Back to Journals » ClinicoEconomics and Outcomes Research » Volume 9
The cost-effectiveness of a NSCLC patient assistance program for pemetrexed maintenance therapy in People's Republic of China
Authors Shi Q, Hu S, Furnback WE, Guzauskas GF, Shen J, Wang BCM
Received 15 August 2016
Accepted for publication 24 October 2016
Published 3 February 2017 Volume 2017:9 Pages 99—106
DOI https://doi.org/10.2147/CEOR.S119818
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Giorgio Colombo
Qiang Shi,1 Shanlian Hu,2 Wesley E Furnback,3 Gregory F Guzauskas,3 Jiejing Shen,1 Bruce CM Wang3
1Lilly Suzhou Pharmaceutical Company, Ltd., Shanghai, People’s Republic of China; 2Shanghai Health Development Research Center, Shanghai, People’s Republic of China; 3Elysia Group, Ltd., Taipei, Taiwan, Republic of China
Background: Eli Lilly and the China Primary Health Care Foundation are currently implementing a patient assistance program (PAP) in China, which allows first-line nonsquamous non-small-cell lung cancer (NSCLC) patients who complete four cycles of pemetrexed induction therapy to receive free, continuous pemetrexed maintenance therapy.
Objective: To estimate the cost-effectiveness of pemetrexed maintenance therapy vs basic standard care (BSC) and the economic impacts of providing a PAP for pemetrexed maintenance therapy to NSCLC patients who have completed pemetrexed induction therapy in a Chinese health care setting.
Methods: We developed a novel decision-analytic model to evaluate the long-term costs and clinical efficacy of pemetrexed plus BSC vs BSC alone. We utilized a three-state (progression-free survival, progressed disease, and dead) partition survival model for both the clinical and economic aspects of the analysis. Cost and health utility estimates were derived from the literature. We performed a scenario analysis to estimate the real-world impact of introducing the PAP in China by comparing the use of the PAP vs non-PAP. Model uncertainty was evaluated using one-way and multivariate probabilistic sensitivity analysis.
Results: Compared to BSC, pemetrexed plus BSC resulted in a gain of 0.22 years of life (95% credible range [CR]: 0.04–0.46) and 0.13 quality-adjusted life years (95% CR: 0.04–0.26) per patient, at an increased cost of $28,105 (95% CR: −$22,720 to $48,646) without a PAP and $3,068 (95% CR: −$1,263 to $9,163) with a PAP. The incremental cost-effectiveness ratio for pemetrexed plus BSC vs BSC alone was cost-prohibitive at $222,700 for non-PAP, but cost-effective at $24,319 with a PAP.
Conclusion: Our study suggests that maintenance pemetrexed therapy following pemetrexed induction for patients with advanced NSCLC is likely to be highly non-cost-effective in the absence of a PAP, but the pending implementation of the PAP promises to make it cost-effective, with a >90% probability of cost-effectiveness at a Chinese willingness-to-pay threshold per quality-adjusted life year.
