Back to Journals » Research and Reports in Urology » Volume 14
The Correlation Between Platelet Count and Survival in Prostate Cancer
Authors Mezei T , Bőde I, Tenke P, Jósa V, Merkel K, Szilasi Z, Tordai A, Máthé D, Baranyai Z
Received 30 January 2022
Accepted for publication 16 April 2022
Published 6 May 2022 Volume 2022:14 Pages 193—202
DOI https://doi.org/10.2147/RRU.S359715
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Guglielmo Mantica
Tünde Mezei,1 Imre Bőde,1 Péter Tenke,1 Valéria Jósa,2 Keresztély Merkel,3 Zsuzsanna Szilasi,4 Attila Tordai,5 Domokos Máthé,6 Zsolt Baranyai7
1Department of Urology, Jahn Ferenc South-Pest Hospital, Budapest, Hungary; 2Department of Otorhinolaryngology and Head and Neck Surgery, Jahn Ferenc South-Pest Hospital, Budapest, Hungary; 3Department of Surgery, Szent Imre Hospital, Budapest, Hungary; 4Department of Otorhinolaryngology and Head and Neck Surgery, HDF Medical Centre, Budapest, Hungary; 5Institute of Pathophysiology, Semmelweis University, Budapest, Hungary; 6Department of Biophysics and Radiation Biology, Semmelweis University, Budapest, Hungary; 7Semmelweis University, Department of Transplantation and Surgery, Budapest, Hungary
Correspondence: Tünde Mezei, Jahn Ferenc South-Pest Hospital, Department of Urology, Budapest, Hungary, Köves str 1, Budapest, 1204, Hungary, Tel +3620/2013038, Email [email protected]
Purpose: A number of studies have confirmed that elevated platelet count accompanying various solid tumours is associated with worse survival. However, only meagre data are available on the relationship between thrombocytosis and survival in prostate cancer.
Methods: We conducted a retrospective analysis on clinical-pathological data accumulated from 316 patients during on average 51 months of follow-up after laparoscopic prostatectomy performed for prostate cancer. We analyzed the relationship between platelet count, risk factors, prostate-specific antigen (PSA) and cancer stage with use the Tumor, Node, Metastase system (TNM), as well as surgical margin, and prognosis.
Results: Thrombocytosis occurred in only one out of the 316 patients. The multivariate Cox proportional hazard model showed that preoperative PSA, risk group, preoperative haemoglobin level, and surgical margin status were significant, independent predictors of biochemical progression-free survival. By contrast, age at diagnosis and thrombocytosis had no such predictive value.
Conclusion: We could not demonstrate an association between elevated platelet count and worse survival in our study population of patients with prostate cancer.
Keywords: prostate cancer, thrombocytosis, prognosis, pathomechanism, solid tumor
Introduction
Cancer of the prostate is the second most common malignancy in males. In 2017, nearly 1.3 million cases were diagnosed and 416,000 related deaths were recorded. During the past decade, the incidence of the disease increased by 42%. Twenty-one per cent of this increment is due to changes in the age distribution and 13% is related to the growth of the population, as well as 8% is the result of changes in age-specific rates.1
While standard assessment tools for risk stratification are informative, prostate cancers may behave uncharacteristically with natural history or relapse patterns that are sometimes unpredictable Similar clinical and histologic patterns at diagnosis may lead to variable clinical outcomes across patients. Consequently, biomarkers that are capable of significantly improving risk stratification, distinguishing indolent versus aggressive prostate cancer, remain an unmet need.2 The measurement of prostate-specific antigen (PSA) level is suitable for establishing the diagnosis and to follow-up the effectiveness of the management of prostate cancer; however, it is inadequate by itself for estimating prognosis. Prostate-specific antigen velocity (PSAV) and PSA doubling time (PSA-DT) may have a prognostic role in treating prostate cancer (PCa) but have limited diagnostic use because of background noise (total prostate volume, and benign prostatic hyperplasia), different intervals between PSA determinations, and acceleration/deceleration of PSAV and PSA-DT over time. Free/total PSA is of no clinical use if the total serum PSA is >10 ng/mL or during follow-up of known PCa. The clinical value of f/t PSA is limited in light of the new diagnostic pathways incorporating Magnetic Resonance Imaging (MRI).3
