Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
The Challenges of Identifying Environmental Determinants of Type 1 Diabetes: In Search of the Holy Grail
Authors Butalia S , Kaplan GG , Khokhar B, Haubrich S, Rabi DM
Received 1 August 2020
Accepted for publication 15 October 2020
Published 9 December 2020 Volume 2020:13 Pages 4885—4895
DOI https://doi.org/10.2147/DMSO.S275080
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Antonio Brunetti
Sonia Butalia,1– 4 Gilaad G Kaplan,1– 3 Bushra Khokhar,2 Sydney Haubrich,5 Doreen M Rabi1– 4,6
1Department of Medicine, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada; 2Department of Community Health Sciences, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada; 3O’Brien Institute for Public Health, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada; 4Libin Cardiovascular Institute, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada; 5Ward of the 21st Century, University of Calgary, Calgary, Alberta, Canada; 6Department of Cardiac Sciences, Faculty of Medicine, University of Calgary, Calgary, Alberta, Canada
Correspondence: Sonia Butalia
Division of Endocrinology and Metabolism, Richmond Road Diagnostic and Treatment Centre, 1820 Richmond Road SW, Calgary, Alberta T2T 5C7, Canada
Tel +1 403-955-8327
Fax +1 403-955-8249
Email [email protected]
Abstract: Type 1 diabetes is the result of autoimmune-mediated destruction and inflammation of the insulin-producing β-cells of the pancreas. The excess morbidity and mortality from its complications coupled with its increasing incidence emphasize the importance to better understand the etiology of this condition. It has a strong genetic component, but a genetic predisposition is not the sole contributor to disease development as only 30% to 50% of identical twins both develop the disease. In addition, there are multiple lines of evidence to support that environmental factors contribute to the pathogenesis of type 1 diabetes. Environmental risk factors that have been proposed include infections, dietary factors, air pollution, vaccines, location of residence, childhood obesity, family environment and stress. Researchers have conducted many observational studies to identify and characterize these potential environmental factors, but findings have been inconsistent or inconclusive. Many studies have had inherent methodological issues in recruitment, participation, defining cases and exposures, and/or data analysis which may limit the interpretability of findings. Identifying and addressing these limitations may allow for greatly needed advances in our understanding of type 1 diabetes. As such, the purpose of this article is to review and discuss the limitations of observational studies that aim to determine environmental risk factors for type 1 diabetes and propose recommendations to overcome them.
Keywords: type 1 diabetes, risk factors, epidemiology
Introduction
Type 1 diabetes is the result of autoimmune-mediated destruction and inflammation of the insulin-producing β-cells of the pancreas.1 It has a strong genetic component, and over the last several decades, multiple loci have been identified in its pathogenesis.1 But genetic predisposition is not the sole contributor to disease development as only 30% to 50% of identical twins both develop the disease and the incidence of type 1 diabetes has been increasing worldwide by 3% to 5% per year, which is far too quickly if genetic factors were only at play.1–8
Multiple lines of evidence support the role of environmental risk factors in the pathogenesis of type 1 diabetes including (1) migration studies showing increased incidence in groups who have moved from areas of low incidence to high incidence9,10; (2) shift to earlier onset of disease11; (3) increased incidence in all age groups4;and (4) greatest increase in rate of incidence is observed in previously low-incidence countries.3,5 Despite this compelling evidence that environmental factors are likely at play, identifying specific factors has been challenging with findings to date being inconsistent or inconclusive.12
Many environmental factors have been postulated in the pathogenesis of type 1 diabetes (Table 1).12 Viruses have been implicated as an environmental trigger of type 1 diabetes; however, in many cases, the temporal relationship between viruses and type 1 diabetes is unclear.13–17 Common childhood vaccinations have also been questioned in the etiology of type 1 diabetes; however, well-conducted studies have negated the relationship.18,19 Dietary factors, like gluten, nitrate, and nitrite intake, have been associated with increased risk of type 1 diabetes; however, studies describe inconsistent results.20–22 Results from cohort studies suggest that exclusive breastfeeding may have a small protective effect in early infancy, potentially when introducing cereal grains to the infant diet, but evidence is inconclusive.23,24 Childhood obesity has also been linked to increasing rates of type 1 diabetes, with promising mechanisms proposed, but further studies are required in this area.25 The increase in type 1 diabetes, as well as other immunologic disorders, may be due to the reduced or lack of exposure to agents that previously children were more exposed to mediated factors such as improved sanitation and hygiene.26 Researchers refer to this as the “hygiene hypothesis”, which suggests that the developing immune system requires stimulation from environmental factors for maturation of immune defenses.27 Surrogate markers of this hypothesis have been assessed, including gut flora/permeability, rural versus urban residence, and birth order or number of siblings.12 Finally, the gut microbiome may play an important role in the pathogenesis of islet autoimmunity (leading to type 1 diabetes) with research in this area still underway.28
 |
Observational studies pose several challenges, and biases may be introduced in design or analyses, leading to heterogeneity in the literature.29 The purpose of this article is to review and discuss the methodological issues present in observational studies and outline how they pose challenges in determining environmental risk factors for type 1 diabetes.
Study Participation and Recruitment
There are two main approaches for enrolling participants into observational studies for type 1 diabetes. They include (1) direct recruitment and (2) administrative databases with advantages and disadvantages for each method.