Keywords: non-small-cell lung cancer, pemetrexed, patient assistance program, cost–utility analysis, basic standard care
Introduction
The global incidence and prevalence of lung cancer are rapidly increasing. In 2015, the estimated total number of incident lung cancer cases was 733,300.1 Approximately 85% of these cases are non-small-cell lung cancer (NSCLC), a common lung cancer which develops slowly compared to small cell lung cancer but is less receptive to chemotherapy treatments.2 A Chinese analysis of 274 patients with NSCLC found 39.8% had squamous cell carcinoma, and 67.9% had advanced stage disease (IIIB–IV).3 The treatment for advanced stage NSCLC typically consists of chemotherapy, surgery, and radiation therapy. After chemotherapy induction cycles, chemotherapy maintenance regimens may use a different drug to expose patients to an agent with a different pharmaceutical mechanism.4–6 Alternatively, successful combination induction regimens may utilize the least toxic agent from the combined induction regimen as maintenance therapy, so that a proven beneficial therapy may continue to be administered while also decreasing a patient’s adverse events.7,8
Pemetrexed is a chemotherapy drug that inhibits the formation of precursor purine and pyrimidine nucleotides, preventing the formation of DNA and RNA that fuels the growth and survival of both normal and cancer cells.9 Pemetrexed first gained approval in the USA in 2004 and is indicated (within the USA) for use in locally advanced or metastatic nonsquamous NSCLC after initial treatment in combination with cisplatin. It is also indicated for use as maintenance therapy for patients whose disease has not progressed after four cycles of platinum-based first-line chemotherapy and after prior single-agent chemotherapy.10 Pemetrexed was approved by the Chinese Food and Drug Administration in 2011 for the initial treatment of patients with locally advanced or metastatic nonsquamous NSCLC when used in combination with cisplatin.11
In China, Eli Lilly and the China Primary Health Care Foundation are currently implementing a patient assistance program (PAP), which allows first-line nonsquamous NSCLC patients who complete four cycles of pemetrexed induction therapy to receive free, continuous pemetrexed maintenance therapy. To date, the clinical and cost implications of the PAP have yet to be determined, and no health economic analyses have considered the topic. The objective of our study is to estimate the cost-effectiveness of pemetrexed maintenance therapy vs basic standard care (BSC) and the economic impacts of providing a PAP for patients who have completed pemetrexed induction therapy in a Chinese health care setting.
Methods
Approach
We developed a novel decision-analytic model to evaluate the long-term costs and clinical efficacy of pemetrexed plus BSC vs BSC alone. Only direct medical costs related to treatment regimens were estimated, including induction therapy, maintenance pemetrexed therapy, BSC therapy, and major adverse events. The analysis was conducted from a Chinese health care perspective using a lifetime horizon to enable assessment of life expectancy. All costs are reported in USD, and long-term costs and outcomes were discounted at 3% per year.12 We used a societal willingness-to-pay threshold of 39,900 USD per additional quality-adjusted life year (QALY), representing a threefold increase of the gross domestic product (GDP) per capita of China as recommended by the World Health Organization.13,14 The range from GDP per capita to three times GDP per capita (13,300–39,900 USD) was considered cost-effective, whereas any value less than GDP per capita ($13,300) was considered “very cost-effective”. All costs were converted to USD using an exchange rate of 1 USD =6.57 RMB.15 The models were programmed in Microsoft® Excel (Microsoft Corporation, Redmond, WA, USA). Ethical approval was not obtained because this is a health economic model based entirely on previously published literature and no patient level data was analyzed.
Population
The PARAMOUNT trial examined the efficacy of pemetrexed continuation maintenance therapy vs placebo in patients with advanced nonsquamous NSCLC whose disease had not progressed during four cycles of pemetrexed–cisplatin induction chemotherapy.16 The trial included 939 NSCLC patients, of which 539 patients did not progress while on induction therapy; then these patients were randomized 2:1 to receive either pemetrexed maintenance therapy or BSC.16 The median follow-up at the overall survival (OS) data cutoff date was 12.5 months (95% confidence interval [CI]: 11.1–13.7 months) for all patients and 24.3 months (95% CI: 23.2–25.1 months) for surviving patients.17
Patients randomized to pemetrexed had a significant reduction in the risk of disease progression compared with the placebo group (hazard ratio [HR]: 0.62; 95% CI: 0.49–0.79; p<0.0001)16 and a statistically significant reduction in the risk of death (HR: 0.78; 95% CI: 0.64–0.96; p=0.0195).17 The median progression-free survival (PFS) and OS for those on pemetrexed maintenance therapy were 4.1 months and 13.9 months compared with 2.8 months and 11 months for BSC, respectively.16,17 In addition, pemetrexed was well-tolerated by patients, although laboratory Grade 3–4 adverse events were more common in the pemetrexed group (33 [9%] of 359 patients) than in the placebo group (1 [<1%] of 180 patients; p<0.0001), as were non-laboratory Grade 3–5 adverse events (32 [9%] of 359 patients in the pemetrexed group; 8 [4%] of 180 patients in the placebo group; p=0.080).16
Model structure
We utilized a three-state (PFS, progressed disease, and dead) partition survival model, a commonly used method in advanced oncology indications, for both the clinical and economic aspects of the analysis. This approach uses the area under two survival curves (PFS and OS) to calculate the proportion of patients in each health state at a given time point.18 Partition survival models bypass the need to estimate discrete transition probabilities and avoid the need for additional assumptions by modeling the survival data directly. The approach is relevant for modeling the range of events typically considered in the analysis of cost-effectiveness of health technologies including OS, disease-free survival, and PFS.
