Back to Journals » Neuropsychiatric Disease and Treatment » Volume 13
The effects of memantine on behavioral disturbances in patients with Alzheimer's disease: a meta-analysis
Authors Kishi T , Matsunaga S, Iwata N
Received 29 May 2017
Accepted for publication 21 June 2017
Published 20 July 2017 Volume 2017:13 Pages 1909—1928
DOI https://doi.org/10.2147/NDT.S142839
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Taro Kishi,* Shinji Matsunaga,* Nakao Iwata
Department of Psychiatry, Fujita Health University School of Medicine, Toyoake, Aichi, Japan
*These authors contributed equally to this work
Background: Memantine is effective in the treatment of behavioral disturbances in patients with Alzheimer’s disease. It has not yet been fully determined which behavioral disturbances respond best to memantine.
Methods: We conducted a meta-analysis of memantine vs control (placebo or usual care) for the treatment of individual behavioral disturbances (delusion, hallucination, agitation/aggression, dysphoria, anxiety/phobia, euphoria, apathy, disinhibition, irritability/lability, aberrant motor activity/activity disturbances, nighttime disturbance/diurnal rhythm disturbances, and eating disturbances). Randomized controlled studies of memantine in patients with Alzheimer’s disease were included in this study. To evaluate these outcomes, standardized mean difference (SMD), with 95% confidence intervals (95% CIs), based upon a random-effects model was evaluated in the meta-analysis.
Results: A total of 11 studies (n=4,261; memantine vs placebo: N=4, n=1,500; memantine + cholinesterase inhibitors [M + ChEIs] vs ChEIs: N=7, n=2,761) were included in the meta-analysis. Compared to control, memantine showed significant improvement in agitation/aggression (SMD =-0.11; 95% CIs =-0.20, -0.03; P=0.01; I2=47%), delusion (SMD =-0.12; 95% CIs =-0.18, -0.06; P=0.0002; I2=0%), disinhibition (SMD =-0.08; 95% CIs =-0.15, -0.00; P=0.04; I2=0%), and nighttime disturbance/diurnal rhythm disturbances (SMD =-0.10; 95% CIs =-0.18, -0.02; P=0.02; I2=36%). Memantine was also marginally superior to control in hallucination (SMD =-0.06; 95% CIs =-0.12, 0.01; P=0.07; I2=0%) and irritability/lability (SMD =-0.09; 95% CIs =-0.19, 0.01; P=0.07; I2=42%). Memantine is similar to control in dysphoria, anxiety/phobia, euphoria, apathy, and eating disturbance.
Conclusion: The meta-analysis suggest that memantine has benefits for the treatment of most of the behavioral disturbances in patients with Alzheimer’s disease. Memantine does not deteriorate negative symptoms as behavioral disturbances in patients with Alzheimer’s disease.
Keywords: memantine, Alzheimer’s disease, behavioral disturbances, meta-analysis
Introduction
Alzheimer’s disease is a neurodegenerative disease.1 The percentage of people with Alzheimer’s disease increases with age: 3% of people aged 65–74 years, 17% of people aged 75–84 years, and 32% of people aged 85 years and older have Alzheimer’s disease.2 It has an insidious onset, with gradual progression of cognitive symptoms and behavioral disturbances.1
There are the following four approved drugs for the treatment of Alzheimer’s disease worldwide: memantine and three cholinesterase inhibitors (donepezil, galantamine, and rivastigmine).1 Memantine has been approved worldwide for treating moderate-to-severe Alzheimer’s disease. It is postulated that memantine exerts its therapeutic effect through its action as a low-to-moderate affinity, noncompetitive (open channel), nonselective, voltage-dependent, N-methyl-d-aspartic acid (NMDA) receptor antagonist, which binds preferentially to NMDA receptor-operated calcium channels.3 Memantine blocks the effects of sustained, pathologically elevated levels of glutamate, which could otherwise lead to neuronal dysfunction.4–6 In addition, memantine may also upregulate NMDA receptor expression, causing activation in the presence of a strong stimulus.7
Our previous meta-analysis showed that memantine monotherapy was superior to placebo in cognitive impairment (standardized mean difference [SMD] =−0.27; 95% confidence intervals [95% CIs] =−0.39 to −0.14) and behavioral disturbances (SMD =−0.12; 95% CIs =−0.22 to −0.01).8 We did an additional meta-analysis to show that although there was a trend favoring the combination therapy with memantine and cholinesterase inhibitors compared to cholinesterase inhibitor monotherapy for treating cognitive impairment (SMD =−0.13; 95% CIs =−0.26 to 0.01), memantine was superior to placebo in behavioral disturbances (SMD =−0.13; 95% CIs =−0.24 to −0.02).9 Thus, there was evidence on the efficacy of memantine for cognitive impairment and behavioral disturbances on patients with Alzheimer’s disease to date.
