Back to Journals » Therapeutics and Clinical Risk Management » Volume 18
The Changes of Thyroid Function and Related Factors in Critical Patients without Thyroid Illness in ICU: A Retrospective Cross-Sectional Study
Received 9 February 2022
Accepted for publication 26 April 2022
Published 16 May 2022 Volume 2022:18 Pages 571—578
DOI https://doi.org/10.2147/TCRM.S361791
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Deyun Wang
Jiang-Nan Zhang, Xi-Le Zhao
Department of Endocrinology, The First Hospital of Fuzhou, Fuzhou, 350009, People’s Republic of China
Correspondence: Jiang-Nan Zhang, Department of Endocrinology, The First Hospital of Fuzhou, No. 190 of DaDao Street, Taijiang District, Fuzhou, 350009, People’s Republic of China, Tel +86 13763892689, Email [email protected]
Objective: To retrospectively analyze the changes of thyroid function and related factors in critical patients with non-thyroid illness, hoping to find some indicators for the further examination of the thyroid function in the intensive care unit situation.
Methods: The clinical data of 52 patients admitted to the ICU of Fuzhou First Hospital in Fujian Province, China, from May 2018 to March 2019 were collected. Patients were allocated into the central hypothyroidism group (CH group, n = 21) and the low T3 syndrome group (LT3S group, n = 31) based on thyroid function. All related medical data were collected, and the correlations between variables were identified using Spearman’s or Pearson’s rank correlation coefficients.
Results: The Acute Physiology and Chronic Health Evaluation (APACHE) II score in the CH group and the LT3S group were 20.6 ± 3.6 and 19.3 ± 3.6, respectively, measured within 24 hours following hospital admission. The mean value of thyroid-stimulating hormone (TSH) in the CH group (0.3 ± 0.3 IU/mL) was significantly lower than that in the LT3S group (1.7 ± 0.9 IU/mL), P < 0.001. Fasting plasma glucose (FPG) level in the CH group was significantly higher than that in the LT3S group (10.3 ± 5.0 mmol/L vs 6.8 ± 2.5 mmol/L, P = 0.002).
Conclusion: Central hypothyroidism may exist in critically ill patients and may be associated with elevated fasting plasma glucose levels; accordingly, it should be included as part of patient assessment. When FPG is higher than 6.4mmol/L on admission, thyroid function should be actively examined.
Keywords: central hypothyroidism, fasting blood glucose, critical illness, thyroid-stimulating hormone
Introduction
In recent years, with the development of critical care medicine, ever more clinical studies have shown that critical diseases, such as severe infection, major cardiac surgery, and chronic obstructive pulmonary disease may affect thyroid function.1,2 It is difficult to assess the thyroid function in patients hospitalized in an intensive care unit (ICU). Many of them have low serum concentrations of both thyroxine (T4) and triiodothyronine (T3), and their serum thyroid-stimulating hormone (TSH) concentration also may be below. At present, low T3 syndrome (LT3S) associated with systemic inflammatory is consistent with the most reported among these conditions.3 LT3S is characterized by decreased serum total triiodothyronine (TT3) and serum-free triiodothyronine (FT3), normal or decreased serum total tetraiodothyronine (TT4), serum-free thyroxine (FT4), and increased reverse triiodothyronine (rT3). Serum TSH in LT3S is typically normal or reduced and may be markedly low, usually not less than 0.05 mU/mL.4–7 Some studies have suggested that the decline in serum TSH levels in critical patients indicate that the central nervous system, such as hypothalamus or pituitary, etc., is weakened,8,9 and the weakened central nervous system will further affect the pituitary gonadal axis and the sympathetic adrenal medulla axis. Levels of downstream hormones, such as serum cortisol, would be affected. Some critical patients may develop hypotension in association with central hypothyroidism and central hypoadrenalism. Though central hypothyroidism is rarely reported in critically ill patients, early identification of abnormal thyroid function and timely initiation of thyroid hormone replacement therapy have an important impact on the prognosis of critically ill patients. Pay attention to the thyroid function of critical patients with non-thyroid diseases and the related factors of central hypothyroidism, and early identification of central hypothyroidism, which can effectively guide the intensive care medicine physicians in the management of patients and the prediction of prognosis. In summary, this study retrospectively analyzes the thyroid function status of critical patients in our hospital’s ICU, trying to find the relevant risk factors for central hypothyroidism.