Hypothetical patients began the model in the PFS health state, where they remained until they either experienced disease progression or death from other causes. Patients in the progressed disease state remained there until they either died from progressed disease or from other causes. Patient survival, quality-adjusted survival, and health care cost were estimated for each model cycle and then summarized over the entire time horizon for both treatment options. Model cycles were 1 month each to account for the high rates of disease progression in NSCLC. We utilized a 5-year time horizon, at which point 95% and 98% of pemetrexed plus BSC and BSC alone patients had died, respectively; we chose this short time horizon due to the rapid rate of disease progression and in recognition of the limits of data extrapolation validity.
Clinical inputs
We fit parametric Weibull curves to digitized copies of PARAMOUNT-reported BSC arm Kaplan–Meier PFS (as reassessed at the time of OS data cutoff) and final OS data17 using a maximum likelihood approach.19 Parametric survival modeling allows extrapolation of hypothetical patient outcomes beyond trial-reported follow-up time. To calculate the pemetrexed plus BSC arm survival, we applied the trial-reported PFS and OS HRs at the time of OS data cutoff to the BSC arm’s parametric curves (Table 1). We also modeled adverse event rates for anemia, neutropenia, and fatigue based on PARAMOUNT findings. Only adverse events with Grade 3–4 toxicity were considered.
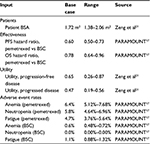  | Table 1 Clinical model inputs Abbreviation: BSA, body surface area; BSC, basic standard care; OS, overall survival; PFS, progression-free survival. |
Quality of life inputs
We derived health state utility values for progression-free and progressed disease health states from a community-based study in advanced NSCLC from the UK, which used the standard gamble interview and visual analog scale to assess quality of life (Table 1).20 Utility values were multiplied by the number of patients in each health state for each month in the time horizon, then summed for comparison between groups. Based on results of an EQ-5D questionnaire given during the PARAMOUNT trial, we assumed no significant differences in health conditions between the two study arms during maintenance therapy, and thus the same utility values were assigned to both treatment groups.
Cost inputs
Cost estimates for the model were largely derived from a previous Chinese perspective cost-effectiveness model of maintenance pemetrexed after cisplatin and pemetrexed chemotherapy for NSCLC (Table 2).21 Drug costs were local to China and were sourced from GBI health.22 For drug costs, we included the cost of induction therapy (four cycles) for both arms. We assigned a body surface area (used for calculating pemetrexed dosage) of 1.72 m2 for all patients. A multiplier of 0.3 was used for BSC costs between progressed and nonprogressed disease.21
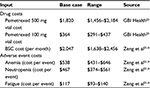  | Table 2 Cost inputs Note: *Data from Zeng X, Peng L, Li J, Chen G, et al, Cost-effectiveness of continuation maintenance pemetrexed after cisplatin and pemetrexed chemotherapy for advanced nonsquamous non-small-cell lung cancer: estimates from the perspective of the Chinese health care system, Clin Ther, 2013;35(1):54–65, Elsevier, copyright 2013.21 Abbreviation: BSC, best supportive care. |
Assumptions
We assumed that the HRs reported in the PARAMOUNT trial remained constant beyond trial-reported follow-up time in extrapolated survival estimates. In addition, we assumed that the PAP covered 100% of maintenance therapy costs, whereas non-PAP patients were charged the full cost.