Increasing evidence suggests that inflammation plays an essential role in cancer development and progression. Several studies demonstrated that inflammation marker neutrophil–lymphocyte ratio (NLR) had prognostic value in localized and advanced PCa; on the contrary, other studies reported that high serum NLR had no prognostic value in patients with PCa.4
Other study shows that systemic immune-inflammation index (SII), which is calculated as SII = platelet × NLR, appear to be a significant diagnostic marker in patients with high-grade PCa.5
Thrombocytosis observed in oncology patients has been reported to entail a worse prognosis in a variety of solid tumours: colorectal,6,7 breast,8 lung,6,9 renal,6,10 cervical,11 ovarian12 and brain tumours.13 Only meagre data are available in the literature on the relationship between thrombocytosis and prostate cancer. M
Our objective was to ascertain whether prostate cancer is similarly associated with elevated platelet count in our study population, as well as whether any relationship exists between thrombocytosis and progression-free survival or overall survival.5 Due to the meagre results available, we try to place our results in the international literature.
Materials and Methods
We performed a retrospective analysis of the clinical-pathology data of the patients treated with laparoscopic prostatectomy at the “Jahn Ferenc” South-Pest Hospital (Budapest, Hungary) between 2011 and 2014. During this period, all surgical operations were performed at our institute by laparoscopy. The criterion for inclusion was the presence of a histologically confirmed prostate tumour (with the exception of clinical T4, N2, and M+ cases); data from 321 patients in total were analyzed. The presence of a synchronous tumour (other than the prostate cancer), any inflammatory disease (such as pneumonia, abscess, cholecystitis, endocarditis, urinary tract infection, Crohn’s disease, ulcerative colitis), thromboembolic events (deep-vein thrombosis, pulmonary embolism, myocardial infarction), corticosteroid therapy or other diseases that may affect the platelet count (myelodysplastic disease, etc.) were criteria for exclusion, which eliminated five patients altogether from the study. The data gathered during follow-up were analyzed along with the abnormalities detected by physical examination, as well as with the changes in PSA levels. PSA level was measured one month after surgery – then, at 3-month intervals in the first, and at 6-month intervals during the second to fifth postoperative year, and at yearly intervals thereafter.
Platelet count was determined preoperatively within 14 days prior to surgery or before the start of neoadjuvant therapy and assessed in the same laboratory.
Thrombocytosis was defined as a platelet count exceeding the upper limit of the normal range (>400 G/L), but we also performed analysis between elevated platelet count and survival at other cut-off values (for example, mean platelet value). Anaemia was diagnosed when the haemoglobin level was lower than 130 g/L. The patients were categorized into low-, intermediate-, and high-risk groups according to the classification by D’Amico.16 Within the framework of multimodal management, a proportion of the high-risk patients received neoadjuvant therapy in the form of androgen deprivation (ADT). During surgery, lymph node dissection was performed as recommended by the guideline.3,17 In particular, lymph node dissection was not performed in low-risk cases but was always carried out in high-risk patients. In intermediate-risk patients, the decision to perform lymphadenectomy was based on the Briganti nomogram.18 Patients exhibiting disease progression received ADT, irradiation, or the combination of these modalities. In conformity with the European consensus, progression-free survival (PFS) was defined as survival until biochemical relapse (>0.2 ng/mL increase of PSA level between two subsequent measurements after radical prostatectomy),19 which was the primary endpoint of our study. Overall survival (OS) was calculated from the time of surgery to the tumour-related death of the patient, or to the end of follow-up.