However, there are various symptoms of behavioral disturbances, such as delusion, hallucination, agitation/aggression, dysphoria, anxiety/phobia, euphoria, apathy, disinhibition, irritability/lability, aberrant motor activity/activity disturbances, nighttime disturbance/diurnal rhythm disturbances, and eating disturbances.10 For example, although a drug, which has sedative effect, seems to be effective for positive symptoms, such as agitation and irritability, this drug seems to exasperate negative symptoms, such as apathy.10 There has not been robust evidence on the efficacy of memantine for individual behavioral disturbances in patients with Alzheimer’s disease. The effect size of anti-dementia drugs for individual behavioral disturbances in patients with Alzheimer’s disease in randomized trials has been extremely small, due to the need to manage subscale scores of behavioral disturbance scale. Therefore, because a meta-analysis can increase the statistical power for group comparisons and can overcome the limitation of sample size in underpowered studies,11 we conducted a meta-analysis to achieve conclusive evidence for the efficacy of memantine on individual behavioral disturbances in patients with Alzheimer’s disease.
Methods
This meta-analysis was performed based upon the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines (International prospective register of systematic reviews [PROSPERO]: CRD42017059245).12 We combined with the data from the studies of memantine monotherapy and the studies of combination therapy with memantine and cholinesterase inhibitors, because studies of the combination therapy included the patients who had several dementia symptoms at the baseline despite taking some cholinesterase inhibitors.
Search strategy and inclusion criteria
To identify relevant studies, two of the authors (TK and SM) independently searched MEDLINE, Cochrane library, Scopus, and PsycINFO without language restrictions from the inception of their databases to April 25, 2017, using the following search strategy: (“Alzheimer Disease” [Mesh] OR “Alzheimer disease” OR “Alzheimer’s disease”) AND (“Memantine”[Mesh] OR “memantine”) AND (“randomized” OR “random” OR “randomly”). The authors also searched ClinicalTrials.gov (http://ClinicalTrials.gov/), ISRCTN registry (https://www.isrctn.com/), and the International Clinical Trials Registry Platform (http://www.who.int/ictrp/en/) to include randomized controlled trials as comprehensively as possible and to minimize the possibility of publication bias. Only randomized placebo- or usual care-controlled trials of memantine treatment in patients with Alzheimer’s disease lasting ≥2 weeks were included. The studies that included more than 50% patients who received the combination therapy were classified as a combination therapy group in this study (Table 1). Two authors (TK and SM) independently assessed inclusion/exclusion criteria and selected the studies. The references of the included articles and review articles were also searched for citations of additional relevant published and unpublished studies, including conference abstracts.
Data synthesis and outcome measures
The primary outcomes were individual behavioral disturbances as follows: delusion, hallucination, agitation/aggression, dysphoria, anxiety/phobia, euphoria, apathy, disinhibition, irritability/lability, aberrant motor activity/activity disturbances, nighttime disturbance/diurnal rhythm disturbances, and eating disturbances. Nine of 11 studies included in the meta-analysis used Neuropsychiatric Inventory,13 and the other two studies14,15 used the Behavioral Pathology in Alzheimer’s Disease Rating Scale.16 For three-arm (memantine 10 mg/day arm, memantine 20 mg/day arm, and placebo arm) studies,17 we combined the data of the memantine 10 mg/day arm with that of memantine 20 mg/day. For four-arm (memantine monotherapy arm, combination therapy with memantine and donepezil arm, donepezil monotherapy arm, and placebo arm) studies,18 we combined the data of the memantine monotherapy arm with that of the combination therapy with memantine (ie, memantine group) and donepezil arm and the data of donepezil monotherapy arm with that of placebo arm (ie, non-memantine group).
