke Wada, Tomoki Hakozaki, Yoshihiko Tashiro, Koji Nogaki, Kosuke Yamada, Kazuhiro Matsuda, Akira Fujimori, Yuta Enami, Masahiko Murakami
Division of Gastroenterological and General Surgery, Department of Surgery, School of Medicine, Showa University, Tokyo, Japan
Correspondence: Takeshi Aoki
Division of Gastroenterological and General Surgery, Department of Surgery, School of Medicine, Showa University, 1-5-8, Hatanodai, Shinagawa-Ku, Tokyo, 142-8555, Japan
Tel +81-3-3784-8000
Fax +81-3-3784-5835
Email [email protected]
Purpose: Bile duct injury is one of the most serious complications of laparoscopic cholecystectomy. Intraoperative indocyanine green (ICG) cholangiography is a safe and useful navigation modality for confirming the biliary anatomy. ICG cholangiography is expected to be a routine method for helping avoid bile duct injuries.
Patients and Methods: We examined 25 patients who underwent intraoperative cholangiography using ICG fluorescence. Two methods of ICG injection are used: intrabiliary injection (percutaneous transhepatic gallbladder drainage [PTGBD], gallbladder [GB] puncture and endoscopic nasobiliary drainage [ENBD]) at a dosage of 0.025 mg during the operation or intravenous injection with 2.5 mg ICG preoperatively.
Results: There were 24 patients who underwent laparoscopic cholecystectomy and 1 patient who underwent hepatectomy. For laparoscopic cholecystectomy, the average operation time was 127 (50– 197) minutes, and estimated blood loss was 43.2 (0– 400) g. The ICG administration route was intravenous injections in 12 cases and intrabiliary injection in 12 cases (GB injection: 3 cases, PTGBD: 8 cases, ENBD:1 case). The course of the biliary tree was able to be confirmed in all cases that received direct injection into the biliary tract, whereas bile structures were recognizable in only 10 cases (83.3%) with intravenous injection. The postoperative hospital stay was 4.6 (3– 9) days, and no postoperative complications (Clavien–Dindo ≧IIIa) were observed. For hepatectomy, a tumor located near the left Glissonian pedicle was resected using a fluorescence image guide. Biliary structures were fluorescent without injury after resecting the tumor. No adverse events due to ICG administration were observed, and the procedure was able to be performed safely.
Conclusion: ICG fluorescence imaging allows surgeons to visualize the course of the biliary tree in real time during cholecystectomy and hepatectomy. This is considered essential for hepatobiliary surgery to prevent biliary tree injury and ensure safe surgery.
Keywords: indocyanine green fluorescent imaging, navigation surgery, near-infrared fluorescent cholangiography
Introduction
The most common laparoscopic operation in Japan is laparoscopic cholecystectomy (LC), which is performed more than 60,000 times each year. Bile duct injury is one of the most serious complications in LC, and its incidence is reported to range from 0.3% to 0.7%.1 Generally, bile duct injury can lead to a miserable course. Bile duct injury can also lead to obstruction, including obstructive jaundice, eventually leading to the need for liver transplantation in the worst cases.2 The main cause of bile duct injury is misidentification of the anatomy.3,4 Preoperative imaging techniques using ultrasonography (US), computed tomography (CT) and magnetic resonance cholangiopancreatography (MRCP) provide a clear view of the anatomy.5,6 However, these simulation devices are mainly used only for preoperative planning.
There are several imaging techniques to confirm the relevant anatomical structures. Intraoperative cholangiography has been widely used to identify the biliary structures.7,8 However, conventional intraoperative cholangiography using radiography has the disadvantage of exposing the patient and medical staff to radiation, in addition to usually requiring a large and expensive C-arm fluoroscopy machine and additional human resources to operate it.9 Therefore, more convenient and safe techniques to visualize the anatomy are needed.
Indocyanine green (ICG) binds to serum and bile proteins in vivo and expresses a fluorescent signal.10 In 2009, Ishizawa et al first reported fluorescence cholangiography using ICG excreted into the bile following preoperative intravenous injection as the source of fluorescence during LC.11 In 2010, our team applied ICG fluorescence cholangiography to LC using a prototype fluorescent imaging system, and in 71.4% of cases, the common bile duct (CBD) and cystic duct (CD) were identified by ICG fluorescence.12 Over the last several years, the efficacy of incisionless near-infrared fluorescent cholangiography (NIFC) has been consistently reported to increase the visualization and identification of extrahepatic biliary structures.