Materials and Methods
Method
We conducted a retrospective monocenter observational study in the 16-bed intensive care unit (ICU) of the Fuzhou First Hospital in Fujian Province, China, from May 2018 to March 2019. The inclusion criteria were as follows: 1) Patients who received serum FT3 detection within 24 hours after admission, and the serum FT3 level was below the lower limit of our hospital’s detection capacity, 3.1pmol/L, and admitted to the ICU of our hospital. 2) The acute physiology and chronic health evaluation (APACHE) II score10 is used to assess the severity of disease, patients who were scored beyond 11 points by two independent physicians within 24 hours after admitting to the ICU are enrolled in this study. The exclusion criteria were as follows: 1) Patients are younger than 18 years of age. 2) Patients who are pregnant. 3) The presence of uremia; hypothalamic and pituitary lesions; cerebral infarction; cerebral hemorrhage and other central nervous system diseases. 4) The survival time is less than 24 hours after undergoing cardiopulmonary resuscitation. 5) Endocrine system diseases such as primary hyperthyroidism or hypothyroidism.
A total of 52 patients were included in this study. Enrolled patients were allocated into two groups according to the thyroid function,11 a low T3 syndrome group (LT3S group) and a central hypothyroidism group (CH group). The group standards were set as follows: 1) patients with a blood rT3 level less than 0.95 ng/mL and a blood TSH level less than 0.37 uIU/mL were divided into the CH group; 2) patients with a blood rT3 level greater than 0.95 ng/mL and a blood TSH level greater than 0.37 uIU/mL were divided into the LT3S group. The normal range of serum rT3 in our hospital is 0.20–0.95 ng/mL, and the normal value of serum TSH is 0.37–4.20 uIU/mL.
Outcomes
All related medical data, such as demographics, comorbidity, clinical examinations, radiological findings, microbiologic investigations, thyroid function examination results and therapeutic management et al were collected, and all the laboratory results were evaluated in duplicate samples in the clinical laboratory of our hospital.
The primary outcomes are the thyroid function, including TSH, rT3, FT3, FT4, TT3, TT4 and the fasting plasma glucose (FPG). The definition of the FPG is that the level of plasma glucose examined after 12-hour admission without eating or drinking.
Statistical Analysis
All statistical analyses were performed using SPSS version 19.0 (SPSS, Chicago, IL, USA). Continuous variables were evaluated for a normal distribution using the Kolmogorov–Smirnov test. Categorical variables were presented as percentages. For between-group comparisons, the Student’s t-test and Wilcoxon test were used for continuous variables, and the Chi-squared test or Fisher’s exact test was used for categorical data. Correlations between variables were identified using Spearman’s or Pearson’s rank correlation coefficients. Parametric data are presented as mean ± standard error, and non-parametric data are presented as median and interquartile range. Significance was defined as a P-value of <0.05. Bivariate correlation analysis was used to determine the correlation between two variables, and P <0.05 was considered statistically significant. The receiver operating characteristic (ROC) curve of critically ill patients with central hypothyroidism was plotted with fasting plasma glucose level within 24 hours of admission. A 95% confidence interval (95% CI) area under the ROC curve (AUC) was used to reflect the predictive power of fasting plasma glucose level within 24 hours of admission for critically ill patients with potential central hypothyroidism.
Results
Demographic Characteristics and Underlying Diseases
A total of 52 patients were enrolled, among which 19 patients were diagnosed with severe pneumonia (6 patients in the LT3S group and 13 patients in the CH group); 4 patients with multiple organ dysfunction syndrome (MODS) (1 in the LT3S group, 3 in the CH group); 8 patients with chronic obstructive pulmonary disease (COPD) (2 patients in each group); 7 patients with acute respiratory distress syndrome (ARDS) (3 in the LT3S group, 4 in the CH group); 3 patients with interstitial pneumonia (2 in the LT3S group, 1 in the CH group); and 1 patient with malignant tumor was in the CH group.