Data extraction
Two authors (TK and SM) independently extracted the data from the included studies. Where possible, we used intention-to-treat (ITT) or a full analysis set (FAS) population. When such data were unavailable, the results for observed case (OC) analysis were extracted from each study. When the data required for meta-analysis were missing, we contacted the investigators (or the industries) of the relevant study and requested unpublished data.
Meta-analysis methods
The meta-analysis was conducted using Review Manager software.19 The random-effects model was selected for this meta-analysis due to the potential heterogeneity across studies. To evaluate these outcomes, SMD, with 95% CIs, based upon a random-effects model, was evaluated in the meta-analysis. We assessed the methodological quality of the trials, according to the Cochrane risk-of-bias criteria in the Cochrane Handbook.11 Study heterogeneity was tested using the I2 statistic, considering I2≥50% to reflect considerable heterogeneity.11 We did not find considerable heterogeneity with respect to all meta-analysis. To detect the confounding factors for the result of primary outcomes for efficacy, two subgroup analysis (including a test for subgroup differences) were performed for the following: severity of disease (mild-to-moderate vs moderate and moderate-to-severe) and therapeutic strategy (memantine monotherapy vs combination therapy with memantine and cholinesterase inhibitors). Finally, we utilized funnel plots to explore potential publication bias.
Results
Study characteristics
Of the 2,239 results obtained in our literature search, we excluded the following: 1,498 as duplicates, 693 after a review of the abstract or title review, and 28 articles after a review of the full text (22 review articles, four single-arm studies, and two same studies). We did not retrieve 10 studies by searching through the review articles and clinical trial registries (Figure S1). Although 30 studies were identified though the literature search, only 11 studies (memantine monotherapy vs placebo: four studies,14,17,20,21 n=1,500; combination therapy with memantine and cholinesterase inhibitors vs cholinesterase inhibitors: seven studies,15,18,22–26 n=2,761) were included in the meta-analysis, since the other 20 studies did not report any available data for performing a meta-analysis.
The main characteristics of studies and patients are summarized in Table 1. The mean duration of the studies was 26.5 weeks (one study was 52 weeks, other studies were 24 weeks), the mean patient age was 76.3 years, and the percentage of males was 34.6%. Although one of the 11 studies was an open-label study (ie, not placebo-controlled study),22 the other 10 studies were double-blinded, randomized, placebo-controlled trials. One study was a memantine extended-release study.23 The dose of memantine was 20 mg/day in all studies, other than Kitamura et al’s17 study (three arms: memantine 10 mg/day arm, memantine 20 mg/day arm, and placebo arm). The Howard et al’s18 study used OC populations in their analysis. Because this study was a four-arm study (memantine monotherapy arm, combination therapy with memantine and donepezil arm, donepezil monotherapy arm, and placebo arm),18 we combined the data of memantine monotherapy arm with that of combination therapy with memantine (ie, memantine group) and donepezil arm and data of donepezil monotherapy arm with that of placebo arm (ie, non-memantine group). Two studies were not sponsored by a pharmaceutical company.18,22 Most of all studies included in the study excluded the patients who had psychiatric disorders other than Alzheimer’s disease.
Evaluations on the methodological quality of the included studies were performed based upon the Cochrane risk-of-bias criteria and are shown in Figures S2 and S3.