Direct Recruitment
Observational studies that investigate the causes of type 1 diabetes often recruit patients directly from diabetes centres or clinics to participate in research.30–32 The advantages of this approach include efficiency in the recruitment of participants, detailed information on disease status, detailed clinical information, information on multiple environmental exposures, and the opportunity to follow patients prospectively. Unfortunately, recruiting patients directly can result in selection bias in that non-attendees of clinics are not included in studies. Non-attendees of diabetes clinics have been shown to differ from those that do attend the clinic in their sociodemographic characteristics such as age, sex, and socioeconomic status, resulting in an unrepresentative sample population.33 While it may be possible to identify potential participants in individual academic centres, few larger national networks of children and adolescents with incident type 1 diabetes exist, so enrolling large cohorts of participants in this manner can be challenging. The Environmental Determinants of Diabetes in the Young (TEDDY) study has done an exemplary job of recruitment by addressing some of these issues.34 The primary goal of TEDDY, a prospective cohort study, is to determine the environmental causes of type 1 diabetes. Participants are recruited from the general population, at birth, and researchers follow them prospectively throughout childhood or until the development of either islet autoimmunity or type 1 diabetes.34
Administrative Databases
Administrative databases have several advantages including population-based sampling, information on multiple exposures, efficiency in recruitment of large sample sizes, and the ability to be linked with other databases be it clinical, laboratory, or other administrative databases. Administrative databases often have independent data recording, which not only minimizes non-response, selection, and recall biases but can also provide both cross-sectional and longitudinal information about prevalence and incidence rates of diabetes at a population level. Several databases and electronic medical records have been used in type 1 diabetes research including the Swedish National Diabetes Register,35 the Danish Adult Diabetes Database,36 the Scottish Care Information–Diabetes Collaboration (SCI-DC) database37 and The Health Improvement Network (THIN).38 Disadvantages of databases include that they are limited to the variables collected, the way the variables are collected (ie data may be collected by category rather than as a continuous variable), lacking in rich clinical detail, and expensive to create, maintain, and manage. Furthermore, studies that use large diabetes databases are often a secondary analysis of data collected for other purposes. This may lead to issues with data quality (eg missing or nonsense data), crude or surrogate measures of important predictors, and misclassification biases. Confounding may be more difficult to address if important covariate information is not available.39 Together, although direct recruitment from clinical settings or from databases has advantages, there are also some disadvantages in doing so as outlined above.
Selection of Cases and Controls
Observational studies often draw inferences from comparing cases and controls. However, the procedures used to select cases and controls in observational studies for type 1 diabetes have the potential to introduce biases (Table 2).
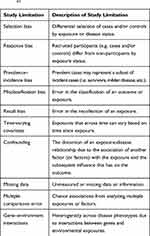 |
Table 2 Study Limitations to Consider in Epidemiologic Studies41 |
Selection Bias
In case–control studies, selection bias occurs when the selection of cases differs from the selection of controls. For example, recruitment of people with type 1 diabetes may occur at speciality diabetes centres through posters in reception areas, direct invitation to participate (eg at clinic appointments), or via registration in an electronic medical record. In contrast, controls may be selected through population registries. Selection bias may occur as cases and controls are selected from two different source populations where cases are recruited from clinics and controls are recruited from the community. For example, in a matched case–control study investigating the risk of atrial fibrillation in people with type 1 diabetes, cases were recruited from a pool of individuals in a diabetes database, while controls (matched by age, sex, and country of residence) were selected from a pool of healthy individuals in the general population.40 When cases are selected from diabetes clinics, the selection may not be representative of the actual population. First, those with type 1 diabetes, particularly adults, do not necessarily receive centralized care. Instead, they may receive care from a variety of care providers, including general practitioners or family physicians, internists, and endocrinologists so they may not attend diabetes clinics. Second, patients with severe presentations of type 1 diabetes (ie severe diabetic ketoacidosis (DKA)) may die prior to attending the clinic. Finally, community controls may be misclassified or early in their disease course. All these factors require consideration to minimize selection bias in observational studies.
Response Bias
Response bias occurs when the exposure occurs differently in participants of a study than it does in non-participants.41 Non-responders are known to differ from responders in a number of sociodemographic characteristics and health-related behaviours like age, sex, socioeconomic status, and smoking, all of which have been postulated to be related to type 1 diabetes onset.42–44 Non-response often occurs in a higher proportion of controls than cases, and many studies do not take this into account. For example, an inverse relationship between socioeconomic status and non-response has been observed.44 Some studies have demonstrated that type 1 diabetes risk is associated with indicators of higher socioeconomic status.44,45 Similarly, smoking, alcohol consumption, and drug use all appear to be higher in non-responders.43,46 In a case–control study, maternal smoking was observed to be protective of type 1 diabetes risk.47 These findings may need to be interpreted with caution, as statistically significant associations may have been influenced by a non-response bias driving the observed significant associations.47,48 Investigators using observational studies to determine environmental causes of type 1 diabetes could minimize response bias by recruiting participants from the general population to help ensure socioeconomic and behavioural diversity.