Analysis
We conducted a cost-effectiveness analysis comparing pemetrexed plus BSC vs BSC alone and considered both costs and clinical outcomes. In the pemetrexed plus BSC arm, we considered scenarios where 1) pemetrexed is unsubsidized using the PAP and 2) pemetrexed is 100% subsidized using the PAP. We calculated life years, QALY, and lifetime direct medical costs for both treatment groups. The incremental health benefit of pemetrexed plus BSC vs BSC alone was calculated as the incremental difference between effectiveness and risk changes, with both risk and benefit measured using QALYs. To calculate the incremental net benefit, PAP and non-PAP QALYs were multiplied by the societal willingness to pay per additional QALY, then we subtracted the costs to the health care payer resulting in either a benefit or cost to society. If the value is positive, it is a net benefit to society; if negative, it is a net cost to society. The incremental cost-effectiveness ratio (ICER) was calculated as the difference in costs divided by the difference in QALYs.
Model uncertainty was evaluated using one-way and multivariate probabilistic sensitivity analysis. One-way sensitivity analysis was performed using values derived from CIs or reasonable ranges as determined from published sources. Multivariate probabilistic sensitivity analysis was performed using Monte Carlo simulation, in which the model inputs were drawn from probability distributions based on parameter ranges representing the uncertainty in the estimate.
An additional analysis was undertaken to better understand the real-world impact of introducing the PAP in China. This analysis compares the use of the PAP vs non-PAP. In the non-PAP arm, all patients are expected to receive BSC after induction therapy (Figure 1). In the PAP arm, the percentage of patients receiving maintenance therapy with pemetrexed was varied from 0% to 100%.
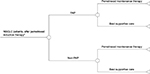  | Figure 1 Real-world PAP impact analysis. Note: aSurviving patients with nonprogressed disease. Abbreviations: NSCLC, non-small-cell lung cancer; PAP, patient assistance program. |
Results
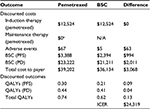
Pemetrexed plus BSC resulted in 1.41 (95% credible range [CR]: 1.19–1.70) life years and 0.74 (95% CR: 0.50–1.01) QALYs, at a cost of $64,238 (95% CR: $52,481–$78,432) without a PAP and $39,202 (95% CR: $31,278–$49,209) with a PAP (Table 3). The BSC group resulted in 1.19 (95% CR: 1.07–1.32) life years and 0.62 (95% CR: 0.43–0.82) QALYs, at a cost of $36,134 (95% CR: $29,817–$43,120).
Compared to BSC, pemetrexed plus BSC resulted in a gain of 0.22 years of life (95% CR: 0.05–0.45) and 0.13 QALYs (95% CR: 0.04–0.25) per patient, at an increased cost of $28,105 (95% CR: −$22,720 to $48,464) without a PAP and $3,068 (95% CR: −$1,263 to $9,163) with a PAP. The ICER for pemetrexed plus BSC vs BSC alone was cost-prohibitive at $222,700 for non-PAP, but cost-effective at $24,319 with a PAP.
Even though pemetrexed was not charged to payers in the PAP scenario, the increase in costs compared to BSC was primarily driven by the extended survival brought by pemetrexed, and secondarily by the cost of adverse events with pemetrexed. The PAP and non-PAP scenarios both accounted for pemetrexed maintenance therapy and thus had equivalent survival outcomes. However, the PAP scenario represented a cost-savings of $25,037 (95% CR: $33,516–$18,402) compared to non-PAP.
The results of the one-way sensitivity analyses are shown in Figure 2, incremental cost, QALYs, and the ICER were primarily influenced by the OS HR.
  | Figure 2 Tornado diagram. Abbreviations: ICER, incremental cost-effectiveness ratio; BSC, basic standard care; OS, overall survival. |
In probabilistic sensitivity analysis, PAP was cost-effective vs BSC in 90.2% of simulations at a $39,900 willingness-to-pay per QALY threshold, and 95.77% of simulations at a $45,662 (¥300,000) threshold. The cost-effectiveness scatterplot and cost-effectiveness acceptability curve graphs are shown in Figures 3 and 4.
  | Figure 3 Cost-effectiveness scatterplot. Abbreviation: QALYs, quality-adjusted life years. |
  | Figure 4 Cost-effectiveness acceptability curve. Abbreviations: WTP, willingness to pay; QALY, quality-adjusted life year. |
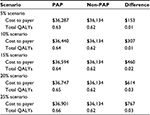
The additional analysis compared a scenario of a PAP vs non-PAP. In the non-PAP arm, all patients received BSC, whereas in the PAP arm, the percentage of patients receiving pemetrexed was varied from 5% to 25%. The results of this analysis (Table 4) show that as the percentage of patients receiving pemetrexed increases, both costs and QALYs increase consistently, resulting in the same ICER for each iteration.