Results of the meta-analysis
Memantine showed significant improvement in agitation/aggression (SMD =−0.11; 95% CIs =−0.20, −0.03; P=0.01, I2=47%; Figure 1), delusion (SMD =−0.12; 95% CIs =−0.18, −0.06; P=0.0002; I2=0%; Figure 2), disinhibition (SMD =−0.08; 95% CIs =−0.15, −0.00; P=0.04; I2=0%; Figure 3), and nighttime disturbance/diurnal rhythm disturbances (SMD =−0.10; 95% CIs =−0.18, −0.02; P=0.02; I2=36%; Figure 4) compared to control. Memantine was also marginally superior to control in hallucination (SMD =−0.06; 95% CIs =−0.12, 0.01; P=0.07; I2=0%; Figure 5) and irritability/lability (SMD =−0.09; 95% CIs =−0.19, 0.01; P=0.07; I2=42%; Figure 6). Memantine is similar to control in aberrant motor activity/activity disturbances, anxiety/phobia, apathy, dysphoria, eating disturbances, and euphoria (Figures 7–12). The data for individual behavioral disturbances scores were simulated with no publication bias.
  | Figure 1 Forest plot of agitation/aggression scores. |
  | Figure 2 Forest plot of delusion scores. |
  | Figure 3 Forest plot of disinhibition scores. |
  | Figure 5 Forest plot of hallucination scores. |
  | Figure 6 Forest plot of irritability/lability scores. |
  | Figure 8 Forest plot of anxiety/phobia scores. |
  | Figure 9 Forest plot of apathy scores. |
  | Figure 10 Forest plot of dysphoria scores. |
  | Figure 11 Forest plot of eating disturbance scores. |
  | Figure 12 Forest plot of euphoria scores. |
Subgroup analysis divided by therapeutic strategy
We did not find considerable heterogeneity with respect to all meta-analysis (Figures 1–12). We also did not find any significant subgroup differences in all subgroup analysis.
Delusion was the outcome, where memantine was superior to control in the monotherapy subgroup and the combination therapy subgroup (Figure 2). Agitation/aggression and disinhibition were the outcomes, where memantine was superior to control in the combination therapy subgroup but not in the monotherapy subgroup (Figures 1 and 3).
Subgroup analysis divided by the severity of disease
We also did not find considerable heterogeneity with respect to all meta-analysis (Figures 1–12). We also did not find any significant subgroup differences in all subgroup analysis. Although we found marginally subgroup differences in subgroup analysis divided by the severity of disease with respect to apathy (P=0.07), this subgroup analysis showed that memantine was similar to control in moderate-to-severe Alzheimer’s disease patients, as well as mild-to-moderate Alzheimer’s disease patients (Figure 9).
Agitation/aggression, delusion, disinhibition, and nighttime disturbance/diurnal rhythm disturbances were outcomes, where memantine was superior to control in the moderate-to-severe Alzheimer’s disease patients’ subgroup, but not in the mild-to-moderate Alzheimer’s disease patients’ subgroup (Figures 1–4).
Discussion
This meta-analysis showed that memantine showed significant efficacy compared to controls in improving delusion, agitation/aggression, disinhibition, and nighttime disturbance/diurnal rhythm disturbances in patients with Alzheimer’s disease. Moreover, memantine seems to benefit the treatment of hallucination and irritability/lability. These symptoms are classified as positive symptoms.10 Memantine was similar to controls for negative symptoms, such as dysphoria, anxiety/phobia, euphoria, apathy, aberrant motor activity/activity disturbances, and eating disturbances. Memantine improves cognitive functions,8,9 and anti-dementia drugs may prevent brain atrophy in patients with Alzheimer’s disease.27 Therefore, we considered that the evidence that memantine did not deteriorate negative symptoms, such as behavioral disturbances in patients with Alzheimer’s disease, was very important for the clinicians and the patients. If the patients receiving memantine have negative symptoms, the evidence suggests that the patients do not need to stop taking memantine.