Prevalence–Incidence Bias
Prevalence–incidence bias may occur in observational studies, particularly in case–control studies where selection of participants occurs after onset of the condition. Prevalent cases of a condition only represent a subset of individuals from the source population. Some groups may not be appropriately represented, like those who are rural-residing, migrated, or those who have died.49 Associations found in prevalence studies may be a reflection of markers of survival or disease progression rather than actual risk factors of a disease.50 Failure to take these unique population factors into account could skew the resulting data.
Prevalent cases may be a more attractive option for investigators because these cases enhance study feasibility (eg study recruitment), but inferences made from the study of prevalent cases only may lead to inaccurate conclusions. For example, in a systematic review and meta-analysis of 23 studies of children, adolescents, and adults, prevalent cases of individuals with type 1 diabetes had lower levels of serum 25-OH vitamin D compared to age- and sex-matched controls.51 However, in a study of participants with incident type 1 diabetes, prevalent type 1 diabetes, and healthy controls, serum 25-OH vitamin D levels were low among all groups, and reduced levels were not specifically associated with type 1 diabetes.52 Following cohorts of study participants prospectively is one of the best ways to minimize the biases often found in prevalence studies.
Misclassification Bias
Misclassification bias results from the errors in classification of the outcome or exposure. Challenges arise using clinical and administrative databases for the study of type 1 diabetes, because surveillance systems may not distinguish diabetes by type.53 Some investigators have used a combination of features for case ascertainment of type 1 diabetes including age, prescription history (eg insulin use), and/or body mass index, but the validity of these criteria has not been determined.54,55 Others have used a genetic risk score to identify cases of type 1 diabetes, but the score has only been validated in people of white European descent, limiting its applicability.56,57 Researchers using clinical or administrative databases to define cases of type 1 diabetes need to carefully select criteria that are valid for the population they are studying, then confirm that the information contained in the database(s) is accurate.
Selection and Definition of Exposure Information
The procedures used to select and define exposure information in observational studies are also subject to introducing several biases (Table 2).
Misclassification Bias
Misclassification (or information) bias occurs with inaccurate classification of the exposure. This bias may be introduced when using databases to ascertain exposure information. For example, studies of infections as an environmental risk factor for type 1 diabetes have been inconsistent and this may be due to misclassification bias.58–60 In one study, no association was observed between early infections in life and subsequent risk of type 1 diabetes.60 The authors used a primary care database to collect general practitioner visits for infections and prescriptions for antibiotics. However, infections were likely underestimated as parents may not consult their general practitioner for every infection, particularly milder presentations, and antibiotics are not clinically indicated for many viral infections.60 Studies using more inclusive criteria, such as serologic markers for enterovirus infection, have demonstrated a significant association with type-1-diabetes-related autoimmunity.13 Misclassification bias could be minimized by collecting data directly instead of extracting secondary data from databases.
Recall Bias
Recall bias is a systematic difference in the recollection of exposure information among cases and controls. A number of characteristics have been shown to influence recall, including age, education, socioeconomic status, and the time interval since exposure.61 Several environmental factors for type 1 diabetes have been assessed using survey data that relies on recall, like maternal characteristics and practices, breastfeeding, childhood factors, and dietary habits.62–67 Recall of dietary habits is especially challenging, as participants cannot always recall their dietary intake accurately.68 Many breastfeeding studies have retrospective designs that require recollection of exposure history from several years prior.69 Mothers of children with type 1 diabetes are more apt to recall times when they deviated from breastfeeding recommendations.69 People may be more likely to recall their exposure information if they are more motivated to discover important links for their condition.70
Time-Varying Covariates and Diagnostic Bias
The timing of exposure is another challenge in studying the environmental factors associated with type 1 diabetes. The latency period between time of exposure and type 1 diabetes onset is unknown. Several timelines have been proposed, including a linear process, relapse and remitting process, or a dose threshold (of an environmental agent) but proposed timelines remain hypothetical.71 Using a feature of the Bradford Hill criteria known as “temporality”, an exposure must occur prior to the outcome; however, the timing, dose, and or/threshold is not known for many environmental factors in type 1 diabetes.72 There is certainty of the timing of some exposures as they are known to have occurred prior to disease onset such as maternal infections in pregnancy and breastfeeding during infancy. It is less clear with other factors that may influence type 1 diabetes risk in a time-dependent fashion. For example, vitamin D levels are known to vary with sun exposure, activity, season, diet, supplementation, and clothing, but some studies of vitamin D have used a single serum sample in their analyses.73 Similarly, studies of other environmental factors have not collected exposures as time-varying covariates. Instead, the studies applied repeated-measures analysis.74
In addition, in case–control studies, the exposure window is often overlooked, and cases and controls have different exposure windows by design. Many studies will match cases with type 1 diabetes to healthy controls on age and sex instead of an index date (eg date of diagnosis). In type 1 diabetes cases, the exposure window ends at disease onset, while controls are given a longer exposure window.75,76 As such, exposures may be overrepresented, which dilutes possible exposure-disease associations. For example, in a case–control study of several environmental risk factors, controls were siblings of children with type 1 diabetes. If there was more than one sibling, the control child would be the one with age closest to the index child. Because controls may have been older, they would have had a longer exposure window at the time of questionnaire administration.76 This bias can be overcome by assigning an index date of a matched case and not including exposure history after that date. For example, a study on the relationship between serum vitamin D and development of insulin-dependent diabetes matched cases with controls based on five factors, including age, sex, and blood serum collection date for the study.77 Adding the collection date as an index may have allowed for a more precise estimation of participant exposure windows.