Statistical Methodology
Statistical analyses for categorical variables were performed by the χ2 or the Fisher’s exact tests and for continuous variables by the Mann–Whitney test. PFS and OS data were analyzed by the Log rank test and Kaplan–Meier estimates were computed. In multivariate survival analyses, Cox proportional hazard models were adjusted for age, PSA levels, Gleason score, risk scores, and wound edge positivity. The analyses were performed with the SPSS Statistics Software, version 22. Result is significant if the p-value is less than 0.05.
Results
Clinical pathology data from 316 patients with prostate cancer were analyzed. Patients with T4 stage disease were not operated upon based on the inclusion criteria, as well as those with T1 stage prostate cancer were missing from the study population. The majority of patients belonged to the intermediate-risk group (Table 1).
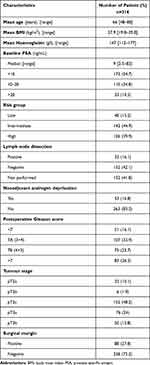 |
Table 1 The Clinical Pathology Data of the Subjects |
The mean duration of follow-up was 51 (5 to 80) months during which disease progression occurred in 25.9% of the patients. Importantly, mean survival was not significantly different between the patient subsets with or without disease progression (48 versus 52 months). Eighty-two per cent of patients with biochemical relapse underwent adjuvant therapy by pelvic irradiation (n = 21), hormone therapy (n = 16), or the combination of these two modalities (n = 30). Six patients (1.89%) died of tumour progression during follow-up. Before surgery, 87 patients (27.5%) were treated with platelet aggregation inhibitors: 68 received acetylsalicylic acid and 19 clopidogrel. The administration of these agents was discontinued before the operation and hence, their effects did not interfere with preoperative laboratory testing. In addition to platelet count, we studied the impact of other factors known to influence survival by performing the Kaplan–Meier univariate analysis (Table 2).
 |
Table 2 The Impact of Platelet Count and of the Known Factors Influencing Survival on PFS (Kaplan–Meier Univariate Analysis) |
Higher haemoglobin levels were associated with significantly longer survival. This correlation was significant (p < 0.0005) even if the median haemoglobin concentration (Hgb <147 g/L) was defined as the cut-off level. Mean platelet count was 214 (114–569) G/L. Thrombocytosis occurred in just one patient and this made further statistical analysis inappropriate. We could not demonstrate a statistically significant correlation between elevated platelet count and survival at other cut-off values either (mean PLT: 214 G/L, p = 0.333, or PLT: 300 G/L, p = 0.149). We also examined whether a lower platelet count is associated with worse survival. Mean platelet count did not increase with the progression of the tumour stage, although there were only six patients with T2b stage disease in the study population (Table 3).
 |
Table 3 Survival Data by Disease Stage and Platelet Count |
The multivariate Cox proportional hazard model showed that preoperative PSA, preoperative haemoglobin, risk group, and surgical margin status were significant, independent predictors of biochemical PFS. By contrast, age at diagnosis and thrombocytosis had no such predictive value (Table 4).
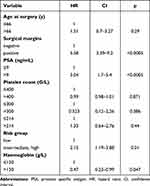 |
Table 4 Multivariate Cox Proportional Hazards Model for the Variables Used to Predict the Risk of Disease Progression |
Discussion
The pathomechanism of the relationship between solid tumour-associated thrombocytosis and survival is not known. Several hypotheses have been proposed. Platelets may be activated by tumour cells in a process known as the tumour cell-induced platelet aggregation. Platelet activation may occur either by direct interaction with tumour cells,20 or indirectly, through the mediation of adenosine diphosphate (ADP), thromboxane A2, or metalloproteinases.21 Additionally, tumour cells may secrete thrombin, or may stimulate pro-thrombotic activity in other tissues, and can thereby contribute to enhanced platelet activation.22 Activated platelets facilitate the growth of tumour cells by releasing a number of angiogenic and tumour growth factors. These include thrombospondin, platelet factor 4, transforming growth factor-beta, vascular endothelial growth factor (VEGF), and platelet-derived growth factor.23–27 These processes induce angiogenesis, which also leads to the acceleration of tumour growth.28–33 Additionally, tumour cells secrete VEGF which activates the coagulation cascade – this has an activating effect also on platelets and enhances the adhesion of the latter to the endothelial surface of blood vessels in the tumour.31 Accordingly, the tumour cells and the platelets are involved in a mutually potentiating interaction. Activated platelets release micro-vesicles which increase the invasive potential of the tumour cells.34 These observations led to the conclusion that the emboli containing platelets and tumour cells contribute to the formation of tumour metastases. Moreover, the platelets form a cloak around tumour cells and thereby prevent their recognition by the natural killer cells.35 Platelets can also interfere with dendritic cells, neutrophils, macrophages and lymphocytes and modulate their immunological function.36 In their studies conducted on patients with ovarian cancer,
IL-6 is a multifunctional pro-inflammatory cytokine which is expressed in clinical specimens obtained from patients with prostate cancer and in multiple cell lines.38 The review by N
Biochemical progression-free survival varies between 38% and 51% at 10 years if patients are treated with radical prostatectomy as monotherapy.3 In our study population, biochemical progression-free survival is 74.1% at the mean follow-up of 51 months.