Although we did not detect any considerable heterogeneity in all of the meta-analysis, we performed two subgroup analysis (severity of disease and therapeutic strategy) to detect confounding factors. We did not find significant subgroup differences. Subgroup analysis could provide the following evidence, although we did not address multiple comparisons: 1) memantine has benefits for the treatment of delusion in patients with not only combination therapy but also memantine monotherapy; 2) patients with combination therapy may have more benefits for the treatment of agitation/aggression, and disinhibition than patients with memantine monotherapy; and 3) patients with moderate-severe Alzheimer’s disease may have more benefit for the treatment of agitation/aggression, delusion, disinhibition and nighttime disturbance/diurnal rhythm disturbances than patients with mild-moderate Alzheimer’s disease.
There were several limitations in this study which need to be addressed. First, patient characteristics differed between the studies examined including: symptom severity, inclusion criteria, race, ethnicity, and study duration. These differences could generate heterogeneity, when combining data for systematic review and meta-analysis. Second, most studies included in this study were industry-sponsored studies. Therefore, there remains a possibility for sponsorship bias in our results. Third, most of all studies included in the study did not report sufficient information about concomitant drugs such as psychotropic drugs (Table 1). Therefore, we did not examine whether concomitant drugs influence on the results of the meta-analysis. Fourth, because mean patients’ age among the studies included in the meta-analysis were very similar (Table 1), we did not perform the meta-regression analysis to examine whether the effect size of memantine was associated with patient age. Fifth, our study focused on memantine treatment for Alzheimer’s disease. We considered that it needed to conduct a network meta-analysis of anti-dementia drugs for Alzheimer’s disease on efficacy and safety because network meta-analysis can combine direct and indirect evidence to address the frequent absence of randomized trials that directly compare all the interventions of interest. This should offer suggestion on which pharmacological interventions for the Alzheimer’s disease is best.
Conclusion
The meta-analysis suggest that memantine has benefits for the treatment of most of the behavioral disturbances in patients with Alzheimer’s disease. Memantine does not deteriorate negative symptoms as behavioral disturbances in patients with Alzheimer’s disease.
Acknowledgments
We thank Mr Shohei Yasuda (Daiichi Sankyo Company, Limited), Mr Masato Kobayashi (Daiichi Sankyo Company, Limited), Mr Kazuto Sato (Daiichi Sankyo Company, Limited), Dr Jun Horiguchi (Department of Psychiatry, Faculty of Medicine, Shimane University), Dr Rei Wake (Department of Psychiatry, Faculty of Medicine, Shimane University), Dr Nathan Herrmann (Sunnybrook Health Sciences Centre, University of Toronto), Dr Neli Boneva (Lundbeck A/S), Ole Michael Lemming (Lundbeck A/S), Dr Robert Howard (Department of Old Age Psychiatry and Psychopathology, King’s College London), and Dr Patrick Phillips (MRC Clinical Trials Unit at UCL Institute of Clinical Trials & Methodology) for providing information for this study. A part of data which we could not get enough information from published articles nor unpublished studies was provided by Daiichi Sankyo Co., Ltd.
Disclosure
No grant support or other sources of funding were used to conduct this study or prepare this manuscript. Dr Taro Kishi has received speaker’s honoraria from Daiichi Sankyo, Dainippon Sumitomo, Eisai, Janssen, Otsuka, Meiji, MSD, and Tanabe-Mitsubishi (Yoshitomi) and has a Fujita Health University School of Medicine research grant and a Health Labour Sciences Research Grant. Dr Shinji Matsunaga has received speaker’s honoraria from Daiichi Sankyo, Dainippon Sumitomo, Eisai, Janssen, Meiji, MSD, Novartis, Otsuka, and Tanabe-Mitsubishi and has a Fujita Health University School of Medicine research grant and a Grant-in-Aid for Young Scientists (B). Dr Nakao Iwata has received speaker’s honoraria from Astellas, Dainippon Sumitomo, Eli Lilly, GlaxoSmithKline, Janssen, Yoshitomi, Otsuka, Meiji, Shionogi, Novartis, and Pfizer, and had research grants from GlaxoSmithKline, Meiji, and Otsuka. The authors report no other conflicts of interest in this work.