Analytical Methods
Multiple issues may arise in the analytical stage of observational studies for environmental factors related to type 1 diabetes onset (Table 2).
Confounding Data
Isolating the independent effect of any given factor to observe its association with type 1 diabetes is challenging because of potential confounding. Systematic reviews and meta-analyses often fail to control for important confounders. For instance, a systematic review and meta-analysis concluded that breastfeeding for more than 3 months was associated with approximately a 30% risk reduction of type 1 diabetes in those that were breastfed.78 A subsequent meta-analysis using individual participant data found a smaller protective benefit for type 1 diabetes, possibly because the data allowed for adjustment of potential confounders. Those that were exclusively breastfed for greater than 2 weeks were at decreased risk of developing type 1 diabetes (odds ratio 0.75, 95% CI, 0.64 to 0.88) but the protection was attenuated in those breastfed exclusively for greater than 3 months (odds ratio 0.87, 95% CI, 0.75 to 1.00).24 Modern cohort studies investigating the environmental determinants of type 1 diabetes address this concern by collecting a variety of data, including child and maternal dietary factors, body mass index, exposure to infectious agents, and psychosocial factors.34,66,79 Other procedures may be used to control for confounding including matching, restriction and multivariable analysis.
Missing Data
In studies using data collected from clinical and administrative databases, investigators are limited to the data elements collected, so the investigators may not be able to collect information on important factors that may influence type 1 diabetes risk.39 For example, data to determine an individual’s socioeconomic status may not be readily available, so investigators may use proxy measures to approximate exposure at the level of the individual.80,81 Such proxy measures may be subject to ecological fallacy, where data about an individual are extrapolated from group data, but is not accurate for the individual (ie an individual’s income is derived from neighbourhood income estimates, but the individual is unemployed, thus leading to ecological fallacy). Similarly, partial missing observations of data elements pose an issue as data may be missing in a biased manner.82 For example, illicit drug users may leave survey questions blank as their true responses are perceived as less socially desirable.70 Some approaches have been suggested to deal with missing data such as (1) using additional data sources; (2) omitting data element with missing data; (3) omitting participants with missing data; (4) using proxy measures; and/or, (5) estimation of missing value.82 None of these alternatives have the strength of a complete data set.
Multiple Comparison Error
Studies that assess multiple risk factors in one study are often underpowered due to small sample sizes, use of lengthy questionnaires, and application of multivariable analyses without an a priori exposure of interest. Multiple comparison errors can occur when statistically significant findings result from chance alone (ie 5% alpha error rate). For example, in one study of environmental risk factors, the authors aimed to assess the influence of over 70 environmental risk factors on 68 participants with type 1 diabetes.76 In this case, multivariable regression analyses were applied without consideration of overfitting models. Further, the precision of risk estimates is limited. This is reflected by wide confidence intervals and, subsequently, potential loss of statistical significance.
Gene–Environment Interactions
The onset of type 1 diabetes is likely the result of interplay among various gene and environmental factors that make the study of interactions more complicated. The higher-risk alleles of type 1 diabetes vary among and between populations.83 Furthermore, Petrone et al have demonstrated HLA DRB1 and DQB1 genes influence the age of onset of type 1 diabetes as well as the degree of beta-cell destruction at diagnosis.84 Few studies have accounted for genetic heterogeneity of their underlying study population like in the prospective birth cohort study of Finnish children where researchers observed an interaction between a polymorphism in the PTPN22 gene and infant feeding.85 A PTPN22 allele was associated with autoantibodies and clinical type 1 diabetes among children exposed to cow’s milk before the age of 6 months, but not in among children exposed later. Many studies have not included genetic heterogeneity, which may account for diluted results. Similarly, the distribution of environmental factors may also vary among populations (eg dietary preferences, family sizes, types of infectious agents, etc.), so more work is required to gain further understanding of the gene–environment interactions.
Recommendations
Consider the following when evaluating environmental risk factors for type 1 diabetes.
Prospective Studies
Well-designed prospective cohort studies are important to evaluate environmental risk factors and further advance our understanding of type 1 diabetes.
- Defining and collecting environmental factors prospectively allows researchers to collect robust data that is targeted to type 1 diabetes.
- These studies allow investigators to address and eliminate many biases discussed above by defining environmental factors a priori, collecting information prospectively, and performing appropriate analyses.
- Collecting information prospectively minimizes recall and exposure biases as it allows researchers to rely less on participant recall and limit the time since exposure.
Preliminary results are available from several promising prospective cohort studies that adhere to many of these recommendations.31,86–92 Many of these studies, which follow large cohorts of healthy individuals to a new diagnosis of type 1 diabetes, are well positioned to determine the environmental factors associated with type 1 diabetes development because they are not subject to the methodological pitfalls of many other observational studies described.
Case–Control Studies
If a case–control study design is used to evaluate environmental determinants of type 1 diabetes, factors to consider are:
- Collecting cases and controls from the same pool of participants.
- If collecting data from a database, the use of a combination of features for case ascertainment is suggested.
