Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
The Characteristics of the Skin Physiological Parameters and Facial Microbiome of “Ideal Skin” in Shanghai Women
Authors Ma L , Niu Y , Yuan C, Bai T, Yang S, Wang M, Li Y, Shao L
Received 15 December 2022
Accepted for publication 24 January 2023
Published 3 February 2023 Volume 2023:16 Pages 325—337
DOI https://doi.org/10.2147/CCID.S400321
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Laiji Ma,1,2 Yujie Niu,1,2 Chunying Yuan,3 Tianming Bai,3 Suzhen Yang,3 Man Wang,4 Yan Li,3 Li Shao1
1School of Perfume and Aroma Technology, Shanghai Institute of Technology, Shanghai, People’s Republic of China; 2The Oriental Beauty Valley Research Institute, Shanghai Institute of Technology, Shanghai, People’s Republic of China; 3R&D Innovation Center, Shandong Freda Biotech Co., Ltd., Jinan, Shandong, People’s Republic of China; 4Department of Nutrition, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital South Campus, Shanghai, People’s Republic of China
Correspondence: Li Shao, School of Perfume and Aroma Technology, Shanghai Institute of Technology, No. 100, Haiquan Road, Fengxian District, Shanghai, 201418, People’s Republic of China, Email [email protected] Yan Li, R&D Innovation Center, Shandong Freda Biotech Co., Ltd, No. 888, Xinluo Street, Lixia District, Jinan, Shandong, 250101, People’s Republic of China, Email [email protected]
Purpose: Everyone pursues perfect skin, but there exist significant differences between cultures, and no commonly accepted standards have been established. Therefore, our study attempted to define the “ideal skin” of oriental women and analyze the relationship between different skin physiological parameters and microbiomes.
Patients and Methods: Based on our customized grading standard, the VISIA CR photos of 111 young women aged from 18 to 25 in Shanghai were collected and scored by the severity of pores, acne, spots, and wrinkles. The volunteers were then divided into “ideal skin” (W1), “normal skin” (W2), and “undesirable skin” (W3) groups. The physiological parameters of facial skin were measured by non-invasive instrumental methods, and the skin microbiome was analyzed by 16S rRNA and ITS high-throughput sequencing.
Results: From “ideal skin” to “undesirable skin”, the skin physiological parameters, α-diversity, and composition of the facial microbiome showed noticeable regular changes. Compared with the “normal skin” (W2) and “undesirable skin” (W3), the “ideal skin” (W1) group had lower sebum content, TEWL, melanin, hemoglobin, and roughness but higher hydration content and skin pH value. Furthermore, the Shannon index of skin bacteria was significantly increased in W1 (P = 0.004), suggesting that the ideal skin had higher species diversity. From W1 to W3, the species composition was changed significantly. The abundance of Actinobacteria was increased, while Proteobacteria and Bacteroidetes were decreased. Correspondingly, the abundances of lipophilic Propionibacterium and Malassezia were increased, while the abundances of Stenotrophomonas, Pseudomonas, Ralstonia, and Streptococcus, were significantly decreased. Additionally, Spearman correlation analysis revealed strong correlations between the physiological parameters and the microbiota. Notably, the Shannon index of skin bacteria was significantly positively correlated with skin hydration (P = 0.03) but negatively correlated with the abundance of Cutibacterium (P = 0.000), hemoglobin content (P = 0.025), and sebum content (P = 0.5). Therefore, the skin hydration content and the abundance of Cutibacterium played an important role in maintaining the α-diversity and skin homeostasis.
Conclusion: Ideal skin had better water-oil balance and barrier function, higher microbial diversity, and more reasonable species distribution. Therefore, daily skincare needs to control skin oil and maintain skin microecological balance to achieve ideal skin conditions for young women aged 18– 25 years old.
Keywords: Shanghai women, ideal skin, definition, physiological parameters, skin microbiome
Introduction
In modern society, healthy skin and a beautiful appearance are considered the foundation of happiness. Beauty has been defined to be equal to happiness, life satisfaction, and a higher salary.1–4 Moreover, beautiful are always considered superior, possessing characteristics such as higher intelligence, higher moral character, more interpersonal skills, and easier access to groups and families.5–8 Therefore, as the zoologist Desmond Morris said, “flawless skin is the most universally desired human feature”.9
However, flawless skin is difficult to obtain, like everything in the world. The skin covers the entire surface of the human body, and it is in direct contact with the external environment. Therefore, various skin problems inevitably occur under the combined influence of endogenous and exogenous factors.10,11 For example, acne appears due to the increased sebum secretion caused by changes in hormone levels, and about 90% of people experience pain caused by acne.12–14 Personal living habits, diet, sleep patterns, stress, and so on have led to the occurrence of acne.15,16 Moreover, pore size is closely related to exuberant sebaceous gland secretion, skin aging, and increased hair follicle volume.17,18 Enlarged pores can make facial skin look rough, loose, and lackluster.17 In daily life, sunlight exposure can cause skin pigmentation and other problems. Facial pigmentation is not only related to sunlight exposure but also to hormone levels and genetics.19,20 The appearance of wrinkles is inevitable in skin aging, resulting from the combined action of endogenous factors (natural aging) and exogenous factors.21 As known, our skin is home to millions of bacteria, fungi, viruses and mites that compose the skin microbiota, which plays essential roles in protecting against invading pathogens, educating our immune system, and the breakdown of natural products.22 When the microecological balance is disturbed, various skin problems, such as sensitive skin, acne, atopic dermatitis, and psoriasis, will occur accordingly.23–27
Many factors affect the skin condition, such as the above-mentioned pigmentation, acne, enlarged pores, and wrinkles. The existence of these problems will decline facial beauty and make it difficult to achieve a perfect state, which has become one of the problems that contemporary women are very concerned about. “Smooth and tender, delicate and transparent, moist and flawless, elastic and healthy skin” is in line with the interpretation of oriental women’s ideal skin. In this era of pursuing youthful beauty, we are willing to invest more time and money in personal care to enhance facial characteristics, specifically to make us look younger, healthier, and more attractive. This has also promoted the successful development of the personal care industry, undoubtedly bringing challenges and opportunities to cosmetic research and development.