Laparoscopic fluorescence imaging systems have become commercially available, and their usefulness during hepatectomy has been widely reported. Our team first demonstrated that ICG fluorescence imaging was extremely useful for clearly demarcating the liver segments prior to anatomical liver resection13 and determining of the surgical margin.14 In 2009, Ishizawa et al reported the usefulness of ICG fluorescence cholangiography during hepatectomy.15 In 2015, Kawaguchi et al reported the usefulness of ICG imaging for the visualization of the bile duct during laparoscopic liver resection.16 Thus, intraoperative cholangiography technique with ICG fluorescence is used not only in LC but also in hepatectomy.
ICG cholangiography is expected to become a routine method for helping avoid or minimize bile duct injuries. In this study, we demonstrated the current applications of intraoperative cholangiography with ICG fluorescence in cholecystectomy and hepatectomy.
Patients and Methods
Intraoperative Cholangiography for LC
This study comprised 24 patients who underwent LC using ICG cholangiography between April 2016 and December 2019 and gave their consent to be included in this study. Patients with severe inflammation and with anatomic variation were selected. All patients underwent MRCP preoperatively to confirm the course of the bile duct. Patients suspected of having CBD stones or cholangitis underwent ERCP specifically. Endoscopic nasobiliary drainage (ENBD) was performed after choledocholithotomy. Percutaneous transhepatic gallbladder drainage (PTGBD) was performed on patients who had been diagnosed with moderate to severe acute cholecystitis according to the diagnosis criteria of the Tokyo guidelines.17
We retrospectively collected data, included patient demographics, indications for and duration of operations, specific LC complications and biliary structures visualized with ICG.
There are two ways to deliver ICG: intrabiliary injection and intravenous injection. Injecting ICG directly into the bile duct is further divided into gallbladder (GB) puncture and bile duct injection. GB puncture involves puncturing the GB during surgery and injecting ICG (0.025 mg/mL). In contrast, with the bile duct injection method, ICG is injected into the bile duct via an extra biliary fistula tube inserted before surgery, such as that for PTGBD or ENBD. In the intravenous injection approach, 2.5 mg/body of ICG is administered 1 hour before surgery.
ICG cholangiography is performed before the CD is exposed and after the CD is confirmed by dissecting the Calot triangle, ICG cholangiography is performed to identify the anatomy of the biliary structure.
Intraoperative Cholangiography During Hepatectomy
One patient in the present study underwent intraoperative ICG cholangiography during hepatectomy. After the bile duct was exposed to transect the liver parenchyma, 2.5 mg/body of ICG was injected intravenously and the course of the bile duct was confirmed.
Results
Intraoperative Cholangiography for LC
The average (range) age was 62.8 (30–82) years old, male/female ratio was 14/10, the average operation time was 127 (50–197) minutes, and estimated blood loss was 43.2 (0–400) g. The ICG administration route was intravenous injections in 12 cases and intrabiliary injection in 12 cases (GB injection: 3 cases, PTGBD: 8 cases, ENBD:1 case). The average postoperative hospital stay was 4.6 (3–9) days, and no postoperative complications (Clavien–Dindo≧IIIa) were observed (Table 1). The course of the bile structure was able to be confirmed in all cases that received direct injection into the biliary tract, whereas bile structures were recognizable in 10 cases (83.3%) with intravenous injection (Table 2). In two cases without clear visualization, the CD was gradually revealed as Calot triangular separation was performed using the fluorescence signal of the CBD as a guidance. No adverse events due to ICG administration were observed, and the procedure was able to be performed safely. In this study, there were four cases with aberrant bifurcation of the biliary system. In three cases, the CD was bifurcated from the right posterior branch (RPB) of the hepatic duct, while in the remaining one, the CD was bifurcated from the lower junction of the CBD. Aberrant bifurcation of the biliary system was ultimately able to be detected after dissection and before resection in all cases (Table 3).
 |
Table 1 Patients’ Characteristics |
 |
Table 2 Visualization of the Biliary Tract with Intraoperative Cholangiography |
 |
Table 3 Visualization of the Anatomical Abnormality of Biliary Tract with Intraoperative Cholangiography |
Case Presentation
Patient 1
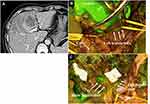
A 28-year-old-man was admitted to our hospital to undergo laparoscopic cholecystectomy for a GB stone. MRCP showed the CD entering the RPB. ICG (2.5 mg/body) was administered 1 hour before surgery in this case. As Calot triangular separation was performed, the course of the bile duct was confirmed by fluorescence. We recognized the CD running from the RPD and CBD. The operation was performed safely without misidentification of the anatomy (Figure 1).