- Studies using databases are vulnerable to misclassification bias, particularly if the method of classification has not been validated for the study population and/or the source data have not been reviewed for accuracy.
- If selecting cases from diabetes clinics, be aware that they may systematically differ from those that would be found in the general population (eg those with severe presentations may not attend or die prior to attending).
- Once cases are selected, match them to controls using an index date to represent exposure windows as accurately as possible, and consider that community controls may also be misclassified or early in their disease course.
Analyses
Performing appropriate analyses is also key to a well-designed observational study.
- It is important to be aware of potential confounders and collect data on them so that they can be controlled for.
- Whenever possible, try to work with a complete dataset so that missing data does not need to be accounted for in a different way.
- As more evidence on gene–environment interactions becomes available, researchers will also need to account for genetic heterogeneity of their underlying study population.
Conclusion
Environmental factors play a role in the development of type 1 diabetes, but identifying strong preventive or risk factors have been challenging. Observational studies to identify environmental risk factors for developing type 1 diabetes may have inherent issues in study recruitment and participation; defining cases and exposures; and data analysis. Such issues may limit the interpretability of findings. Investigators looking to conduct research in this area can take steps to identify and address the limitations inherent in observational research. Well-designed studies may allow for greatly needed advances in our understanding of type 1 diabetes.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Canadian Institutes for Health Research (CIHR) Operating Grant (MOP-133723). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Paschou SA, Papadopoulou-Marketou N, Chrousos GP, Kanaka-Gantenbein C. On type 1 diabetes mellitus pathogenesis. Endocr Connect. 2018;7(1):R38.
2. Gale EAM. The rise of childhood type 1 diabetes in the 20th century. Diabetes. 2002;51(12):3353–3361. doi:10.2337/diabetes.51.12.3353
3. Jarosz-Chobot P, Polanska J, Szadkowska A, et al. Rapid increase in the incidence of type 1 diabetes in Polish children from 1989 to 2004, and predictions for 2010 to 2025. Diabetologia. 2011;54(3):508–515. doi:10.1007/s00125-010-1993-4
4. The Diamond Project Group. Incidence and trends of childhood type 1 diabetes worldwide 1990–1999. Diabet Med. 2006;23(8):857–866. doi:10.1111/j.1464-5491.2006.01925.x
5. Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltész G. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet. 2009;373(9680):2027–2033. doi:10.1016/S0140-6736(09)60568-7
6. EURODIAB ACE Study Group. Variation and trends in incidence of childhood diabetes in Europe. Lancet. 2000;355(9207):873–876.
7. Onkamo P, Väänänen S, Karvonen M, Tuomilehto J. Worldwide increase in incidence of type I diabetes – the analysis of the data on published incidence trends. Diabetologia. 1999;42(12):1395–1403. doi:10.1007/s001250051309
8. Forlenza GP, Rewers M. The epidemic of type 1 diabetes: what is it telling us? Curr Opin Endocrinol Diabetes Obes. 2011;18(4):248–251. doi:10.1097/MED.0b013e32834872ce
9. Söderström U, Åman J, Hjern A. Being born in Sweden increases the risk for type 1 diabetes – a study of migration of children to Sweden as a natural experiment. Acta Paediatr. 2012;101(1):73–77. doi:10.1111/j.1651-2227.2011.02410.x
10. Raymond N, Jones J, Swift P, et al. Comparative incidence of type I diabetes in children aged under 15 years from South Asian and White or Other ethnic backgrounds in Leicestershire, UK, 1989 to 1998. Diabetologia. 2001;44(Supp S3):B32–B36. doi:10.1007/PL00002951
11. Karvonen M, Pitkäniemi J, Tuomilehto J. The onset age of type 1 diabetes in Finnish children has become younger. The Finnish Childhood Diabetes Registry Group. Diabetes Care. 1999;22(7):1066–1070. doi:10.2337/diacare.22.7.1066
12. Butalia S, Kaplan GG, Khokhar B, Rabi DM. Environmental risk factors and type 1 diabetes: past, present, and future. Can J Diabetes. 2016;40(6):586–593. doi:10.1016/j.jcjd.2016.05.002
13. Yeung W-CG, Rawlinson WD, Craig ME. Enterovirus infection and type 1 diabetes mellitus: systematic review and meta-analysis of observational molecular studies. BMJ. 2011;342:d35.