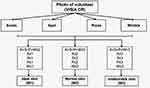
The pursuit of ideal skin has become the goal pursued by beauty, while what kind of skin belongs to perfect or ideal skin remains unclear. There are great differences between different cultures, and no commonly accepted standards have been established. Therefore, in our present study, we attempted to define the “ideal skin” of oriental women based on the understanding and aesthetics of oriental people for perfect skin. According to VISIA CR photos, female volunteers aged 18–25 in Shanghai (n=111) were divided into “ideal skin” (W1), “normal skin” (W2), and “undesirable skin” (W3) groups based on our customized scoring standard using pores, acne, spots, and wrinkles as evaluating indicators. We further analyzed the skin physiological parameters and facial microbiome of oriental women to obtain the characteristics of the ideal skin. This research would provide a scientific basis for skin care.
Materials and Methods
Volunteer Recruitment
This study was based on the investigation of the correlation between skin health status and skin and gut microbiota of qualified volunteers living in Shanghai (aged 18–60, n=494). Our research complied with the Declaration of Helsinki. Ethical approval was provided by the Institutional Ethics Committee of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital South Campus (Shanghai Fengxian District Central Hospital, Approval No.: 2021-KY-15). Written informed consent was obtained from each participant prior to sample collection. The inclusion criteria included the following items: healthy adults with 18–60 years of age who live in Shanghai; being aware of the research purpose; read, understood and signed the informed consent forms. The exclusion criteria included the following items: received anti-inflammatory drugs or antibiotics orally or by injection within 3 months; facially applied anti-inflammatory drugs within 2 months; undergoing treatments for asthma or other chronic respiratory diseases; lactating or pregnant women; volunteers already enrolled in other clinical traits. All volunteers needed to finish the Self-Evaluation Questionnaire carefully, referring to questions about their skin condition, life habits, and physiology. All skin parameters and microbial samples were collected in July 2021.
Considering that skin physiological characteristics is regulated and influenced by hormone levels,19 especially by estrogen level for women. Generally, estrogen secretion usually peaks at the age of 30, collagen secretion peaks at the age of 20, and overlapping peaks appear at the age of 25. Therefore, the age of 25 is considered the beginning of skin aging.28,29 Therefore, we chose the age group of the ideal skin research group to be 18–25 years old. In order to minimize the influence of gender and age on skin biophysical parameters and skin microbes, female volunteers (n=111) aged 18–25 in Shanghai were selected as the research subjects.
Definition and Grouping of “Ideal Skin”
In general, the skin status was evaluated from aspects of acne, pores, spots, and wrinkles. Therefore, in our current study, the VISIA CR photos of volunteers were collected and scored using pores, acne, spots, and wrinkles as evaluating indicators. The estimated standard was slightly modified based on the pore score,18 Fitzpatrick’s wrinkle scale, acne grading standard, and the chloasma area and severity index (MASI) score.30 The grading standard is shown in Table 1, and our customized scoring standard is illustrated in Figure 1. By integrating various skin statuses, we defined “ideal skin” was that “skin with almost no visible pores, no visible wrinkles, and no invisible acne and spots”. Here, the skin color was not included in our study.
 |
Table 1 Grading Standard of Facial Skin Status |
In our study, the VISIA CR photos of the volunteers were scored by a team of seven cosmetic specialists, who were uniformly trained according to our customized scoring standard (Figure 1 and Table 1), then the volunteers were divided into “ideal skin” group (W1), “normal skin” group (W2) and “undesirable skin” group (W3). The grouping and basic information of each group was shown in Table 2.
 |
Table 2 Basic Information of the Participants in Each Group |
Collection of Skin Physiological Parameters and Skin Microbial Samples
The method for skin physiological parameters and microbial sample collection was according to Shao et.al,31 described briefly as follows: all subjects were required not to use skin care products and cosmetics after washing their faces the night before the measurement and the day of the test. Volunteers were asked to stay in an environment of constant temperature and humidity (21±1°C and 50±5%) for 30 min, and an instrument was used to measure cheek-related parameters. The VISIA-CR facial image analyzer was used to take pictures of the front and side of the volunteer’s face, and then Corneometer® CM 825, Tewameter® TM 300, Sebumeter® SM 815, Glossymeter® GL200, memetreter® MX 18, pH 905 and Cutometer dual MPA 580 were used to measure hydration, transdermal water loss (TEWL), sebum, roughness, melanin, hemoglobin, pH and elasticity (R2), respectively.
The microbial samples were collected in an area of 3*3 cm2 on the left cheek of the volunteer, a sterile cotton swab with sterile collection solution containing 0.9% NaCl and 0.1% Tween-20 was used to repeatedly scrape at least 30 times, and then the samples were immediately stored at −80°C for subsequent DNA extraction.
DNA Extraction and PCR
Microbial genomic DNA was extracted using the Fast DNA® Spin Kit for Soil (MP Biomedicals, USA) according to the kit instructions. DNA quality, concentration, and purity were examined using 1% agarose gel electrophoresis and NanoDrop 2000 (Thermo Fisher Scientific, USA). The V3-V4 variable region in the bacterial 16S rRNA gene was amplified by PCR using the forward primer 338F (5ʹ-ACTCCTACGGAGGCAGCAG-3ʹ) and the reward primer 806R (5ʹ- GGACTACHVGGGTWTCTAAT-3ʹ). Meanwhile, the fungal endogenous transcribed spacer (ITS1-ITS2) was amplified by PCR with the forward primer ITS1F (5ʹ-CTTGGTCATTTAGAGGAAGTAA-3ʹ) and the reward primer ITS2R (5ʹ-GCTGCGTTCTTCATCGATGC-3ʹ). PCR products were purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, USA) according to the manufacturer’s instructions.