So far, all instances of solid tumour-related thrombocytosis were associated with worse survival. However, the definition of thrombocytosis is dependent on the selected cut-off value, and this often makes the comparison of the published data difficult. For example, SHIMODAIRA et al used 300 G/L as cut-off value,43 whereas GUTIONTOV et al used 187 G/L.44 According to the literature, the prevalence of thrombocytosis ranges between 8% and 49% in colorectal cancer, 2.7% and 8.2% in hepatic tumours, and 16% and 27% in pancreatic tumours.7 In contrast with the findings from studies conducted in other malignancies, we could not demonstrate a statistical relationship between elevated platelet count and poor survival in patients with prostate cancer. We measured a platelet count corresponding to the upper limit of the normal range (400 G/L) in just one patient (0.3%) – this proportion is lower than that characteristic of the general population without malignancy (1.5 to 2.2%).45
Between 2011 and 2014 there were 55 patients in our institute who were diagnosed with prostate cancer with bone metastasis (Tany, Nany, M1). 87% of the patients had multiple and 13% had oligo metastasis. Mean PLT count was 237 G/L (114–355). Two patients had thrombocytopenia (3,6%) and none of them had thrombocytosis. Neither thrombocytopenia (p = 0.248) nor thrombocytosis was related to overall survival based on Kaplan–Meier analysis.
By contrast, S
Our objective was to ascertain whether any relationship exists between thrombocytosis and worse survival in prostate cancer. Prostate cancer has a good prognosis, as the five-year survival rate is 97.8%.51 In our study population, the survival rate is 98.1% after 51 months. We could not demonstrate a statistical relationship between elevated platelet count and poor survival in patients with prostate cancer, because they have a good survival compared with other solid tumors. The 5 year survival rate is 20.5% in lung cancer and 64.6% in colon cancer.51 In both lung and colon cancer, a relationship has been observed between thrombocytosis and worse prognosis.6,7,9 BAILEY et al found the same trend after they investigated 40,000 patients with thrombocytosis in their study, that is, lung and colorectal cancer were much more commonly diagnosed in patients with thrombocytosis than in the general population, whereas breast and prostate cancer were much less commonly diagnosed.45 In a case where a patient’s prognosis is already good, there might be less inclination to use platelet counts as a measure of prognosis.
In contrast with other solid tumours, the absence of thrombocytosis in prostate cancer appears intriguing. The prostate is enveloped by a dual-layer capsule: the inner capsule is a thin, fibrous shell – the visceral layer of the pelvic fascia, actually. The venous plexus of the prostate gland is located between the two layers of the capsule. The inner capsule might have a protective function and if this is the case, the tumour is not exposed to the factors that possibly induce thrombocytosis. However, this is contradicted by the fact that we could not detect thrombocytosis even in Stage T3 disease where the tumour extends beyond the capsule. Another possible explanation is the existence of a possible prostate-blood barrier,52 which has been suggested during the management of chronic prostatitis. The active component of the blood-prostate barrier is still unknown; some researchers consider that vascular endothelial cells and the basement membrane constitute this barrier, whereas epithelial cells are deemed essential by others.53 However, even if this prostate-blood barrier existed and functioned similarly to the blood-brain barrier, this hypothesis is contradicted by the fact that thrombocytosis has been detected in patients with brain tumours.13 Furthermore, it should also be noted that prostate cancer most often metastasizes to the bone marrow, that is, to a location where platelets are generated.