14. Pak C, McArthur R, Eun H-M, Yoon J-W. Association of cytomegalovirus infection with autoimmune type 1 diabetes. Lancet. 1988;332(8601):1–4. doi:10.1016/S0140-6736(88)92941-8
15. Ramondetti F, Sacco S, Comelli M, et al. Type 1 diabetes and measles, mumps and rubella childhood infections within the Italian insulin-dependent diabetes registry. Diabet Med. 2012;29(6):761–766. doi:10.1111/j.1464-5491.2011.03529.x
16. Honeyman MC, Coulson BS, Stone NL, et al. Association between rotavirus infection and pancreatic islet autoimmunity in children at risk of developing type 1 diabetes. Diabetes. 2000;49(8):1319–1324. doi:10.2337/diabetes.49.8.1319
17. Rodriguez-Calvo T, Sabouri S, Anquetil F, von Herrath MG. The viral paradigm in type 1 diabetes: who are the main suspects? Autoimmun Rev. 2016;15(10):964–969. doi:10.1016/j.autrev.2016.07.019
18. Beyerlein A, Strobl AN, Winkler C, et al. Vaccinations in early life are not associated with development of islet autoimmunity in type 1 diabetes high-risk children: results from prospective cohort data. Vaccine. 2017;35(14):1735–1741. doi:10.1016/j.vaccine.2017.02.049
19. Morgan E, Halliday SR, Campbell GR, Cardwell CR, Patterson CC. Vaccinations and childhood type 1 diabetes mellitus: a meta-analysis of observational studies. Diabetologia. 2016;59(2):237–243. doi:10.1007/s00125-015-3800-8
20. Virtanen SM. Dietary factors in the development of type 1 diabetes. Pediatr Diabetes. 2016;17(S22):49–55. doi:10.1111/pedi.12341
21. Lund-Blix NA, Dong F, Mårild K, et al. Gluten intake and risk of islet autoimmunity and progression to type 1 diabetes in children at increased risk of the disease: the Diabetes Autoimmunity Study in the Young (DAISY). Diabetes Care. 2019;dc182315.
22. Bahadoran Z, Ghasemi A, Mirmiran P, Azizi F, Hadaegh F. Nitrate-nitrite-nitrosamines exposure and the risk of type 1 diabetes: a review of current data. World J Diabetes. 2016;7(18):433–440. doi:10.4239/wjd.v7.i18.433
23. Meijer CR, Discepolo V, Troncone R, Mearin ML. Does infant feeding modulate the manifestation of celiac disease and type 1 diabetes? Curr Opin Clin Nutr Metab Care. 2017;20(3):222–226. doi:10.1097/MCO.0000000000000367
24. Cardwell CR, Stene LC, Ludvigsson J, et al. Breast-feeding and childhood-onset type 1 diabetes: a pooled analysis of individual participant data from 43 observational studies. Diabetes Care. 2012;35(11):2215–2225.
25. Buzzetti R, Zampetti S, Pozzilli P. Impact of obesity on the increasing incidence of type 1 diabetes. Diabetes Obes Metab. 2020;22(7):1009–1013. doi:10.1111/dom.14022
26. Bach J-F. The hygiene hypothesis in autoimmunity: the role of pathogens and commensals. Nat Rev Immunol. 2017;18:105.
27. Alexandre-Silva GM, Brito-Souza PA, Oliveira ACS, Cerni FA, Zottich U, Pucca MB. The hygiene hypothesis at a glance: early exposures, immune mechanism and novel therapies. Acta Trop. 2018;188:16–26. doi:10.1016/j.actatropica.2018.08.032
28. Vatanen T, Franzosa EA, Schwager R, et al. The human gut microbiome in early-onset type 1 diabetes from the TEDDY study. Nature. 2018;562(7728):589–594. doi:10.1038/s41586-018-0620-2
29. Lipsitch M, Jha A, Simonsen L. Observational studies and the difficult quest for causality: lessons from vaccine effectiveness and impact studies. Int J Epidemiol. 2016;45(6):2060–2074.
30. Hamman RF, Bell RA, Dabelea D, et al. The SEARCH for diabetes in youth study: rationale, findings, and future directions. Diabetes Care. 2014;37(12):3336–3344. doi:10.2337/dc14-0574
31. Ziegler AG, Meier-Stiegen F, Winkler C, Bonifacio E. Prospective evaluation of risk factors for the development of islet autoimmunity and type 1 diabetes during puberty–TEENDIAB: study design. Pediatr Diabetes. 2012;13(5):419–424. doi:10.1111/j.1399-5448.2011.00763.x
32. Hagopian WA, Erlich H, Lernmark Å, et al. The Environmental Determinants of Diabetes in the Young (TEDDY): genetic criteria and international diabetes risk screening of 421 000 infants. Pediatr Diabetes. 2011;12(8):733–743.
33. Hynes L, Byrne M, Dinneen SF, McGuire BE, O’Donnell M, Mc Sharry J. Barriers and facilitators associated with attendance at hospital diabetes clinics among young adults (15–30 years) with type 1 diabetes mellitus: a systematic review. Pediatr Diabetes. 2016;17(7):509–518. doi:10.1111/pedi.12198
34. The TEDDY Study Group. The Environmental Determinants of Diabetes in the Young (TEDDY) study: study design. Pediatr Diabetes. 2007;8(5):286–298. doi:10.1111/j.1399-5448.2007.00269.x
35. Petrie D, Lung TW, Rawshani A, et al. Recent trends in life expectancy for people with type 1 diabetes in Sweden. Diabetologia. 2016;59(6):1167–1176. doi:10.1007/s00125-016-3914-7
36. Ishtiak-Ahmed K, Carstensen B, Pedersen-Bjergaard U, Jørgensen ME. Incidence trends and predictors of hospitalization for hypoglycemia in 17,230 adult patients with type 1 diabetes: a Danish Register Linkage Cohort Study. Diabetes Care. 2017;40(2):226. doi:10.2337/dc16-0862
37. Boulanger M, Al-Shahi Salman R, Kerssens J, Wild SH, Group obotSDRNE. Association between diabetes mellitus and incidence of intracerebral haemorrhage and case fatality rates: a retrospective population-based cohort study. Diabetes Obes Metab. 2017;19(8):1193–1197. doi:10.1111/dom.12934
38. Vajravelu ME, Keren R, Weber DR, Verma R, De León DD, Denburg MR. Incidence and risk of celiac disease after type 1 diabetes: a population-based cohort study using the health improvement network database. Pediatr Diabetes. 2018;19(8):1422–1428. doi:10.1111/pedi.12770
39. Wild S, Fischbacher C, McKnight J. Using large diabetes databases for research. J Diabetes Sci Technol. 2016;10(5):1073–1078. doi:10.1177/1932296816645120
40. Dahlqvist S, Rosengren A, Gudbjornsdottir S, et al. Risk of atrial fibrillation in people with type 1 diabetes compared with matched controls from the general population: a prospective case-control study. Lancet Diabetes Endocrinol. 2017;5(10):799–807. doi:10.1016/S2213-8587(17)30262-0