Patient 2
A 42-year-old-woman was diagnosed with acute cholecystitis and admitted to our hospital for an emergency operation. MRCP showed the independent RPB entering the CBD and the bifurcation of the CD from the independent RPB. As we performed an emergency operation for acute cholecystitis, the GB was full. The GB was punctured during the operation to drain the contents and facilitate grasping. ICG (0.025 mg/mL) was then injected directly into the GB. The bifurcation of CD, RPB and CBD was confirmed by fluorescence (Figure 2).
Patient 3
A 42-year-old-man was found to have hepatocellular carcinoma in segment 4 on preoperative CT. The tumor was located near the left portal vein. We planned to perform the S4 subsegmentectomy to preserve the remnant liver function. This approach was required in order to transect the liver parenchyma while avoiding injuring the left Glissonian pedicle. During the transection of the liver parenchyma, the left Glissonian pedicle was difficult to recognize, since it was compressed by a huge tumor. Therefore, we identified the course of the hilar bile duct via the fluorescence biliary road map using ICG. The tumor was then completely resected with a safe surgical margin using the fluorescence image guidance. On the transection plane, no bile leakage was shown. The postoperative course was uneventful, and the patient was discharged on the 11th postoperative day (Figure 3).
Discussion
For hepatobiliary surgery, it is important to recognize the accurate anatomy structures as the misidentification of the anatomy can lead to serious complications. The confirmation of the course of the biliary trees is needed to ensure safe surgery. We herein report an overview of the current application of ICG fluorescence during cholecystectomy and hepatectomy.
ICG fluorescence imaging is widely used in various fields such as for the identification of sentinel lymph nodes in several malignancies, the evaluation of adequate perfusion after cardiovascular grafting and hepatobiliary surgery.1,11–16 In 2008, our team first showed that ICG fluorescence imaging was extremely useful for clearly demarcating the liver segments prior to anatomical liver resection.13 Subsequently, the efficacy of NIFC for the identification of subcapsular hepatic tumors and securing the surgical margin in hepatobiliary surgery was widely reported.12,14
Ishizawa et al first demonstrated the effectiveness of intraoperative cholangiography.15 Being able to obtain fluorescent images of the biliary tract without the need for catheterization of the bile duct is extremely useful. Over the last several years, the report on incisionless NIFC has been consistently shown to increase the visualization and identification of extrahepatic biliary structures.11,15,18–26 Recently Dip et al reported the first randomized trial comparing LC performed under white-light imaging alone versus combined white and NIFC after the intravenous injection of ICG dye.27 NIFC improves the visualization of extrahepatic bile duct anatomy over white-light alone. In most of our cases, the biliary tree was able to be detected by ICG cholangiography. While this included four cases with aberrant bifurcation of the biliary system that was detected preoperatively, the operations were performed safely, with abnormal bifurcation of the biliary trees successfully recognized allowing for the avoidance of bile duct injury. In our series with severe inflammation or surrounding thick tissues, the course of the CD was indistinct at first. However, it was gradually revealed with fluorescence signal after Calot triangular separation, and the bifurcation of the CD and CBD was clearly observed. The main cause of bile duct injury during LC was reported to be the misidentification of the biliary anatomy,3,4 and aberrant bifurcation of the biliary system was able to be identified before resection in all cases in this study. NIFC was helpful in preventing bile duct injury, especially in aberrant or severe inflammation cases.