The mean age of 52 patients was 64.5 ± 12.2 years and included 41 males and 11 females. The CH group comprised 21 patients including 16 males and 5 females with an average age of 64.4 ± 10.5 years. The APACHE II score for this group was 20.6 ± 3.6 within 24 hours after the admission. The basic diseases recorded for this group were as follows: 2 (9.5%) patients had diabetes mellitus; 6 (28.6%) patients had hypertension; 3 (14.3%) patients had organic heart disease; 3 (14.3%) patients had cancer; 5 (23.8%) patients had a tracheotomy.
There were 31 patients in the LT3S group including 25 males and 6 females with an average age of 64.6 ± 13.5 years. The APACHE II score for this group was 19.3 ± 3.6 within 24 hours after the admission. The basic diseases recorded for this group were as follows: 4 patients had diabetes (12.9%); 8 patients had hypertension (25.8%); 1 patient had organic heart disease (3.2%); no patients had a tumor (0.0%); 5 patients had a tracheotomy (17.2%). There were no significant differences in age, gender composition, APACHE II score, or basic diseases between the two groups (P > 0.05). The choice of treatment therapy, using vasoactive drugs, taking corticosteroids, and taking glucose solution, were no significant differences between the two groups (P > 0.05) (Table 1).
 |
Table 1 Comparison of Clinical Data, Thyroid Function and Laboratory Examination of the Two Groups |
Comparison of Thyroid Function Between Two Groups Within 24 Hours After Admission
The mean value of TSH in the CH group was significantly lower than in the LT3S group (0.3 ± 0.3 uIU/mL vs 1.7 ± 0.9 uIU/mL, P < 0.001). The mean value of rT3 in the CH group (0.6 ± 0.1 ng/mL) was significantly lower than in the LT3S group (P = 0.002). The FT3, FT4, and other indexes showed no significant difference between the two groups.
Comparison of Laboratory Examinations Between the Two Groups Within 24 Hours After Admission
Within 24 hours of admission, compared with the LT3S group, the mean FPG level (10.3 ± 5.04 mmol/L vs 6.8 ± 2.5 mmol/L, P = 0.002) and the Hb1Ac level (8.1 ± 2.1 vs 6.1 ± 1.7, P<0.001) in the CH group was significantly higher. However, there were no significant differences in blood cholesterol, serum triglyceride, low-density lipoprotein (LDL), high-density lipoprotein (HDL), or other related endocrine and metabolic indexes between the two groups. There was no statistical difference between the two groups concerning white blood cells, C-reactive protein (CRP), and other indicators representing the severity of infection (Table 1).
Bivariate Correlation Analysis Between FPG Level and Thyroid-Stimulating Hormone, Increased Reverse Triiodothyronine, Serum Free Triiodothyronine, and Serum Free Tetraiodothyronine
Bivariate correlation analysis showed that FPG level was negatively correlated with serum TSH level (r = −0.348, P = 0.012) and negatively correlated with serum rT3 level (r = −0.394, P = 0.004). There was no correlation with serum FT3 level (r = −0.289, P = 0.04) or serum FT4 level (r = −0.036, P = 0.802) (Figure 1).
Prediction of Fasting Plasma Glucose for Central Hypothyroidism in Critically Ill Patients
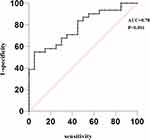
The prediction of central hypothyroidism in critically ill patients by fasting plasma glucose was made within 24 hours after the admission using the area under the receiver operating characteristic curve (ROC). The area under the ROC curve of FPG was 0.78 (95% CI 0.64–0.88, P<0.001) (Figure 2).
When the FPG is >6.4mmol/L, the sensitivity and specificity of FPG in predicting central hypothyroidism in critically ill patients was 95% (95% CI 75.1–99.9%) and 54.9% (95% CI 36–72.7%) (Figure 2).