Gene Sequencing and Data Processing
Library construction was performed using the NEXTFLEX Rapid DNA-Seq Kit (Bioo Scientific, USA). Sequencing was performed using MiseqPE300 (Illumination, USA). The raw data were uploaded to the NCBI SRA database (Accession Number: SRP330206). The raw sequencing data were quality-controlled using Fastp software (v0.19.6) and spliced using FLASH software (v1.2.11). The sequences were classified using Uparse software (v7.0.1090) and annotated according to the similarity of 97%, the Silva 16S rRNA and Unite ITS databases were aligned, and the alignment threshold was set to 0.7.
The raw data from high-throughput sequencing were collated and filtered, and the validated sequences were obtained for subsequent analysis. Approximately 2,861,229 (16S rRNA) and 4,282,096 (ITS) valid sequences were obtained. All samples were flattened out according to the minimum sequence number, and then, in turn, a total of bacteria co-clustered 9068 OTUs, belonging to 57 phyla, 1672 genera, and 3472 species. In addition, fungi co-clustered 2807 OTUs, belonging to 12 phyla, 554 genera, and 938 species.
Statistical Analysis
All data are represented as mean ± standard deviation (X±SD) unless otherwise indicated. The statistical significance level was 0.05 unless otherwise noted. SPSS 25.0 software was used for data analysis of skin physiological parameters. Non-parametric test and Kruskal–Wallis test were selected to analyze the differences between groups in skin physiological parameters. While non-parametric Wilcoxon rank-sum test was used to compare microbial diversity and composition between the two groups. To compare the β-diversity between two groups, unweighted distance metrics were used. Spearman rank correlation test was used to determine the Spearman correlation between the biophysical parameters and the microbiota. These data were analyzed on the online platform of MajorbioI-Sanger Cloud Platform (www.i-sanger.com).
Results
Volunteer Grouping
According to our customized scoring standard, 111 volunteers were divided into three groups as follows: “ideal skin” (W1), “normal skin” (W2), and “undesirable skin” (W3). The characteristics of each group are shown in Table 1, and the results indicated that the proportion of “ideal skin” accounted only for 29.7%, which was similar to our prediction. Obviously, most females had more or less skin problems, and their skin did not reach perfect status. Combined with the questionnaire, frequently concerning skin problems included wrinkles (52.58%), enlarged pores (51.94%), dryness (50.00%), blackheads (49.35%), acne scarring (43.87%), and pigmentation (24.52%). It was proved that the evaluation indicators we selected were reasonable. In addition, there was no significant difference in the average age of each group.
Comparison of Skin Physiological Parameters in Each Group
The cheek skin’s physiological parameters of the three groups were shown in Figure 2. As we seen in Figure 2, from the “ideal skin” group to the “undesirable skin” group, namely from W1 to W3, sebum, TEWL, melanin and R2 showed a clear upward tendency (P > 0.05), roughness (P < 0.05) and hemoglobin (P < 0.001) were increased significantly, while for skin pH value, little changes (P> 0.05) were observed among the three groups. Compared with the “normal skin” (W2) and “undesirable skin” (W3) groups, the “ideal skin” group (W1) had higher skin hydration content and lower sebum content, TEWL, melanin, roughness and hemoglobin, showing better water-oil balance and skin barrier function.
Alpha Diversity Analysis
The α-diversity among group W1, W2 and W3 was analyzed by Wilcoxon rank-sum test. As it was shown in Table 3, the Shannon index of bacteria was significantly decreased from “ideal skin” to “undesirable skin” (from W1 to W3). The Shannon index of the W3 group was decreased, which was significantly different from that of W1 (P = 0.0048) and W2 (P = 0.016). However, the Ace and Chao indexes of bacteria revealed increased tendency, although no significant differences (P > 0.05). The Simpson index of the W3 group was increased significantly, which was different from W1 and W2 (P < 0.05). Coverage index was decreased in W1, which was significantly different from W2 (P < 0.05) and W3 (P < 0.05). It could be seen that from “ideal skin” to “undesirable skin” (from W1 to W3), the diversity of bacteria was decreased, the Shannon index and Ace index of fungi were also decreased, while there was no significant difference (P > 0.05), and the diversity of fungi was not obviously different. This finding suggested that the “ideal skin” had higher α-diversity and higher species diversity than normal and undesirable skin.
 |
Table 3 Alpha Diversity Index (Mean ± SD) of “Ideal Skin” (W1), “Normal Skin” (W2) and “Undesirable Skin” (W3) |
No Difference of β-Diversity Among Three Groups
Based on the unweighted_unifrac algorithm distance metrics, principal coordinate analysis (PCoA) was used to evaluate the β-diversity among three groups. The results showed that there was no significant difference in β-diversity between groups (P > 0.05) (data not shown), indicating that the overall species composition and relative abundance of bacteria and fungi among W1, W2 and W3 was similar.