In our series, the detection rate of CD, CBD and CHD using NIFC with intravenous injection was 83.3% and the accuracy rate of the identification of biliary structure by NIFC was reported to be 96.9%, 75.7% and 52.3%, respectively.26 In contrast, biliary structure with ICG injection into bile duct directly was able to be identified in all cases in this study. Previous reports concerning fluorescence cholangiography with direct ICG injection into the bile duct are summarized in Table 4.28–31 One of the advantages of this method over intravenous injection is the ability to obtain a clear view of the biliary structure because of the absence of fluoresce noise from the liver. The rate of the identification of the biliary tract for cases with ICG direct injection in this study was better than that described in a previous report,28–31 possibly due to a lower concentration of ICG being used and the avoidance of an excessive fluorescent signal. NIFS with direct ICG injection was shown to be particularly effective especially for patients who underwent PTGBD preoperatively for acute cholecystitis. For patients who undergo ICG injection via direct GB puncture, it is important to avoid dye spillage, as this worsens the visibility of the operation field. In our cases, we were able to keep the operation field clean by closing the puncture site with surgical clips just after the removal of the catheter to prevent dye spillage.
 |
Table 4 Relevant Articles About Fluorescence Cholangiography with Direct ICG Injection into Biliary Tract |
Intraoperative cholangiography with ICG fluorescence is also useful for hepatectomy. NIFC is a good tool for performing real-time biliary navigation during hepatectomy, allowing for the confirmation of the anatomy of the bile duct and providing surgeons with spatial relationships between the bile duct and surrounding tissues. In one of our cases, we needed to preserve the left Glissonian pedicle, which was difficult to recognize, since it was compressed by a huge tumor. We were able to completely resect the tumor with a safe surgical margin and preservation of the left Glissonian pedicle using the fluorescence image guidance. For this particular case, NIFS may also have been useful for real-time cholangiography, demonstrating the biliary roadmap during hepatectomy.
NIFC has several potential advantages over conventional radiographic cholangiography. The most important point is to achieve overlay imaging of the biliary tract with fluorescence in real-time during surgery. Surgeons can then perform the operation using the fluorescence signal as a guidance. This helps avoid misidentification of the anatomy, which is the most common cause of bile duct injury. Second, this technique is much more convenient than conventional radiographic cholangiography, as it does not need a large and expensive C-arm fluoroscopy machine or workers to control it and can thus save time and additional human resources. Finally, this is a safe technique, as adverse events due to ICG administration are rare.
However, one disadvantage of this method is the difficulty in identifying biliary trees, especially in cases with severe inflammation or thick tissue. In the present study, the course of the bile tree was able to be confirmed in all cases by direct injection into the biliary tract, whereas bile structures were recognizable in 10 cases (83.3%) with intravenous injection. Near-infrared light was reported to only be able to penetrate tissue to a depth of about 5–10 mm.1 The biliary structure in liver parenchyma or in cases covered by thick tissue is difficult to visualize, so peeling back the surrounding tissue is required to detect the fluorescence. Of note, the body mass index (BMI) in our case where biliary structures could be detected was 30.4. While some previous reports have suggested no relationship between the BMI and the identification of biliary structures under fluorescent cholangiography,18 others have noted that all patients in whose structures were not able to visualize were obese.32,33
Another issue with this method is that when intravenous injection is chosen, there are no established standards concerning the timing or concentration of ICG. In the most reported cases of intravenous injection, 2.5 mg/body of ICG was administered 30–60 minutes before surgery (Table 5).1,12,19,25,27,29,32–37 In our present cases, 2.5 mg/body of ICG was administered 1 hour before surgery approximately. In this retrospective study, the interval from intravenous administration of ICG to the detection of each biliary structure was investigated in detail, and the fluorescence signal from CD could be confirmed at 48.59 minutes, that from CBD at 42.03 minutes and that from CHD at 48.02 minutes after the administration of ICG. The CD could be detected in only 33.3% before ICG injection. However, the CD, CBD and CHD were observed in 75% of cases by ICG injection and NIFS imaging. After dissection of the Calot triangle, the detection rate of the biliary structures was 83.3% (Table 6). One reason for the failed detection after dissection may have been because the intravenously administered ICG had not yet been excreted into the biliary system. Furthermore, the high degree of fluorescence noise from the liver may have hampered the identification of the biliary structure in these cases. Masaru et al reported that the identification of the biliary tract could be improved by performing ICG administration on the day before the operation rather than just prior to surgery.38 The ICG concentration and fluorescence intensity do not always match. While there have been several reports on ICG fluorescent cholangiography, standards have yet to be established. A detailed examination of the optimal dose and administration time should be conducted in a future study.
 |
Table 5 Relevant Articles About Fluorescence Cholangiography with IV Injection |
 |
Table 6 Visualization of the Biliary Tract with IV Injection |
To our knowledge, there have been no other reports that evaluate the two methods of ICG administration―intravenous versus intrabiliary injection―at a single facility.