Discussion
Central hypothyroidism is rarely reported in critically ill patients. In this study, the thyroid function of critically ill patients admitted to the ICU was monitored, and it was found that selected critically ill patients had central hypothyroidism. According to domestic and foreign research reports, the mechanism of central hypothyroidism in critically ill patients may be as follows. 1) Critically ill patients are prone to hemodynamic changes, hypoxia of varying degrees, and acid-based balance disorders, resulting in cerebral vascular ischemia and hypoxia of brain cells, impaired hypothalamus function, inhibited hypothalamus TRH expression, resulting in reduced TSH,12 as well as decreased thyroid hormone secretion. 2) Critically ill patients with an infection will have elevated inflammatory factors such as interleukin (IL)-2, IL-4, tumor necrosis factor (TNF), and interferon γ, which can inhibit TSH biological activity and lead to a decline in TSH.13 3) Some drugs can cause decreased blood thyroxine, and the dopamine hydrochloride injection, which is one of the vasoactive drugs and cardiotonic drugs, has been shown to induce clinically relevant and iatrogenic hypothyroidism in critically ill adults and children.14 Even at low doses, the dopamine hydrochloride injection can greatly inhibit TSH secretion and reduce plasma T4 and T3 concentrations in adult and pediatric ICU patients. The most frequently reported cause of thyroid dysfunction in critically ill patients is low T3 syndrome. Surprisingly, over the past three decades, many endocrinologists have argued that low T3 syndrome is a beneficial physiological response,5,15–17 but actual evidence for this notion is unclear. The difference between low T3 syndrome and central hypothyroidism, respectively, is whether serum TSH levels are significantly reduced and elevated serum rT3 levels, which requires thyroid hormone replacement therapy.
Recent studies have clearly shown that thyroid hormones play an important role in the development of disease in critically ill patients. Thyroid hormone plays a crucial role in cell metabolism and immune function, including regulating cell-mediated immune function.18,19 A study showed that Hashimoto’s thyroiditis represents an independent prognostic parameter in intrathyroidal papillary thyroid cancer, but cannot improve prognostic specificity.20 Some researchers found that total thyroidectomy associated with prophylactic central neck dissection, with increased postoperative complications in patients over 75 years old, advocating a tailored surgical approach in elderly population.21,22 When the body is stimulated by infection or other non-infectious inducements, a large number of uncontrolled inflammatory mediators in the blood will cause excessive total T3 consumption in the body, leading to an immune imbalance, multi-functional organ damage, and other adverse consequences. Studies have shown that thyroid hormone level is related to the disease severity of critically ill patients, and the thyroid hormone levels of critically ill patients can be used as a judgment indicator of disease prognosis.23 Accordingly, thyroid function monitoring should be a focus for critically ill patients, as the timely detection of central hypothyroidism will have an important impact on the prognosis of critically ill patients. In this study, according to the difference in thyroid hormones TSH and rT3, the CH group and LT3S group were defined, but the final result of thyroid hormone difference between the two groups showed that only TSH and rT3 were significantly different, which was without expectation, but also provided some data references for the further study.
To discover factors related to the occurrence of central hypothyroidism in critically ill patients, the blood glucose, blood lipid, and plasma protein of critically ill patients were analyzed. Results of our study showed differences in FPG levels between patients with central hypothyroidism and those with low T3 syndrome. Further analysis showed that FPG level was negatively correlated with serum TSH level and serum FT3 level. In this study, the number of patients with diabetes was small, accounting for only approximately 10.5% of all enrolled patients; however, there were still a majority of patients with significantly elevated levels of fasting plasma glucose. Standard teaching tells us that thyroid function should not be measured during critical illness unless absolutely necessary. However, the sooner we can distinguish potential thyroid problems, the sooner we can adopt replacement treatments.23 Some indicators with potential value, such as fasting plasma glucose levels, may remind us to be alert to fluctuations in thyroid function and remind us to carry out complete thyroid function tests.
Consistent with existing studies, 40–50% of critically ill ICU patients will develop stress hyperglycemia, leading to drastic changes in blood glucose.24 Concurrently, basic and clinical studies25,26 showed that severe blood glucose fluctuations increased oxidative stress and the expression of protein kinase C activity. The levels of TNF-α, IL-6, C-reactive protein, and other inflammatory factors also increased significantly. Tumor necrosis factor can inhibit the proliferation of thyroid cells, and Il-6 inhibits the synthesis of peroxidase messenger ribonucleic acid in the thyroid gland and blocks the release of TSH, thereby reducing the production of total T3 in patients. Therefore, the results of this study suggest that fasting glucose control has clinical significance for reducing the occurrence of central hypothyroidism in critically ill patients.