If we accept that thrombocytosis is caused by the IL-6 produced by prostate cancer cells, while IL-6 is only produced by the hormone-refractory cells, it can explain why none of our patients had thrombocytosis. However, it does not account for the discrepancies found in the literature.
The findings of this study have to be seen in light of some limitations. First, this is a single-center, retrospective study. In particular, we studied patients treated by surgery, therefore our study population was not representative of cases with early-stage or advanced metastatic disease.
Conclusion
One can only wonder why we found lower platelet counts in prostate cancer than those published for other solid tumours. Elucidating the underlying cause of this difference might contribute to a better understanding of the relationship between thrombocytosis and solid tumours. It appears worthwhile to repeat our study in a larger patient population with a wider range of tumour stages.
Data Sharing Statement
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval
Ethical approval for this study was obtained from The Hungarian Scientific and Research Ethics Committee of Medical Research Council, approval number: 8951-0/2015/EKU (0444/15).
Informed consent was obtained from the subjects in accordance with the Declaration of Helsinki. All participants provided written informed consent prior to enrolment in the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Fitzmaurice C, Abate D, Abbasi N, et al.; Global Burden of Disease Cancer C. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol. 2019;5(12):1749–1768.
2. Eggener SE, Rumble RB, Armstrong AJ, et al. Molecular biomarkers in localized prostate cancer: ASCO guideline. J Clin Oncol. 2020;38(13):1474–1494.
3. Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014;65(1):124–137.
4. Tang L, Li X, Wang B, et al. Prognostic value of neutrophil-to-lymphocyte ratio in localized and advanced prostate cancer: a systematic review and meta-analysis. PLoS One. 2016;11(4):e0153981.
5. Sonmez G, Demirtas T, Tombul ST, Akgun H, Demirtas A. Diagnostic efficiency of systemic immune-inflammation index in fusion prostate biopsy. Actas Urol Esp. 2021;45(5):359–365.
6. Zhang X, Lv Z, Yu H, Zhu J. The clinicopathological and prognostic role of thrombocytosis in patients with cancer: a meta-analysis. Oncol Lett. 2017;13(6):5002–5008.
7. Baranyai Z, Josa V, Toth A, et al. Paraneoplastic thrombocytosis in gastrointestinal cancer. Platelets. 2016;27(4):269–275.
8. Taucher S, Salat A, Gnant M, et al. Impact of pretreatment thrombocytosis on survival in primary breast cancer. Thromb Haemost. 2003;89(6):1098–1106.
9. Maraz A, Furak J, Varga Z, Kahan Z, Tiszlavicz L, Hideghety K. Thrombocytosis has a negative prognostic value in lung cancer. Anticancer Res. 2013;33(4):1725–1729.
10. Bensalah K, Leray E, Fergelot P, et al. Prognostic value of thrombocytosis in renal cell carcinoma. J Urol. 2006;175(3 Pt 1):859–863.
11. Cheng J, Zeng Z, Ye Q, et al. The association of pretreatment thrombocytosis with prognosis and clinicopathological significance in cervical cancer: a systematic review and meta-analysis. Oncotarget. 2017;8(15):24327–24336.
12. Ye Q, Cheng J, Ye M, Liu D, Zhang Y. Association of pretreatment thrombocytosis with prognosis in ovarian cancer: a systematic review and meta-analysis. J Gynecol Oncol. 2019;30(1):e5.