Discussion
We developed a novel cost-effectiveness model to estimate the incremental cost and benefits associated with a PAP program for pemetrexed maintenance therapy in NSCLC patients from a Chinese societal perspective, based on the PARAMOUNT clinical trial and other published sources. We found that although pemetrexed maintenance therapy comes at considerable cost and is not cost-effective for the Chinese health care system at full price to payers, the addition of a PAP significantly drives the overall cost downward, making the survival benefits of pemetrexed maintenance therapy cost-effective and more widely available to patients.
A previous cost-effectiveness analysis looked at pemetrexed maintenance therapy without a PAP and similarly concluded that maintenance therapy was not cost-effective, suggesting a price reduction or dose adjustment was needed before widespread adoption by the Chinese health care system.20 Our analysis considers the former recommendation, with potential manufacturer assistance in the form of a 100% discount for maintenance therapy given the patient completes a full course of nondiscounted induction therapy. Once a PAP is implemented in China, our model suggests that pemetrexed maintenance therapy will be highly likely (>90%) to be cost-effective vs standard care.
Limitations
Our model has a number of limitations worth noting. First, the clinical data used in our model was limited to a single clinical trial and did not include Chinese patients.16,17 Although PARAMOUNT was a relatively large cancer trial, the incorporation of data from other studies may provide more robust estimates. Regarding the absence of Chinese patients, Lee et al22 and Liubao et al24 concluded that the influence of such differences between Chinese individuals and other nationalities were not significantly different.22,24 Similarly, our quality-of-life estimations were informed by a single study.
Second, we assumed that the survival HRs reported by PARAMOUNT investigators, summarizing approximately 2 years of follow-up, were held constant over the 5-year time horizon of our model. In the absence of long-term follow-up data, simplifying but data-driven assumptions are often necessary in modeling studies. Nonetheless, our modeled parametric fits to the rapid rates of progression and death in PARAMOUNT resulted in all patients progressing before the second year, and <1% of patients surviving to 5 years; thus, the brief time horizon of our analysis limited the impacts of extrapolating the distant future from relatively brief clinical trial.16,17
Third, multiple assumptions were undertaken in the original cost estimations presented by Zeng et al.21 BSC costs were not available for Chinese patients with advanced nonsquamous NSCLC, and so costs for patients with advanced gastric cancer were used.21
Finally, we did not consider other available regimens such as erlotinib for maintenance therapy of NSCLC following pemetrexed induction therapy as we evaluated patients receiving pemetrexed who would otherwise be receiving BSC if not for the PAP.
Conclusion
From the perspective of the Chinese health care system, our study suggests that maintenance pemetrexed therapy after pemetrexed induction for patients with advanced NSCLC is likely to be highly non-cost-effective in the absence of a PAP, but the pending implementation of the PAP promises to make it cost-effective, with a >90% probability of cost-effectiveness at a Chinese willingness-to-pay threshold per QALY. Ongoing and future comparative clinical trials will provide valuable insight into the optimal treatment in second-line NSCLC, but presently, pemetrexed maintenance therapy combined with a PAP offers the best treatment option for NSCLC patients.
Acknowledgment
This study was funded by Lilly Suzhou Pharmaceutical Co., Ltd.
Disclosure
BCMW, GFG, and WEF are paid consultants to Lilly Suzhou Pharmaceutical Co., Ltd. QS and JS are employees of Lilly Suzhou Pharmaceutical Co., Ltd. The authors report no other conflicts of interest in this work.