41. Porta M, ed. A Dictionary of Epidemiology.
42. Rinne ST, Wong ES, Lemon JM, Perkins M, Bryson CL, Liu CF. Survey nonresponders incurred higher medical utilization and lower medication adherence. Am J Manag Care. 2015;21(1):e1–e8.
43. Cheung KL, Ten Klooster PM, Smit C, de Vries H, Pieterse ME. The impact of non-response bias due to sampling in public health studies: a comparison of voluntary versus mandatory recruitment in a Dutch national survey on adolescent health. BMC Public Health. 2017;17(1):276. doi:10.1186/s12889-017-4189-8
44. Agerholm J, Bruce D, Burstrom B. Comparing healthcare utilization among health survey respondents with the total population - are respondents representative? BMC Health Serv Res. 2016;16(1):510. doi:10.1186/s12913-016-1745-3
45. Xu G, Liu B, Sun Y, et al. Prevalence of diagnosed type 1 and type 2 diabetes among US adults in 2016 and 2017: population based study. BMJ. 2018;362:k1497.
46. Christensen AI, Ekholm O, Gray L, Glümer C, Juel K. What is wrong with non-respondents? Alcohol-, drug- and smoking-related mortality and morbidity in a 12-year follow-up study of respondents and non-respondents in the Danish Health and Morbidity Survey. Addiction. 2015;110(9):1505–1512. doi:10.1111/add.12939
47. Marshall AL, Chetwynd A, Morris JA, et al. Type 1 diabetes mellitus in childhood: a matched case control study in Lancashire and Cumbria, UK. Diabet Med. 2004;21(9):1035–1040. doi:10.1111/j.1464-5491.2004.01282.x
48. Rosenbauer J, Herzig P, Giani G. Early infant feeding and risk of type 1 diabetes mellitus—a nationwide population-based case–control study in pre-school children. Diabetes Metab Res Rev. 2008;24(3):211–222. doi:10.1002/dmrr.791
49. Grimes DA, Schulz KF. Bias and causal associations in observational research. Lancet. 2002;359(9302):248–252. doi:10.1016/S0140-6736(02)07451-2
50. Rothman KJ. Epidemiology: An Introduction. New York: Oxford University Press; 2002.
51. Feng R, Li Y, Li G, et al. Lower serum 25 (OH) D concentrations in type 1 diabetes: a meta-analysis. Diabetes Res Clin Pract. 2015;108(3):e71–e75. doi:10.1016/j.diabres.2014.12.008
52. Bierschenk L, Alexander J, Wasserfall C, Haller M, Schatz D, Atkinson M. Vitamin D levels in subjects with and without type 1 diabetes residing in a solar rich environment. Diabetes Care. 2009;32(11):1977–1979. doi:10.2337/dc09-1089
53. Saydah S, Imperatore G. Emerging approaches in surveillance of type 1 diabetes. Curr Diab Rep. 2018;18(9):61. doi:10.1007/s11892-018-1033-1
54. Harding JL, Shaw JE, Peeters A, Cartensen B, Magliano DJ. Cancer risk among people with type 1 and type 2 diabetes: disentangling true associations, detection bias, and reverse causation. Diabetes Care. 2015;38(2):264. doi:10.2337/dc14-1996
55. Rawshani A, Rawshani A, Franzén S, et al. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med. 2017;376(15):1407–1418. doi:10.1056/NEJMoa1608664
56. Thomas NJ, Jones SE, Weedon MN, Shields BM, Oram RA, Hattersley AT. Frequency and phenotype of type 1 diabetes in the first six decades of life: a cross-sectional, genetically stratified survival analysis from UK biobank. Lancet Diabetes Endocrinol. 2018;6(2):122–129.