13. Brockmann MA, Giese A, Mueller K, et al. Preoperative thrombocytosis predicts poor survival in patients with glioblastoma. Neuro Oncol. 2007;9(3):335–342.
14. Mounce LT, Hamilton W, Bailey SE. Cancer incidence following a high-normal platelet count: cohort study using electronic healthcare records from English primary care. Br J Gen Pract. 2020;70(698):e622–e8.
15. Watts EL, Perez-Cornago A, Kothari J, Allen NE, Travis RC, Key TJ. Hematologic markers and prostate cancer risk: a prospective analysis in UK Biobank. Cancer Epidemiol Biomarkers Prev. 2020;29(8):1615–1626.
16. D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280(11):969–974.
17. Heidenreich A, Bellmunt J, Bolla M, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol. 2011;59(1):61–71.
18. Briganti A, Larcher A, Abdollah F, et al. Updated nomogram predicting lymph node invasion in patients with prostate cancer undergoing extended pelvic lymph node dissection: the essential importance of percentage of positive cores. Eur Urol. 2012;61(3):480–487.
19. Boccon-Gibod L, Djavan WB, Hammerer P, et al. Management of prostate-specific antigen relapse in prostate cancer: a European Consensus. Int J Clin Pract. 2004;58(4):382–390.
20. Suzuki-Inoue K. Essential in vivo roles of the platelet activation receptor CLEC-2 in tumour metastasis, lymphangiogenesis and thrombus formation. J Biochem. 2011;150(2):127–132.
21. Medina C, Jurasz P, Santos-Martinez MJ, et al. Platelet aggregation-induced by caco-2 cells: regulation by matrix metalloproteinase-2 and adenosine diphosphate. J Pharmacol Exp Ther. 2006;317(2):739–745.
22. Danckwardt S, Hentze MW, Kulozik AE. Pathologies at the nexus of blood coagulation and inflammation: thrombin in hemostasis, cancer, and beyond. J Mol Med. 2013;91(11):1257–1271.
23. Assoian RK, Sporn MB. Type beta transforming growth factor in human platelets: release during platelet degranulation and action on vascular smooth muscle cells. J Cell Biol. 1986;102(4):1217–1223.
24. Dubernard V, Arbeille BB, Lemesle MB, Legrand C. Evidence for an alpha-granular pool of the cytoskeletal protein alpha-actinin in human platelets that redistributes with the adhesive glycoprotein thrombospondin-1 during the exocytotic process. Arterioscler Thromb Vasc Biol. 1997;17(10):2293–2305.
25. Hernandez E, Lavine M, Dunton CJ, Gracely E, Parker J. Poor prognosis associated with thrombocytosis in patients with cervical cancer. Cancer. 1992;69(12):2975–2977.
26. Ikeda M, Furukawa H, Imamura H, et al. Poor prognosis associated with thrombocytosis in patients with gastric cancer. Ann Surg Oncol. 2002;9(3):287–291.
27. Kaplan KL, Broekman MJ, Chernoff A, Lesznik GR, Drillings M. Platelet alpha-granule proteins: studies on release and subcellular localization. Blood. 1979;53(4):604–618.
28. Gupta GP, Massague J. Platelets and metastasis revisited: a novel fatty link. J Clin Invest. 2004;114(12):1691–1693.
29. Jurasz P, Alonso-Escolano D, Radomski MW. Platelet–cancer interactions: mechanisms and pharmacology of tumour cell-induced platelet aggregation. Br J Pharmacol. 2004;143(7):819–826.
30. Tsuruo T, Fujita N. Platelet aggregation in the formation of tumor metastasis. Proc Jpn Acad Ser B Phys Biol Sci. 2008;84(6):189–198.
31. Verheul HM, Hoekman K, Lupu F, et al. Platelet and coagulation activation with vascular endothelial growth factor generation in soft tissue sarcomas. Clin Cancer Res. 2000;6(1):166–171.
32. Banks RE, Forbes MA, Kinsey SE, et al. Release of the angiogenic cytokine vascular endothelial growth factor (VEGF) from platelets: significance for VEGF measurements and cancer biology. Br J Cancer. 1998;77(6):956–964.