References
Chen W, Zheng R, Zeng H, Zhang S. Epidemiology of lung cancer in China. Thorac Cancer. 2015;6(2):209–215. | ||
Shi Y, Sun Y, Ding C, et al. China experts consensus on icotinib for non-small cell lung cancer treatment (2015 version). Ann Transl Med. 2015;3(18):260. | ||
Chen Y, Han S, Zheng MJ, Xue Y, Liu WC. Clinical characteristics of 274 non-small cell lung cancer patients in China. Onkologie. 2013;36(5):248–254. | ||
Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet. 2009;374:1432–1440. | ||
Fidias PM, Dakhil SR, Lyss AP, et al. Phase III study of immediate compared with delayed docetaxel after front-line therapy with gemcitabine plus carboplatin in advanced non-small-cell lung cancer. J Clin Oncol. 2009;27:591–598. | ||
Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010;11:521–529. | ||
Brodowicz T, Krzakowski M, Zwitter M, et al. Cisplatin and gemcitabine first-line chemotherapy followed by maintenance gemcitabine or best supportive care in advanced non-small cell lung cancer: a phase III trial. Lung Cancer. 2006;52:155–163. | ||
Pérol M, Chouaid C, Pérol D, et al. Randomized, phase III study of gemcitabine or erlotinib maintenance therapy versus observation, with predefined second-line treatment, after cisplatin-gemcitabine induction chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2012;30:3516–3524. | ||
Chattopadhyay S, Moran RG, Goldman ID. Pemetrexed: biochemical and cellular pharmacology, mechanisms, and clinical applications. Mol Cancer Ther. 2007;6(2):404–417. | ||
Alimta (pemetrexed) [prescribing information]. Indianapolis, IN: Eli Lilly and Company; 2015. | ||
Shi Y, Sun Y. Medical management of lung cancer: experience in China. Thorac Cancer. 2015;6(1):10–16. | ||
Gold M, Seigel J, Russell L, Weinstein M. Cost-Effectiveness in Health and Medicine. Oxford, UK: Oxford University Press; 1996. | ||
Edejer T-T, Baltussen R, Adam T, et al, editors. WHO Guide to Cost-Effectiveness Analysis. Geneva, Switzerland: World Health Organization; 2003. | ||
Central Intelligence Agency. World Factbook. China. GDP Per Capita (PPP) 2014. Available from: https://www.cia.gov/library/publications/the-world-factbook/geos/ch.html. Accessed October 17, 2016. | ||
Google Finance. FX Rate. Available from: https://www.google.com/finance?q=USDCNY&ei=QAWOV_mrDsWy2AaTvqL4Dg. Accessed February 9, 2016. | ||
Paz-Ares L, de Marinis F, Dediu M, et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol. 2012;13:247–255. | ||
Paz-Ares LG, de Marinis F, Dediu M, et al. PARAMOUNT: final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol. 2013;31(23):2895–2902. | ||
Glasziou PP, Simes RJ, Gelber RD. Quality adjusted survival analysis. Stat Med. 1990;9(11):1259–1276. | ||
Hoyle MW, Henley W. Improved curve fits to summary survival data: application to economic evaluation of health technologies. BMC Med Res Methodol. 2011;11:139. | ||
Nafees B, Stafford M, Bhalla S, Watkins J. Health utilities in the UK for second line non-small cell lung cancer. Value Health. 2006;9:A296. | ||
Zeng X, Peng L, Li J, Chen G, et al. Cost-effectiveness of continuation maintenance pemetrexed after cisplatin and pemetrexed chemotherapy for advanced nonsquamous non-small-cell lung cancer: estimates from the perspective of the Chinese health care system. Clin Ther. 2013;35(1):54–65. | ||
Lee SG, Jee YG, Chung HC, et al. Cost-effectiveness analysis of adjuvant therapy for node positive breast cancer in Korea: docetaxel, doxorubicin and cyclophosphamide (TAC) versus fluororacil, doxorubicin and cyclophosphamide (FAC). Breast Cancer Res Treat. 2009;114:589–595. | ||
GBI Health. Max Retail Pricing. GB Group Limited. Available from: http://source.gbihealth.com/Pricing/MaxRetailPricing. Accessed October 19, 2016. | ||
Liubao P, Xiaomin W, Chongqing T, et al. Cost-effectiveness analysis of adjuvant therapy for operable breast cancer from a Chinese perspective: doxorubicin plus cyclophosphamide versus docetaxel plus cyclophosphamide. Pharmacoeconomics. 2009;27:873–886. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.