In the early 1990s, the Critical View of Safety (CVS) technique for identification was introduced, which greatly reduces the bile duct injury rate.39 Though it is essential to clearly identify the CVS, the initial dissection in the Calot triangle to confirm a clear view of CVS is sometimes associated with a risk of bile duct injury due to the misidentification of biliary structures, especially in cases with severe inflammation. However, using NIFC, surgeons can perform the operation using the fluorescence signal emitted by the biliary structures for guidance. In our department, after the CVS was confirmed, the gallbladder was dissected away from the liver bed from the fundus down toward the CD. The CD was identified and isolated in a 360°view of the gallbladder–CD junction. Furthermore, the biliary structure was confirmed by NIFC before cutting off the CD. Our procedure involving the combination of the dome-down technique40 and NIFC is thought to be a safe method that reduces the risk of bile duct injury in cases with aberrant bifurcation of the biliary tract or severe inflammation around the biliary tract.
Conclusion
ICG fluorescence image allows surgeons to visualize the course of the biliary tree in real time during hepatobiliary surgery. This is considered essential for preventing bile duct injury and ensuring safety during cholecystectomy and hepatectomy.
Abbreviations
LC, laparoscopic cholecystectomy; US, ultrasonography; CT, computed tomography; MRCP, magnetic resonance cholangiopancreatography; ICG, indocyanine green; CBD, common bile duct; CD, cystic duct; NIFC, near-infrared fluorescent cholangiography; GB, gallbladder; ERCP, endoscopic retrograde cholangiopancreatography; ENBD, endoscopic nasobiliary drainage; PTGBD, percutaneous transhepatic gallbladder drainage; RPB, right posterior brunch; BMI, body mass index; CVS, critical view of safety.
Data Sharing Statement
The datasets generated and/or analyzed during the current study are not publicly available due to protecting individual patient privacy but are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study has been reviewed by the Ethics Committee of Showa University Hospital; all procedures performed in studies involving human participants were conducted according to the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent for Publication
Written informed consent for publication of the participant images and clinical details were obtained from each patient.
Acknowledgments
The authors would like to thank Japan Medical Communication for the English language review.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no competing interests.
References
1. Ishizawa T, Bandai Y, Ijichi M, et al. Fluorescent cholangiography illuminating the biliary tree during laparoscopic cholecystectomy. Br J Surg. 2010;97:1369–1377. doi:10.1002/bjs.7125
2. Booij KA, de Reuver PR, Yap K, et al. Morbidity and mortality after minor bile duct injury following laparoscopic cholecystectomy. Endoscopy. 2015;47(1):40–46. doi:10.1055/s-0034-1390908
3. Way LW, Stewart L, Gantert W, et al. Causes and prevention of laparoscopic bile duct injuries: analysis of 252 cases from a human factors and cognitive psychology perspective. Ann Surg. 2013;237(4):460–469. doi:10.1097/01.SLA.0000060680.92690.E9
4. Nijssen MA, Schreinemakers JM, Meyer Z, et al. Complications after laparoscopic cholecystectomy: a video evaluation study of whether the critical view of safety was reached. World J Surg. 2015;39(7):1798–1803. doi:10.1007/s00268-015-2993-9
5. Wigmore SJ, Redhead DN, Yan XJ, et al. Virtual hepatic resection using three-dimensional reconstruction of helical computed tomography angioportograms. Ann Surg. 2001;233:221–226. doi:10.1097/00000658-200102000-00011