This study includes some limitations. Firstly, the included patients lacked TRH test results and the head imaging results, such as MRI and enhanced CT, and patients lacked the relative typical clinical manifestations of hypothyroidism (such as hypothermia, hypothyroidism, and hypothyroidism). It is difficult to distinguish from primary central hypothyroidism and the interpretation of thyroid function is also partly controversial; secondly, patients with diabetes were included in this study, which is detrimental to the interpretation of the level of fasting plasma glucose; thirdly, the sample size of the included studies is small, and the positive results of this study suggest the significance of further large-scale trials; fourthly, participants were divided into two groups, namely CH and LT3S and the grouping standards were controversy. These groups were not mutually exclusive, which may cause selective bias; fifthly, many participants had COPD or ARDS and steroids are used quite common in these settings. Considering that steroids are known to suppress TSH (inducing a CH-like state) as well as elevation of blood glucose, so there maybe have some confoundings.
In conclusion, the results of this study suggest that central hypothyroidism may exist in critically ill patients and should be taken seriously. The development of central hypothyroidism may be associated with elevated fasting plasma glucose levels. When fasting plasma glucose was higher than 6.4 mmol/L on hospital admission, the presence of central hypothyroidism will be more significant. Thyroid function should be actively examined, central hypothyroidism and low T3 syndrome should be identified in a timely manner, and corresponding treatment should be administered to provide favorable help to critically ill patients that can help them recover from their primary disease and, accordingly.
Ethics Approval and Consent to Participate
This study was conducted with approval from the Ethics Committee of The First Hospital of Fuzhou. This study was conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from all the study participants.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Berk J, Wade R, Baser HD, Lado J. Case report: severe reversible cardiomyopathy associated with systemic inflammatory response syndrome in the setting of diabetic hyperosmolar hyperglycemic non-ketotic syndrome. BMC Cardiovasc Disord. 2015;15:123. doi:10.1186/s12872-015-0112-3
2. Drechsler C, Schneider A, Gutjahr-Lengsfeld L, et al. Thyroid function, cardiovascular events, and mortality in diabetic hemodialysis patients. Am J Kidney Dis. 2014;63(6):988–996. doi:10.1053/j.ajkd.2013.10.009
3. Kanji S, Neilipovitz J, Neilipovitz B, et al. Triiodothyronine replacement in critically ill adults with non-thyroidal illness syndrome. Can J Anaesth. 2018;65(10):1147–1153. doi:10.1007/s12630-018-1177-0
4. De Groot LJ. Non-thyroidal illness syndrome is a manifestation of hypothalamic-pituitary dysfunction, and in view of current evidence, should be treated with appropriate replacement therapies. Crit Care Clin. 2006;22(1):57–86. doi:10.1016/j.ccc.2005.10.001