Differences in Taxonomic Profiles of Skin Bacteria and Fungi Among Three Groups
The bacterial composition of the three groups was analyzed at different taxonomic levels. At the phylum level, the cheek bacteria of the three groups were mainly composed of Actinobacteria, Proteobacteria, Firmicutes and Bacteroidetes, accounting for more than 97% (Figure 3A). From the “ideal skin” group to the “undesirable skin” group, the abundance of Actinobacteria was increased significantly (Figure 3C, P < 0.001), the abundance of Firmicutes was increased (Figure 3E, P > 0.05), and the abundances of Proteobacteria (Figure 3D, P < 0.05) and Bacteroidetes (Figure 3F, P < 0.05) were significantly decreased. Compared with W1 and W2, Group W3 showed significant changes in species abundance, with a significant increase in Actinobacteria (P = 0.01351) and a pretty significant decrease in Proteobacteria (P = 0.000003402), while a significant decrease in Bacteroidetes (P = 0.01425) was observed. The fungi on the cheek skin of the three groups were mainly composed of Ascomycota and Basidiomycota, accounting for 90.2% (Figure 3B), while the difference analysis of species composition showed the difference was not significant. In other words, at the phylum level, the “ideal skin” group contained more Proteobacteria and Bacteroidetes bacteria and relatively fewer Actinobacteria and Firmicutes bacteria than the “normal skin” and “undesirable skin” groups, while the fungal composition was not so significant.
At the genus level, the composition of cheek bacterial species was mainly composed of Cutibacterium, Staphylococcus, Pseudomonas, Rhodococcus, Neisseriaceae, Streptococcus, Corynebacterium, and Stenotrophomonas (Figure 4A). From the “ideal skin” group to the “undesirable skin” group, Cutibacterium was significantly increased (Figure 4C, P < 0.01) and Staphylococcus had a specific increasing trend (Figure 4D, P > 0.05), Pseudomonas (Figure 4E, P < 0.05), Rhodococcus (Figure 4F, P < 0.001), Streptococcus (Figure 4G, P < 0.01), Ralstonia (Figure 4I, P <0.001) and Stenotrophomonas (Figure 4H, P < 0.001) were significantly decreased. The species composition of cheek fungi was mainly composed of Cutaneotrichosporon, Aspergillus, Cladosporium and Malassezia (Figure 4B and 4J). The results of the between-group differential species analysis showed that the relative abundance of Malassezia was increased significantly (P < 0.05) from the “ideal group” (W1) to the “undesirable group” (W3). From the perspective of ideal skin, the abundance of Cutibacterium, Staphylococcus, and Malassezia in the “ideal skin” group was lower, however, the abundances of Pseudomonas, Rhodococcus, Stenotrophomonas, Ralstonia and Streptococcus were relatively high in the “ideal skin” group compared with the “normal skin” and “undesirable skin” groups.
Correlation Analysis of Skin Microbiota and Physiological Parameters
The Spearman correlation between skin physiological parameters (sebum, hydration, TEWL, roughness, R2, hemoglobin, melanin and pH) and the skin bacteria and fungi was analyzed and summarized into the Spearman correlation heatmap (Figure 5). As shown in Figure 5A, Cutibacterium was positively correlated with sebum content (r = 0.1202, P = 0.24) and hemoglobin (r = 0.1773, P = 0.084), and negatively correlated with hydration content (r = −0.3152, P = 0.0018). This finding was consistent with the trend analysis of the previous physiological parameters and bacterial flora, the sebum content was increased, and the relative abundance of Cutibacterium genus was also increased significantly from W1 to W3. Similarly, we saw a positive correlation between Staphylococcus and TEWL (r = 0.0613, P < 0.05), while hemoglobin was positively associated with Stenotrophomonas (r = −0.2094, P < 0.05), and it was negatively correlated with Pseudomonas (r = −0.2782, P < 0.01), Ralstonia (r = −0.2129, P < 0.001), and Streptococcus (r = 0.0519, P < 0.001). For fungi (Figure 5B), hemoglobin was significantly positively correlated with Cutaneotrichosporon (r = 0.2740, P < 0.01) and unclassified_k_Fungi (r = 0.1556, P < 0.001), while it was significantly negatively correlated with Aspergillus (r = −0.2099, P < 0.01). Moreover, Malassezia was positively correlated with hemoglobin (r = 0.2390, P < 0.05).
 |
Figure 5 Spearman correlation analysis of (A) cheek bacteria, (B) fungi and physiological parameters. Difference test method: Wilcoxon rank-sum test, *P < 0 0.05; **P < 0 0.01; ***P < 0 0.001. |
In addition, the correlation between physiological parameters and the Shannon index of bacteria was analyzed (Table 4). The Shannon index of bacteria was significantly positively correlated with hydration (r = 0.222 P = 0.03), but negatively correlation with sebum content (r = −0.069, P = 0.5) and hemoglobin content (r = −0.228, P = 0.025). Meanwhile, the Shannon index was significantly negatively with the abundance of Cutibacterium (r = −0.7303, P = 0.000), but significantly positively correlated with the abundances of Streptococcus (r = 0.4536, P = 0.000) and Ralstonia (r = 0.25, P = 0.014). Therefore, the skin hydration content and the abundance of Cutibacterium genus played an important role in maintaining the α-diversity and skin homeostasis.
 |
Table 4 Spearman Correlation Analysis Between Skin Physiological Parameters, Microbiota and the Shannon Index of Bacteria |
Discussion
The skin is not only a “barometer” of the body’s health, but also is endowed with more connotations. Generally, having good-looking and healthy skin means happier, better, and more accessible resources and opportunities. Therefore, in this era of pursuing youthful beauty, we are willing to spend more money on personal care, which promote the rapid development of the personal care industry. However, there is no standard for what kind of skin is perfect or ideal. Interestingly, beauty standards may vary between cultures, while different ethnic groups share a common attractiveness standard. Males are expected to be most sexually attracted to female skin free of lesions, eruptions, warts, moulds, cysts, tumors, acne, and hirsutism.9 Based on the understanding and aesthetics of orientals for perfect skin, Our study was the first attempt to define the “ideal skin” of oriental women. Based on our customized scoring standard, female volunteers aged 18–25 in Shanghai (n=111) were divided into “ideal skin”, “normal skin” and “undesirable skin” groups with pores, acne, spots and wrinkles as evaluation indicators, to analysis the skin physiological parameters and cheek microbial flora.