References
Scheltens P, Blennow K, Breteler MM, et al. Alzheimer’s disease. Lancet. 2016;388(10043):505–517. | ||
Sposato LA, Kapral MK, Fang J, et al. Declining incidence of stroke and dementia: coincidence or prevention opportunity? JAMA Neurol. 2015;72(12):1529–1531. | ||
Kishi T, Matsuda Y, Iwata N. Memantine add-on to antipsychotic treatment for residual negative and cognitive symptoms of schizophrenia: a meta-analysis. Psychopharmacology (Berl). 2017;234(14):2113–2125. | ||
Danysz W, Parsons CG. The NMDA receptor antagonist memantine as a symptomatological and neuroprotective treatment for Alzheimer’s disease: preclinical evidence. Int J Geriatr Psychiatry. 2003;18(suppl 1):S23–S32. | ||
Sani G, Serra G, Kotzalidis GD, et al. The role of memantine in the treatment of psychiatric disorders other than the dementias: a review of current preclinical and clinical evidence. CNS Drugs. 2012;26(8):663–690. | ||
Di Iorio G, Baroni G, Lorusso M, Montemitro C, Spano MC, di Giannantonio M. Efficacy of memantine in schizophrenic patients: a systematic review. J Amino Acids. 2017;2017:7021071. | ||
Joshi I, Yang YM, Wang LY. Coincident activation of metabotropic glutamate receptors and NMDA receptors (NMDARs) downregulates perisynaptic/extrasynaptic NMDARs and enhances high-fidelity neurotransmission at the developing calyx of held synapse. J Neurosci. 2007;27(37):9989–9999. | ||
Matsunaga S, Kishi T, Iwata N. Memantine monotherapy for Alzheimer’s disease: a systematic review and meta-analysis. PLoS One. 2015;10(4):e0123289. | ||
Matsunaga S, Kishi T, Iwata N. Combination therapy with cholinesterase inhibitors and memantine for Alzheimer’s disease: a systematic review and meta-analysis. Int J Neuropsychopharmacol. 2014;18(5):yu115. | ||
Cerejeira J, Lagarto L, Mukaetova-Ladinska EB. Behavioral and psychological symptoms of dementia. Front Neurol. 2012;3:73. | ||
Higgins J, Green S [webpage on the Internet]. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. Available from: http://handbook.cochrane.org. Accessed March 2011. | ||
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. | ||
Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–2314. | ||
Nakamura Y, Homma A, Kitamura S, Yoshimura I. Phase III study of memantine hydrochloride, a new NMDA receptor antagonist, in patients with moderate to severe Alzheimer’s disease – efficacy and safety. Jpn J Geriatr Psychiatry. 2011;22(4):464–473. | ||
Nakamura Y, Kitamura S, Nagakubo T, Kobayashi M, Homma A. Study of memantine hydrochloride in combination with donepezil hydrochloride in patients with moderate to severe Alzheimer’s disease – efficacy and safety. Jpn J Geriatr Med. 2016;54(11):1147–1158. | ||
Auer SR, Monteiro IM, Reisberg B. The empirical behavioral pathology in Alzheimer’s disease (E-BEHAVE-AD) rating scale. Int Psychogeriatr. 1996;8(2):247–266. | ||
Kitamura S, Homma A, Nakamura Y, Yoshimura I. Phase II study of memantine hydrochloride, a new NMDA receptor antagonist, in patients with moderate to severe Alzheimer’s disease – efficacy, safety and recommended dose. Jpn J Geriatr Psychiatry. 2011;22(4):453–463. | ||
Howard R, McShane R, Lindesay J, et al. Donepezil and memantine for moderate-to-severe Alzheimer’s disease. N Engl J Med. 2012;366(10):893–903. | ||
The Cochrane Collaboration. Review Manager (RevMan) [Computer Program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014. | ||
Peskind ER, Potkin SG, Pomara N, et al. Memantine treatment in mild to moderate Alzheimer disease: a 24-week randomized, controlled trial. Am J Geriatr Psychiatry. 2006;14(8):704–715. | ||