6. Lamade W, Vetter M, Hassenpflug P, et al. Navigation and image-guided HBP surgery: a review and preview. J Hepatobiliary Pancreat Surg. 2002;9:592–599. doi:10.1007/s005340200079
7. Connor S, Garden OJ. Bile duct injury in the era of laparoscopic cholecystectomy. Br J Surg. 2006;93:158–168. doi:10.1002/bjs.5266
8. Waage A, Nilsson M. Iatrogenic bile duct injury: a population based study of 152, 776 cholecystectomies in the Swedish Inpatient Registry. Arch Surg. 2006;141:1207–1213. doi:10.1001/archsurg.141.12.1207
9. Flum DR, Flowers C, Veenstra DL. A cost-effectiveness analysis of intraoperative cholangiography in the prevention of bile duct injury during laparoscopic cholecystectomy. J Am Coll Surg. 2003;196:385–393. doi:10.1016/S1072-7515(02)01806-9
10. Tanaka T, Choi HS, Fujii H, et al. Image-guided oncologic surgery using invisible light: completed pre-clinical development for sentinel lymph node mapping. Ann Surg Oncol. 2006;13:1671–1681. doi:10.1245/s10434-006-9194-6
11. Ishizawa T, Bandai Y, Kokudo N. Fluorescent cholangiography using indocyanine green for laparoscopic cholecystectomy: an initial experience. Arch Surg. 2009;144:381–382. doi:10.1001/archsurg.2009.9
12. Aoki T, Murakami M, Yasuda D, et al. Intraoperative fluorescent imaging using indocyanine green for liver mapping and cholangiography. J Hepatobiliary Pancreat Sci. 2010;17:590–594. doi:10.1007/s00534-009-0197-0
13. Aoki T, Yasuda D, Shimizu Y, et al. Image-guided liver mapping using fluorescence navigation system with indocyanine green for anatomical hepatic resection. World J Surg. 2008;32:1763–1767. doi:10.1007/s00423-020-01967-z
14. Aoki T, Murakami M, Koizumi T. Determination of the surgical margin in laparoscopic liver resections using infrared indocyanine green fluorescence. Langenbecks Arch Surg. 2018;403(5):671–680. doi:10.1007/s00423-018-1685-y
15. Ishizawa T, Tamura S, Masuda K, et al. Intraoperative fluorescent cholangiography using indocyanine green: a biliary road map for safe surgery. J Am Coll Surg. 2009;208:1–4. doi:10.1016/j.jamcollsurg.2008.09.024
16. Kawaguchi Y, Velayutham V, Fuks D, et al. Usefulness of indocyanine green-fluorescence imaging for visualization of the bile duct during laparoscopic liver resection. J Am Coll Surg. 2015;221:e113–e117. doi:10.1016/j.jamcollsurg.2015.09.001
17. Kimura Y, Takada T, Strasberg SM, et al. TG13 current terminology, etiology, and epidemiology of acute cholangitis and cholecystitis. J Hepatobiliary Pancreat Sci. 2013;20(1):8–23. doi:10.1007/s00534-012-0564-0
18. Dip F, Roy M, Lo Menzo E, et al. Routine use of fluorescent incisionless cholangiography as a new imaging modality during laparoscopic cholecystectomy. Surg Endosc. 2015;29:1621–1626. doi:10.1007/s00464-014-3853-7
19. Dip F, Nguyen D, Montorfano L, et al. Accuracy of near infrared-guided surgery in morbidly obese subjects undergoing laparoscopic cholecystectomy. Obes Surg. 2016;26:525–530. doi:10.1007/s11695-015-1781-9
20. Dip FD, Asbun D, Rosales-Velderrain A, et al. Cost analysis and effectiveness comparing the routine use of intraoperative fluorescent cholangiography with fluoroscopic cholangiogram in patients undergoing laparoscopic cholecystectomy. Surg Endosc. 2014;28:1838–1843. doi:10.1007/s00464-013-3394-5
21. Figueiredo JL, Siegel C, Nahrendorf M, et al. Intraoperative near-infrared fluorescent cholangiography (NIRFC) in mouse models of bile duct injury. World J Surg. 2010;34:336–343. doi:10.1007/s00268-009-0332-8
22. Kaneko J, Ishizawa T, Masuda K, et al. Indocyanine green reinjection technique for use in fluorescent angiography concomitant with cholangiography during laparoscopic cholecystectomy. Surg Laparosc Endosc Percutan Tech. 2012;22:341–344. doi:10.1097/SLE.0b013e3182570240
23. Pesce A, Piccolo G, La Greca G, et al. Utility of fluorescent cholangiography during laparoscopic cholecystectomy: a systematic review. World J Gastroenterol. 2015;21:7877–7883. doi:10.3748/wjg.v21.i25.7877