Our study found that skin physiological parameters presented regular changes from “ideal skin” to “undesirable skin”, although the differences of some parameters were insignificant (Figure 2, P > 0.05). The “ideal skin” group had higher skin hydration content and lower sebum, TEWL, indicating that “ideal skin” had a reasonable water-oil balance and better skin barrier function. In contrast, in the “normal skin” and “undesirable skin” groups, decreased skin hydration content and increased TEWL reflected impaired skin barrier. Skin with low hydration content is more prone to wrinkled, scaly, or rough features, and severe cases may also experience cracking, redness, or itching.32,33 Increased hemoglobin reflects increased blood flow in the blood vessels, with the potential for inflammation and pigmentation of the skin. The increase in sebum content is mainly related to the development of facial pores.16 The increase of oil secretion leads to enlarged pores, making faces look rough, slack, and lackluster. In the “normal skin” and “undesirable skin” groups, the skin roughness was increased significantly. From the “ideal skin” group to the “undesirable skin” group, the content of melanin was increased, and the skin was also prone to problems, such as pigmentation. Judging from the grouping criteria, most of the volunteers in the “undesirable skin” group had acne, pigmentation spots, and large pores, which was consistent with the changes in physiological parameters, such as increased sebum secretion and hemoglobin content. It was proved that different skin conditions have different physiological parameters. For example, skin aging includes chronological skin aging and photoaging,34 the volunteers in our study ranged from 18–25 years, so the effects of chronological skin aging on skin physiological parameters and microorganisms can be excluded. Photoaging is mainly caused by ultraviolet radiation, the most immediate change caused by UV radiation is the accumulation of skin pigment, followed by the damage to skin extracellular matrix.35,36
Moreover, we were pleasantly surprised to find that the microbiota flora changed regularly from the “ideal skin” group to the “undesirable skin” group. Our data showed that the α-diversity of skin bacteria was more abundant in the “ideal skin” group, compared with the “normal skin” and “undesirable skin” groups, since the Chao index of species richness (P > 0.05) and the Shannon index of species diversity (P < 0.01) were significantly different. In studies of problematic skin, such as acne, atopic dermatitis, and dandruff, healthy groups have higher α-diversity compared with unhealthy groups, which is consistent with our findings.37–40 A study compared the composition and diversity of microbes among 4 kinds of subjective skin types, and the sebum content and bacterial diversity of the facial skin both significantly differed among the 4 kinds of subjective skin types, and the relative abundance of Propionibacterium was significantly higher in the oily skin group than in the dry skin group.41 Similarly, environmental pollution (air cleanliness) could also impact the flora since the α-diversity of the population would be higher in clean air.42,43 Skin aging is associated with changes in cutaneous physiology including interactions with skin microbial community. It was indicated that the α-diversity was higher and a striking reduction in the relative abundance of the majority skin genus Propionibacterium of the older adults.42 The Shannon index was significantly positively correlated with hydration but negatively correlated with sebum content and hemoglobin content (Table 4). Therefore, we could control the balance of water and oil to keep more high diversity of skin flora. The ß-diversity of the three groups did not show any difference, which might be related to the fact that our research subjects were all healthy people, and there was little difference in the composition of species.
Species composition was changed significantly from the “ideal skin” group to the “undesirable skin” group. The “ideal skin” group had lower in the abundance of Cutibacterium, Staphylococcus, and Malassezia, but relatively high in the abundances of Pseudomonas, Rhodococcus, Stenotrophomonas, Ralstonia and Streptococcus (Figure 4). Studies have shown that changes in species composition are often important factors in some diseases or skin conditions.44 For example, Propionibacterium acnes, the primary strain of the genus Propionibacterium on the skin, is also known as sentinel bacteria, and its abundance changes have a significant impact on the flora and play an essential role in maintaining skin microecological homeostasis.45 In our present study, the abundance of Propionibacterium was increased significantly from the “ideal skin” group to the “undesirable skin” group. Previous studies have found that the abundance of Propionibacterium is negatively correlated with the α-diversity.46 Our study showed that the α-diversity of the “undesirable skin” group was decreased, suggesting that the abundance of Propionibacterium would affect the diversity of the entire flora in certain circumstances. In addition, a large number of studies have shown that in areas with high sebum secretion, Propionibacterium is the dominant bacteria, which can use sebum to promote its own reproduction, and the imbalance of Propionibacterium acnes can easily lead to the occurrence of acne.47 Meanwhile, the abundance of Malassezia was significantly increased from the “ideal skin” group to the “undesirable skin” group (P < 0.05). Volunteers with acne and enlarged pores in the “undesirable skin” group accounted for most. There were a lot of Malassezia, Propionibacterium acnes and Streptococcus in the hair follicles of acne patients.48 When the number of Malassezia was high, the skin was more prone to aging,49 which was also consistent with our findings. A higher frequency of Propionibacterium, Paracoccus and Corynebacterium was also detected in sensitive skin, compared with non-sensitive skin.33 Zheng et al50 revealed that the abundance of Malassezia in the adolescent acne group was significantly higher compared with the healthy control group. Another research also demonstrated that there was also a close relationship between sensitive skin and its skin bacteria and fungi.51 These studies support our findings that ideal and healthy skin tended to have lower P. acnes and Malassezia abundances compared with the problematic skin. Human skin harbors a diverse milieu of commensals, including bacteria, fungi, viruses and mites, which live together as an intricate ecological community. In this study, we mainly focused on the impact of different skin states on the cutaneous bacterial, fungi abundance and diversity, but did not extend our research to viruses and skin mites. Indeed, viruses and mites also have important role for skin health.52,53 For example, the Demodex mites are significantly increased in the skin of rosacea patients, causing sensitive skin.54,55 Our research revealed that with the skin condition deteriorates, sebum content was significantly increased, so we could speculate that the abundance of mites would also increase, which might affect the microbial homeostasis and further the function of the skin. More studies are needed to reveal the effects of skin conditions on mites, and to get an insight into the association between skin condition and skin microecology.