33. Trikha M, Nakada MT. Platelets and cancer: implications for antiangiogenic therapy. Semin Thromb Hemost. 2002;28(1):39–44.
34. Janowska-Wieczorek A, Wysoczynski M, Kijowski J, et al. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int J Cancer. 2005;113(5):752–760.
35. Nieswandt B, Hafner M, Echtenacher B, Mannel DN. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 1999;59(6):1295–1300.
36. Schlesinger M. Role of platelets and platelet receptors in cancer metastasis. J Hematol Oncol. 2018;11(1):125.
37. Stone RL, Nick AM, McNeish IA, et al. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med. 2012;366(7):610–618.
38. Culig Z. Proinflammatory cytokine interleukin-6 in prostate carcinogenesis. Am J Clin Exp Urol. 2014;2(3):231–238.
39. Nguyen DP, Li J, Tewari AK. Inflammation and prostate cancer: the role of interleukin 6 (IL-6). BJU Int. 2014;113(6):986–992.
40. Adler HL, McCurdy MA, Kattan MW, Timme TL, Scardino PT, Thompson TC. Elevated levels of circulating interleukin-6 and transforming growth factor-beta1 in patients with metastatic prostatic carcinoma. J Urol. 1999;161(1):182–187.
41. Wise GJ, Marella VK, Talluri G, Shirazian D. Cytokine variations in patients with hormone treated prostate cancer. J Urol. 2000;164(3 Pt 1):722–725.
42. Chung TD, Yu JJ, Spiotto MT, Bartkowski M, Simons JW. Characterization of the role of IL-6 in the progression of prostate cancer. Prostate. 1999;38(3):199–207.
43. Shimodaira K, Nakashima J, Nakagami Y, et al. Prognostic value of platelet counts in patients with metastatic prostate cancer treated with endocrine therapy. Urol J. 2020;17(1):42–49.
44. Gutiontov SI, Choe KS, Miller JL, Liauw SL. Improved outcomes after radiotherapy for prostate cancer: anticoagulation, antiplatelet therapy, and platelet count as key factors in disease progression. Cancer Med. 2020;9(13):4667–4675.
45. Bailey SE, Ukoumunne OC, Shephard EA, Hamilton W. Clinical relevance of thrombocytosis in primary care: a prospective cohort study of cancer incidence using English electronic medical records and cancer registry data. Br J Gen Pract. 2017;67(659):e405–e13.
46. Sylman JL, Boyce HB, Mitrugno A, et al. A temporal examination of platelet counts as a predictor of prognosis in lung, prostate, and colon cancer patients. Sci Rep. 2018;8(1):6564.
47. Vidal AC, Howard LE, de Hoedt A, et al. Neutrophil, lymphocyte and platelet counts, and risk of prostate cancer outcomes in white and black men: results from the SEARCH database. Cancer Causes Control. 2018;29(6):581–588.
48. Wong CK, Namdarian B, Chua J, et al. Levels of a subpopulation of platelets, but not circulating endothelial cells, predict early treatment failure in prostate cancer patients after prostatectomy. Br J Cancer. 2012;107(9):1564–1573.
49. Nieder C, Haukland E, Pawinski A, Dalhaug A. Anaemia and thrombocytopenia in patients with prostate cancer and bone metastases. BMC Cancer. 2010;10:284.
50. Leblanc R, Peyruchaud O. The role of platelets and megakaryocytes in bone metastasis. J Bone Oncol. 2016;5(3):109–111.
51. website NCI. Available from: https://www.cancer.gov.
52. Fulmer BR, Turner TT. A blood-prostate barrier restricts cell and molecular movement across the rat ventral prostate epithelium. J Urol. 2000;163(5):1591–1594.
53. Shang Y, Cui D, Yi S. Opening tight junctions may be key to opening the blood-prostate barrier. Med Sci Monit. 2014;20:2504–2507.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