24. Roy M, Dip F, Nguyen D, et al. Fluorescent incisionless cholangiography as a teaching tool for identification of Calot’s triangle. Surg Endosc. 2017;31:2483–2490. doi:10.1007/s00464-016-5250-x
25. Spinoglio G, Priora F, Bianchi PP, et al. Real-time near-infrared (NIR) fluorescent cholangiography in single-site robotic cholecystectomy (SSRC): a single-institutional prospective study. Surg Endosc. 2013;27:2156–2162. doi:10.1007/s00464-012-2733-2
26. Zroback C, Chow G, Meneghetti A, et al. Fluorescent cholangiography in laparoscopic cholecystectomy: the initial Canadian experience. Am J Surg. 2016;211:933–937. doi:10.1016/j.amjsurg.2016.01.013
27. Dip F, LoMenzo E, Sarotto L, et al. Randomized trial of near-infrared incisionless fluorescent cholangiography. Ann Surg. 2019;270:992–999. doi:10.1097/SLA.0000000000003178
28. Graves C, Ely S, Idowu O, et al. Direct gallbladder indocyanine green injection fluorescence cholangiography during laparoscopic cholecystectomy. J Laparoendosc Adv Surg Tech A. 2017;27(19):1069–1073. doi:10.1089/lap.2017.0070
29. Liu YY, Liao CH, Diana M, et al. Near-infrared cholecystocholangiography with direct intragallbladder indocyanine green injection: preliminary clinical results. Surg Endosc. 2018;32:1506–1514. doi:10.1007/s00464-017-5838-9
30. Quaresima S, Balla A, Palmieri L, et al. Routine near infra-red indocyanine green fluorescent cholangiography versus intraoperative cholangiography during laparoscopic cholecystectomy: a case-matched comparison. Surg Endosc. 2020;34:1959–1967. doi:10.1007/s00464-019-06970-0
31. Škrabec CG, Aranda FP, Espín F, et al. Fluorescent cholangiography with direct injection of indocyanine green (ICG) into the gallbladder: a safety method to outline biliary anatomy. Langenbecks Arch Surg. 2020;405:827–832.
32. Daskalaki D, Fernandes E, Wang X, et al. Indocyanine green (ICG) fluorescent cholangiography during robotic cholecystectomy: results of 184 consecutive cases in a single institution. Surg Innov. 2014;21(6):615–621. doi:10.1177/1553350614524839
33. Osayi SN, Wendling MR, Drosdeck JM, et al. Near-infrared fluorescent cholangiography facilitates identification of biliary anatomy during laparoscopic cholecystectomy. Surg Endosc. 2015;29(2):368–375. doi:10.1007/s00464-014-3677-5
34. Larsen SS, Schulze S, Bisgaard T. Non-radiographic intraoperative fluorescent cholangiography is feasible. Dan Med J. 2014;61(8):A4891.
35. Boni L, David G, Mangano A, et al. Clinical applications of indocyanine green (ICG) enhanced fluorescence in laparoscopic surgery. Surg Endosc. 2015;29(7):2046–2055. doi:10.1007/s00464-014-3895-x
36. van Dam DA, Ankersmit M, van de Ven P, et al. Comparing near-infrared imaging with indocyanine green to conventional imaging during laparoscopic cholecystectomy: a Prospective Crossover Study. J Laparoendosc Adv Surg Tech A. 2015;25(6):486–492. doi:10.1089/lap.2014.0248
37. Diana M, Soler L, Agnus V, et al. Prospective evaluation of precision multimodal gallbladder surgery navigation: virtual reality, near-infrared fluorescence, and X-ray-based intraoperative cholangiography. Ann Surg. 2017;266(5):890–897. doi:10.1097/SLA.0000000000002400
38. Masaru M, Yoshikuni K, Yuta K, et al. Indocyanine green administration a day before surgery may increase bile duct detectability on fluorescence cholangiography during laparoscopic cholecystectomy. J Hepatobiliary Pancreat Sci. 2020;00:1–9.
39. Strasberg SM, Hertl M, Soper NJ. An analysis of the problem of biliary injury during laparoscopic cholecystectomy. J Am Coll Surg. 1995;180:101–125.
40. Fullum TM, Kim S, Dan D, et al. Laparoscopic “Dome-down” cholecystectomy with the LCS-5 Harmonic scalpel. JSLS. 2005;9(1):51–57.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.