There is a close correlation between the distribution and abundance of microflora and skin physiological parameters, especially sebum, skin hydration, TEWL and pH value.56 Compared with the “ideal skin” group (W1), the “undesirable skin” group (W3) had increased sebum and decreased skin water content. The corresponding abundances of lipophilic P. acnes and Malassezia were significantly increased, while the abundances of bacteria preferring humid environments, such as Stenotrophomonas, Pseudomonas, Ralstonia and Streptococcus, were significantly reduced. It has demonstrated that P. acnes is significantly positively associated with sebum secretion, pore size and the number of porphyrin spots46, which is consistent with our findings. Hemoglobin reflects vascular activity, and the increase in skin hemoglobin content often manifests as skin inflammation, which affects the appearance of the skin. The hemoglobin content in the “undesirable skin” group was significantly increased. Correlation analysis showed that the hemoglobin content was positively correlated with the abundance of Cutibacterium and Malassezia, while it was significantly negatively correlated with Stenotrophomonas, Pseudomonas, Ralstonia, Streptococcus and other strains that preferred humid environments. It has been found that the spot area is negatively correlated with Propionibacterium.42 Streptococcus is negatively correlated with sebum content and positively correlated with skin hydration content,16,49,56 which is consistent with our study. From the correlation analysis, TEWL was increased in the “undesirable skin” group, which was significantly positively correlated with the abundance of Staphylococcus. Increased Staphylococcus abundance was also associated with the impaired skin barrier. Song et al57 have also found that TEWL was positively correlated with the abundance of Staphylococcus in sensitive skin, especially Staphylococcus aureus, which can easily cause damage to the skin barrier. It can be seen that there is a close relationship between the abundance of flora and the skin physiological parameters. The changes in the skin’s physiological parameters affect the changes in bacterial abundance, and in turn, the changes in the metabolites further affect the function of the skin, which are interlinked and affect each other.
In our present study, we selected the volunteers in 18–25 years old, considered as the best age for skin. Although everyone wants to have ideal skin of 18–25 years old, we have to admit that in the natural law of skin aging, various care methods only slow down skin aging. Therefore, we should also follow the characteristics of skin at all ages to find the ideal skin state at that age, and adopt reasonable maintenance methods to make the skin look younger. In the further research, we will also pay attention to the “ideal skin” research of different age groups to provide a theoretical basis for scientific skincare for different age groups.
Conclusions
In our present study, we attempted to define the “ideal skin” of oriental women and obtain helpful information on the skin’s physiological characteristics and the diversity and composition of the facial microbiome of ideal skin. The “ideal skin” group had a more appropriate water-oil balance, which was more suitable for the growth and reproduction of microorganisms. Therefore, it had higher microbial diversity and reasonable species composition. The skin changes of young women aged 18–25 were mainly reflected in physiological functions. The increase in skin oil would lead to changes in skin microecology, which might be one factor that further results in skin problems. Therefore, if young women aged 18–25 want to achieve the ideal skin condition, daily skincare needs to control skin oil and maintain skin microecological balance.
Data Sharing Statement
The sequence dataset has been deposited on the NCBI Sequence Reads Archive (SRA) Database (Accession Number: SRP330206).
Acknowledgments
The authors sincerely acknowledge all the study participants who provided skin physiological parameters and specimens for the study. In addition, we would like to thank Shandong Freda Biotech Co., Ltd. and Shanghai Collaborative Innovation Center of Fragrance, Flavor, and Cosmetics (1021ZK202002008) for providing financial support.
Disclosure
The authors declare no competing interests for this work.
References
1. Bovet J. The evolution of feminine beauty. In: Exploring Transdisciplinarity in Art and Sciences. Springer; 2018:327–357. doi:10.1007/978-3-319-76054-4_17:.