van Dyck CH, Tariot PN, Meyers B, Malca Resnick E; Memantine MEM-MD-01 Study Group. A 24-week randomized, controlled trial of memantine in patients with moderate-to-severe Alzheimer disease. Alzheimer Dis Assoc Disord. 2007;21(2):136–143. | ||
Araki T, Wake R, Miyaoka T, et al. The effects of combine treatment of memantine and donepezil on Alzheimer’s disease patients and its relationship with cerebral blood flow in the prefrontal area. Int J Geriatr Psychiatry. 2014;29(9):881–889. | ||
Grossberg GT, Manes F, Allegri RF, et al. The safety, tolerability, and efficacy of once-daily memantine (28 mg): a multinational, randomized, double-blind, placebo-controlled trial in patients with moderate-to-severe Alzheimer’s disease taking cholinesterase inhibitors. CNS Drugs. 2013;27(6):469–478. | ||
Herrmann N, Gauthier S, Boneva N, Lemming OM; 10158 Investigators. A randomized, double-blind, placebo-controlled trial of memantine in a behaviorally enriched sample of patients with moderate-to-severe Alzheimer’s disease. Int Psychogeriatr. 2013;25(6):919–927. | ||
Porsteinsson AP, Grossberg GT, Mintzer J, Olin JT; Memantine MEM-MD-12 Study Group. Memantine treatment in patients with mild to moderate Alzheimer’s disease already receiving a cholinesterase inhibitor: a randomized, double-blind, placebo-controlled trial. Curr Alzheimer Res. 2008;5(1):83–89. | ||
Tariot PN, Farlow MR, Grossberg GT, et al; Memantine Study Group. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA. 2004;291(3):317–324. | ||
Kishi T, Matsunaga S, Oya K, Ikuta T, Iwata N. Protection against brain atrophy by anti-dementia medication in mild cognitive impairment and Alzheimer’s disease: meta-analysis of longitudinal randomized placebo-controlled trials. Int J Neuropsychopharmacol. 2015;18(12):yv070. |
Supplementary materials
  | Figure S1 PRISMA flow diagram. |
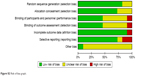  | Figure S2 Risk of bias graph. |
  | Figure S3 Risk of bias summary. |
References
Nakamura Y, Homma A, Kitamura S, Yoshimura I. Phase III study of memantine hydrochloride, a new NMDA receptor antagonist, in patients with moderate to severe Alzheimer’s disease – Efficacy and safety. Japanese Journal of Geriatric Psychiatry. 2011;22:464–473. | ||
MA3301 (2011) Confirmatory randomized, double-blind, placebo-controlled, parallel-group study of SUN Y7017 (memantine hydrochloride) in patients with mild to moderate dementia of the Alzheimer’s type (JapicCTI-050079). Available from: http://www.clinicaltrials.jp/user/cteDetail.jsp?clinicalTrialId=4669. Accessed July 14, 2017. | ||
Nakamura Y, Kitamura S, Nagakubo T, Kobayashi M, Homma A. Study of Memantine Hydrochloride in Combination with Donepezil Hydrochloride in Patients with Moderate to Severe Alzheimer’s disease – Efficacy and safety. Japanese Journal of Geriatric Medicine. 2016;54:1147–1158. | ||
Forest Laboratories (2004) An Evaluation of the Safety and Efficacy of Memantine in Agitated Patients With Moderate to Severe Alzheimer’s Disease. Available from: https://clinicaltrials.gov/ct2/show/NCT00097916. NLM identifier: NCT00097916. Accessed July 14, 2017. | ||
Florida Atlantic University (2007) Delaying the Progression of Driving Impairment in Individuals With Mild Alzheimer’s Disease. Available from: https://clinicaltrials.gov/ct2/show/NCT00476008. NLM identifier: NCT00476008. Accessed July 14, 2017. | ||
Washington University School of Medicine (2008) Corticolimbic Degeneration and Treatment of Dementia. Available from: https://clinicaltrials.gov/ct2/show/NCT00768261. NLM identifier: NCT00768261. Accessed July 14, 2017. | ||