5. Chopra IJ. Nonthyroidal illness syndrome or euthyroid sick syndrome? Endocr Pract. 1996;2(1):45–52.
6. Surks MI, Hupart KH, Pan C, Shapiro LE. Normal free thyroxine in critical nonthyroidal illnesses measured by ultrafiltration of undiluted serum and equilibrium dialysis. J Clin Endocrinol Metab. 1988;67(5):1031–1039. doi:10.1210/jcem-67-5-1031
7. Melmed S, Geola FL, Reed AW, Pekary AE, Park J, Hershman JM. A comparison of methods for assessing thyroid function in nonthyroidal illness. J Clin Endocrinol Metab. 1982;54(2):300–306. doi:10.1210/jcem-54-2-300
8. Spratt DI, Bigos ST, Beitins I, Cox P, Longcope C, Orav J. Both hyper- and hypogonadotropic hypogonadism occur transiently in acute illness: bio- and immunoactive gonadotropins. J Clin Endocrinol Metab. 1992;75(6):1562–1570. doi:10.1210/jcem.75.6.1464665
9. Spratt DI, Cox P, Orav J, Moloney J, Bigos T. Reproductive axis suppression in acute illness is related to disease severity. J Clin Endocrinol Metab. 1993;76(6):1548–1554. doi:10.1210/jcem.76.6.8501163
10. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. doi:10.1097/00003246-198510000-00009
11. Qin S-L, He Q, Hu L, et al. The relationship between inflammatory factors, oxidative stress and DIO-1 concentration in patients with chronic renal failure accompanied with or without euthyroid sick syndrome. J Int Med Res. 2018;46(10):4061–4070. doi:10.1177/0300060518778190
12. Suda S, Muraga K, Kanamaru T, et al. Low free triiodothyronine predicts poor functional outcome after acute ischemic stroke. J Neurol Sci. 2016;368:89–93. doi:10.1016/j.jns.2016.06.063
13. Meyer S, Schuetz P, Wieland M, Nusbaumer C, Mueller B, Christ-Crain M. Low triiodothyronine syndrome: a prognostic marker for outcome in sepsis? Endocrine. 2011;39(2):167–174. doi:10.1007/s12020-010-9431-4
14. Suda S, Shimoyama T, Nagai K, et al. Low free triiodothyronine predicts 3-month poor outcome after acute stroke. J Stroke Cerebrovasc Dis. 2018;27(10):2804–2809. doi:10.1016/j.jstrokecerebrovasdis.2018.06.009
15. Reinhardt W, Mann K. [Non-thyroid illness or changed thyroid hormone parameter syndrome with non-thyroid illnesses]. Med Klin. 1998;93(11):662–668. Norwegian. doi:10.1007/BF03044878
16. Docter R, Krenning EP, de Jong M, Hennemann G. The sick euthyroid syndrome: changes in thyroid hormone serum parameters and hormone metabolism. Clin Endocrinol. 1993;39(5):499–518. doi:10.1111/j.1365-2265.1993.tb02401.x
17. Chopra IJ, Huang TS, Boado R, Solomon DH, Chua Teco GN. Evidence against benefit from replacement doses of thyroid hormones in nonthyroidal illness (NTI): studies using turpentine oil-injected rat. J Endocrinol Invest. 1987;10(6):559–564. doi:10.1007/BF03346994
18. De Vito P, Balducci V, Leone S, et al. Nongenomic effects of thyroid hormones on the immune system cells: new targets, old players. Steroids. 2012;77(10):988–995. doi:10.1016/j.steroids.2012.02.018
19. van der Spek AH, Fliers E, Boelen A. Thyroid hormone metabolism in innate immune cells. J Endocrinol. 2017;232(2):R67–R81. doi:10.1530/JOE-16-0462
20. Marotta V, Sciammarella C, Chiofalo MG, et al. Hashimoto’s thyroiditis predicts outcome in intrathyroidal papillary thyroid cancer. Endocr Relat Cancer. 2017;24(9):485–493. doi:10.1530/ERC-17-0085
21. Gambardella C, Patrone R, Di Capua F, et al. The role of prophylactic central compartment lymph node dissection in elderly patients with differentiated thyroid cancer: a multicentric study. BMC Surg. 2019;18(Suppl 1):1–8. doi:10.1186/s12893-018-0433-0
22. Pezzolla A, Docimo G, Ruggiero R, et al. [Incidental thyroid carcinoma: a multicentric experience]. Recenti Prog Med. 2010;101(5):194–198. Italian.
23. Economidou F, Douka E, Tzanela M, Nanas S, Kotanidou A. Thyroid function during critical illness. Hormones. 2011;10(2):117–124. doi:10.14310/horm.2002.1301
24. Bateman RM, Sharpe MD, Jagger JE, et al. 36th international symposium on intensive care and emergency medicine: Brussels, Belgium. Crit Care. 2016;20:13–182.
25. Liu T, Pei Y, Peng Y, Chen J, Jiang S, Gong J. Oscillating high glucose enhances oxidative stress and apoptosis in human coronary artery endothelial cells. J Endocrinol Invest. 2014;37(7):645–651. doi:10.1007/s40618-014-0086-5
26. Monnier L, Colette C. Glycemic variability: should we and can we prevent it? Diabetes Care. 2008;31(Suppl 2):S150–S154. doi:10.2337/dc08-s241
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.