2. Hosoda M, Coats G, Coats G. The effects of physical attractiveness on job‐related outcomes: a meta‐analysis of experimental studies. Pers Psychol. 2003;56(2):431–462. doi:10.1111/j.1744-6570.2003.tb00157.x
3. Dion K, Berscheid E, Walster E. What is beautiful is good. J Pers Soc Psychol. 1972;24(3):285. doi:10.1037/h0033731
4. Eagly AH, Makhijani ARD, Makhijani MG, et al. What is beautiful is good, but: a meta-analytic review of research on the physical attractiveness stereotype. Psychol Bull. 1991;110(1):109–128. doi:10.1037/0033-2909.110.1.109
5. Voegeli R, Schoop R, Prestat-Marquis E, Rawlings AV, Shackelford TK, Fink B. Differences between perceived age and chronological age in women: a multi-ethnic and multi-centre study. Int J Cosmet Sci. 2021;43(5):547–560. doi:10.1111/ics.12727
6. Magin P, Heading G, Heading G, et al. ‘Perfect skin’, the media and patients with skin disease: a qualitative study of patients with acne, psoriasis and atopic eczema. Aust J Prim Health. 2011;17(2):181–185. doi:10.1071/PY10047
7. Langlois JH, Kalakanis L, Rubenstein AJ, Larson A, Hallam M, Smoot MJ. Maxims or myths of beauty? A meta-analytic and theoretical review. Psychol Bull. 2000;126(3):390. doi:10.1037/0033-2909.126.3.390
8. Watkins LM, Johnston LJI. assessment, Screening job applicants: the impact of physical attractiveness and application quality. Int J Sel Assess. 2000;8(2):76–84. doi:10.1111/1468-2389.00135
9. Fink B, Neave NJI. The biology of facial beauty. Int J Cosmet Sci. 2005;27(6):317–325. doi:10.1111/j.1467-2494.2005.00286.x
10. Park SJB. Biochemical, structural and physical changes in aging human skin, and their relationship. Biogerontology. 2022;23(3):1–14. doi:10.1007/s10522-022-09959-w
11. Dimitriu PA, Iker B, Malik K, Leung H, Mohn W, Hillebrand GGJM. New insights into the intrinsic and extrinsic factors that shape the human skin microbiome. mBio. 2019;10(4):e00839–e00919. doi:10.1128/mBio.00839-19
12. Botros PA, Tsai G, Pujalte GGA. Evaluation and management of acne. Prim Care. 2015;42(4):465–471. doi:10.1016/j.pop.2015.07.007
13. Chilicka K, Rusztowicz M, Rogowska AM, Szyguła R, Asanova B, Nowicka D. Efficacy of hydrogen purification and cosmetic acids in the treatment of acne vulgaris: a preliminary report. J Clin Med. 2022;11(21):6296. doi:10.3390/jcm11216296
14. Chilicka K, Rogowska AM, Szygula R, Rusztowicz M, Nowicka D. Efficacy of oxybrasion in the treatment of acne vulgaris: a preliminary report. J Clin Med. 2022;11(13):3824. doi:10.3390/jcm11133824
15. Briganti S, Flori E, Mastrofrancesco A, Ottaviani MJED. Acne as an altered dermato‐endocrine response problem. Exp Dermatol. 2020;29(9):833–839. doi:10.1111/exd.14168
16. Kim BY, Choi JW, Park KC, Youn SW. Sebum, acne, skin elasticity, and gender difference - which is the major influencing factor for facial pores. Skin Res Technol. 2013;19(1):e45–e53. doi:10.1111/j.1600-0846.2011.00605.x
17. Roh M, Han M, Kim D, Chung KJB. Sebum output as a factor contributing to the size of facial pores. Br J Dermatol. 2006;155(5):890–894. doi:10.1111/j.1365-2133.2006.07465.x
18. Lee SJ, Seok J, Jeong SY, Park KY, Li K, Seo SJ. Facial pores: definition, causes, and treatment options. Dermatol Surg. 2016;42(3):277–285. doi:10.1097/DSS.0000000000000657
19. Zouboulis C, Chen W-C, Thornton M, Qin K, Rosenfield RJ. Sexual hormones in human skin. Horm Metab Res. 2007;39(2):85–95. doi:10.1055/s-2007-961807
20. Del Bino S, Duval C, Bernerd FJ. Clinical and biological characterization of skin pigmentation diversity and its consequences on UV impact. Int J Mol Sci. 2018;19(9):2668. doi:10.3390/ijms19092668
21. Puizina-Ivic NJADA. Skin aging. Acta Dermatovenerologica Alpina Panonica Et Adriatica. 2008;17(2):47. https://www.ncbi.nlm.nih.gov/pubmed/18709289
22. Byrd AL, Belkaid Y, Segre JA. The human skin microbiome. Nat Rev Microbiol. 2018;16(3):143–155. doi:10.1038/nrmicro.2017.157
23. Farage MAJ. The prevalence of sensitive skin. Front Med. 2019;6:98. doi:10.3389/fmed.2019.00098
24. Fitz-Gibbon S, Tomida S, Chiu BH, et al. Propionibacterium acnes strain populations in the human skin microbiome associated with acne. J Invest Dermatol. 2013;133(9):2152–2160. doi:10.1038/jid.2013.21
25. Bjerre R, Bandier J, Skov L, Engstrand L, Johansen JJB. The role of the skin microbiome in atopic dermatitis: a systematic review. Br J Dermatol. 2017;177(5):1272–1278. doi:10.1111/bjd.15390
26. Lewis DJ, Chan WH, Hinojosa T, Hsu S, Feldman SRJ. Mechanisms of microbial pathogenesis and the role of the skin microbiome in psoriasis: a review. Clin Dermatol. 2019;37(2):160–166. doi:10.1016/j.clindermatol.2019.01.011
27. Wang W-M, Jin H-ZJCM. Skin microbiome: an actor in the pathogenesis of psoriasis. Chin Med J. 2018;131(1):95–98. doi:10.4103/0366-6999.221269
28. Kitagaki ZH. Overview on key components and structural characteristics related to skin aging. Flavour Fragr J. 2020;3:173.