New York University School of Medicine (2009) Effects of Memantine on Magnetic Resonance (MR) Spectroscopy in Subjects at Risk for Alzheimer’s Disease. Available from: https://clinicaltrials.gov/ct2/show/NCT00933608. NLM identifier: NCT00933608. Accessed July 14, 2017. | ||
Kitamura S, Homma A, Nakamura Y, Yoshimura I. Phase II study of memantine hydrochloride, a new NMDA receptor antagonist, in patients with moderate to severe Alzheimer’s disease – Efficacy, safety and recommended dose. Japanese Journal of Geriatric Psychiatry. 2011;22:453–463. | ||
Zheng Y, Yu J. [The efficacy and safety of combination therapy of memantine and donepezil among old Alzheimer’s disease patients]. Modern Practical Medicine. 2011;4:415–416. Chinese. | ||
Matsunaga S, Kishi T, Iwata N. Memantine monotherapy for Alzheimer’s disease: a systematic review and meta-analysis. PLoS One. 2015;10:e0123289. | ||
Tsoi KK, Chan JY, Leung NW, Hirai HW, Wong SY, Kwok TC. Combination Therapy Showed Limited Superiority Over Monotherapy for Alzheimer Disease: A Meta-analysis of 14 Randomized Trials. J Am Med Dir Assoc. 2016;17:863.e861–e868. | ||
IE2201(after 2000) Phase I study of SUN Y7017 (memantine hydrochloride) in patients with dementia of the Alzheimer’s type. Available from: http://www.pmda.go.jp/drugs/2011/P201100018/43057400_22300AMX00423_K101_2.pdf. Accessed July 14, 2017. | ||
MEMARY® (memantine) [package insert]. Japan: Daiichi Sankyo Company, Limited; 2015. Available from: http://adinfo.tri-kobe.org/download/drug/memary.pdf. Accessed July 14, 2017. | ||
Araki T, Wake R, Miyaoka T, et al. The effects of combine treatment of memantine and donepezil on Alzheimer’s disease patients and its relationship with cerebral blood flow in the prefrontal area. Int J Geriatr Psychiatry. 2014;29(9):881–889. | ||
Grossberg GT, Manes F, Allegri RF, et al. The safety, tolerability, and efficacy of once-daily memantine (28 mg): a multinational, randomized, double-blind, placebo-controlled trial in patients with moderate-to-severe Alzheimer’s disease taking cholinesterase inhibitors. CNS Drugs. 2013;27(6):469–478. | ||
Herrmann N, Gauthier S, Boneva N, Lemming OM; 10158 Investigators. A randomized, double-blind, placebo-controlled trial of memantine in a behaviorally enriched sample of patients with moderate-to-severe Alzheimer’s disease. Int Psychogeriatr. 2013;25(6):919–927. | ||
Howard R, McShane R, Lindesay J, et al. Donepezil and memantine for moderate-to-severe Alzheimer’s disease. N Engl J Med. 2012; 366(10):893–903. | ||
Peskind ER, Potkin SG, Pomara N, et al. Memantine treatment in mild to moderate Alzheimer disease: a 24-week randomized, controlled trial. Am J Geriatr Psychiatry. 2006;14(8):704–715. | ||
Porsteinsson AP, Grossberg GT, Mintzer J, Olin JT; Memantine MEM-MD-12 Study Group. Memantine treatment in patients with mild to moderate Alzheimer’s disease already receiving a cholinesterase inhibitor: a randomized, double-blind, placebo-controlled trial. Curr Alzheimer Res. 2008;5(1):83–89. | ||
Tariot PN, Farlow MR, Grossberg GT, et al; Memantine Study Group. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA. 2004;291(3):317–324. | ||
van Dyck CH, Tariot PN, Meyers B, Malca Resnick E; Memantine MEM-MD-01 Study Group. A 24-week randomized, controlled trial of memantine in patients with moderate-to-severe Alzheimer disease. Alzheimer Dis Assoc Disord. 2007;21(2):136–143. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