29. ling L. The relationship between changes in sex hormone level and physiology and disease of women in different age groups. Henan J Prevent Med. 2010;21(3):179–181. doi:10.13515/j.cnki.hnjpm.2010.03.012
30. Tamega A, Miot L, Bonfietti C, Gige T, Marques MEA, Miot HA. Venereology, Clinical patterns and epidemiological characteristics of facial melasma in Brazilian women. J Eur Acad Dermatol Venereol. 2013;27(2):151–156. doi:10.1111/j.1468-3083.2011.04430.x
31. Shao L, Jiang S, Li Y, et al. Regular late bedtime significantly affects the skin physiological characteristics and skin bacterial microbiome. Clin Cosmet Investig Dermatol. 2022;15:1051. doi:10.2147/CCID.S364542
32. Blume-Peytavi U, Kottner J, Sterry W, et al. Age-associated skin conditions and diseases: current perspectives and future options. Gerontologist. 2016;56(Suppl 2):S230–S242. doi:10.1093/geront/gnw003
33. Bai Y, Wang Y, Zheng H, Tan F, Yuan C. Correlation between facial skin microbiota and skin barriers in a Chinese female population with sensitive skin. Infect Drug Resist. 2021;14:219–226. doi:10.2147/IDR.S287844
34. Hashizume H. Skin aging and dry skin. J Dermatol. 2004;31(8):603–609. doi:10.1111/j.1346-8138.2004.tb00565.x
35. Maddodi N, Jayanthy A, Setaluri V. Shining light on skin pigmentation: the darker and the brighter side of effects of UV radiation. Photochem Photobiol. 2012;88(5):1075–1082. doi:10.1111/j.1751-1097.2012.01138.x
36. Watson RE, Gibbs NK, Griffiths CE, Sherratt MJ. Damage to skin extracellular matrix induced by UV exposure. Antioxid Redox Signal. 2014;21(7):1063–1077. doi:10.1089/ars.2013.5653
37. Kong HH, Oh J, Deming C, et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012;22(5):850–859. doi:10.1101/gr.131029.111
38. Dreno B, Araviiskaia E, Berardesca E, et al. Microbiome in healthy skin, update for dermatologists. J Eur Acad Dermatol Venereol. 2016;30(12):2038–2047. doi:10.1111/jdv.13965
39. Nagase S, Ogai K, Urai T, et al. Distinct skin microbiome and skin physiological functions between bedridden older patients and healthy people: a single-center study in Japan. Front Med. 2020;7:101. doi:10.3389/fmed.2020.00101
40. Langan EA, Kunstner A, Miodovnik M, et al. Combined culture and metagenomic analyses reveal significant shifts in the composition of the cutaneous microbiome in psoriasis. Br J Dermatol. 2019;181(6):1254–1264. doi:10.1111/bjd.17989
41. Zheng Y, Wu W, Song L, et al. Relationship between subjective facial skin types and skin microbiota in 31 healthy female undergraduates aged 20–25 years in Beijing. Chin J Dermatol. 2019;2019:467–474. doi:10.3760/cma.j.issn.0412-4030.2019.07.005
42. Shibagaki N, Suda W, Clavaud C, et al. Aging-related changes in the diversity of women’s skin microbiomes associated with oral bacteria. Sci Rep. 2017;7(1):1–10. doi:10.1038/s41598-017-10834-9
43. Wu Y, Wang Z, Zhang Y, Ruan L, Li A, Liu X. Microbiome in healthy women between two districts with different air quality index. Front Microbiol. 2020;11:548–618. doi:10.3389/fmicb.2020.548618
44. Rosenthal M, Goldberg D, Aiello A, Larson E, Foxman B. Skin microbiota: microbial community structure and its potential association with health and disease. Infect Genet Evol. 2011;11(5):839–848. doi:10.1016/j.meegid.2011.03.022
45. Rozas M, de Ruijter H, Fabrega A, et al. Dysbiosis to healthy skin: major contributions of Cutibacterium acnes to skin homeostasis. Microorganisms. 2021;9(3):628. doi:10.3390/microorganisms9030628
46. Zheng Y, Liang H, Zhou M, Song L, He C. Skin bacterial structure of young females in China: the relationship between skin bacterial structure and facial skin types. Exp Dermatol. 2021;30(10):1366–1374. doi:10.1111/exd.14105
47. Sfriso R, Egert M, Gempeler M, Voegeli R, Campiche R. Revealing the secret life of skin - with the microbiome you never walk alone. Int J Cosmet Sci. 2020;42(2):116–126. doi:10.1111/ics.12594
48. Akaza N, Akamatsu H, Takeoka S, et al. Malassezia globosa tends to grow actively in summer conditions more than other cutaneous Malassezia species. J Dermatol. 2012;39(7):613–616. doi:10.1111/j.1346-8138.2011.01477.x
49. Li Z, Bai X, Peng T, et al. New insights into the skin microbial communities and skin aging. Front Microbiol. 2020;11:565. doi:10.3389/fmicb.2020.565549
50. Cong F. Variation of skin microbial community in adolescent acne. Microbiol China. 2019;46(12):3414–3423. doi:10.13344/j.microbiol.china.190129
51. Keum HL, Kim H, Kim HJ, et al. Structures of the skin microbiome and mycobiome depending on skin sensitivity. Microorganisms. 2020;8(7):1032. doi:10.3390/microorganisms8071032
52. Schommer NN, Gallo RLJ. Structure and function of the human skin microbiome. Trends Microbiol. 2013;21(12):660–668. doi:10.1016/j.tim.2013.10.001
53. De Pessemier B, Grine L, Debaere M, Maes A, Paetzold B, Callewaert CJM. Gut–skin axis: current knowledge of the interrelationship between microbial dysbiosis and skin conditions. Microorganisms. 2021;9(2):353. doi:10.3390/microorganisms9020353
54. Jarmuda S, O’Reilly N, Żaba R, Jakubowicz O, Szkaradkiewicz A, Kavanagh KJ. Potential role of Demodex mites and bacteria in the induction of rosacea. J Med Microbiol. 2012;61(11):1504–1510. doi:10.1099/jmm.0.048090-0
55. Yasak Guner R, Tosun M, Akyol M, Hayta SBJ. Demodex infestation as a cause of sensitive skin in a dermatology outpatient clinic. J Cosmet Dermatol. 2022;21(4):1610–1615. doi:10.1111/jocd.14246
56. Mukherjee S, Mitra R, Maitra A, et al. Sebum and hydration levels in specific regions of human face significantly predict the nature and diversity of facial skin microbiome. Sci Rep. 2016;6:1–11. doi:10.1038/srep36062
57. Zheng Y, Liang H, Li Z, Tang M, Song L. Skin microbiome in sensitive skin: the decrease of Staphylococcus epidermidis seems to be related to female lactic acid sting test sensitive skin. J Dermatol Sci. 2020;97(3):225–228. doi:10.1016/j.jdermsci.2019.12.004
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.