57. Oram RA, Patel K, Hill A, et al. A type 1 diabetes genetic risk score can aid discrimination between type 1 and type 2 diabetes in young adults. Diabetes Care. 2016;39(3):337. doi:10.2337/dc15-1111
58. Sioofy-Khojine AB, Lehtonen J, Nurminen N, et al. Coxsackievirus B1 infections are associated with the initiation of insulin-driven autoimmunity that progresses to type 1 diabetes. Diabetologia. 2018;61(5):1193–1202. doi:10.1007/s00125-018-4561-y
59. Laitinen OH, Honkanen H, Pakkanen O, et al. Coxsackievirus B1 is associated with induction of beta-cell autoimmunity that portends type 1 diabetes. Diabetes. 2014;63(2):446–455. doi:10.2337/db13-0619
60. Cardwell CR, Carson DJ, Patterson CC. No association between routinely recorded infections in early life and subsequent risk of childhood-onset type 1 diabetes: a matched case–control study using the UK general practice research database. Diabet Med. 2008;25(3):261–267. doi:10.1111/j.1464-5491.2007.02351.x
61. Vetter TR, Mascha EJ. Bias, confounding, and interaction: lions and tigers, and bears, Oh My! Anesth Analg. 2017;125(3):1042–1048. doi:10.1213/ANE.0000000000002332
62. Lamb MM, Frederiksen B, Seifert JA, Kroehl M, Rewers M, Norris JM. Sugar intake is associated with progression from islet autoimmunity to type 1 diabetes: the diabetes autoimmunity study in the young. Diabetologia. 2015;58(9):2027–2034.
63. Piescik-Lech M, Chmielewska A, Shamir R, Szajewska H. Systematic review: early infant feeding and the risk of type 1 diabetes. J Pediatr Gastroenterol Nutr. 2017;64(3):454–459. doi:10.1097/MPG.0000000000001293
64. Hargreave M, Kjaer SK, Jorgensen ME, Jensen A. Type 1 diabetes risk in children born to women with fertility problems: a cohort study in 1.5 million Danish children. Acta Obstet Gynecol Scand. 2016;95(12):1441–1446. doi:10.1111/aogs.13028
65. Lund-Blix NA, Dydensborg Sander S, Størdal K, et al. Infant feeding and risk of type 1 diabetes in two large scandinavian birth cohorts. Diabetes Care. 2017;40(7):920. doi:10.2337/dc17-0016
66. Nygren M, Carstensen J, Koch F, Ludvigsson J, Frostell A. Experience of a serious life event increases the risk for childhood type 1 diabetes: the ABIS population-based prospective cohort study. Diabetologia. 2015;58(6):1188–1197. doi:10.1007/s00125-015-3555-2
67. Ferrara CT, Geyer SM, Liu Y-F, et al. Excess BMI in childhood: a modifiable risk factor for type 1 diabetes development? Diabetes Care. 2017;40(5):698.
68. Naska A, Lagiou A, Lagiou P. Dietary assessment methods in epidemiological research: current state of the art and future prospects. F1000Res. 2017;6:926. doi:10.12688/f1000research.10703.1
69. Norris JM, Scott FW. A meta-analysis of infant diet and insulin-dependent diabetes mellitus: do biases play a role? Epidemiology. 1996;7(1):87–92. doi:10.1097/00001648-199601000-00015
70. Coughlin SS. Recall bias in epidemiologic studies. J Clin Epidemiol. 1990;43(1):87–91. doi:10.1016/0895-4356(90)90060-3
71. Van Belle TL, Coppieters KT, Von Herrath MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev. 2011;91(1):79–118.
72. Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965;58(5):295–300. doi:10.1177/003591576505800503
73. Greer RM, Portelli SL, Hung BS-M, et al. Serum vitamin D levels are lower in Australian children and adolescents with type 1 diabetes than in children without diabetes. Pediatr Diabetes. 2013;14(1):31–41. doi:10.1111/j.1399-5448.2012.00890.x
74. Cardwell C, Carson D, Patterson C. Higher incidence of childhood-onset type 1 diabetes mellitus in remote areas: a UK regional small-area analysis. Diabetologia. 2006;49(9):2074–2077.
75. Ahadi M, Tabatabaeiyan M, Moazzami K. Association between environmental factors and risk of type 1 diabetes - a case-control study. Endokrynol Pol. 2011;62(2):134–137.
76. Vlajinac H, Šipetić S, Marinković J, Bjekić M, Kocev N, Sajić S. The belgrade childhood diabetes study – comparison of children with type 1 diabetes with their siblings. Paediatr Perinat Epidemiol. 2006;20(3):238–243. doi:10.1111/j.1365-3016.2006.00713.x
77. Gorham ED, Garland CF, Burgi AA, et al. Lower prediagnostic serum 25-hydroxyvitamin D concentration is associated with higher risk of insulin-requiring diabetes: a nested case–control study. Diabetologia. 2012;55(12):3224–3227. doi:10.1007/s00125-012-2709-8
78. Gerstein HC. Cow’s milk exposure and type I diabetes mellitus: a critical overview of the clinical literature. Diabetes Care. 1994;17(1):13–19. doi:10.2337/diacare.17.1.13
79. Hummel S, Ziegler AG. Early determinants of type 1 diabetes: experience from the BABYDIAB and BABYDIET studies. Am J Clin Nutr. 2011;94(suppl_6):1821S–1823S. doi:10.3945/ajcn.110.000646
80. Haynes A, Bulsara MK, Bower C, Codde JP, Jones TW, Davis EA. Independent effects of socioeconomic status and place of residence on the incidence of childhood type 1 diabetes in Western Australia. Pediatr Diabetes. 2006;7(2):94–100. doi:10.1111/j.1399-543X.2006.00153.x
81. Patterson CC, Dahlquist G, Soltész G, Green A. Is childhood-onset type I diabetes a wealth-related disease? An ecological analysis of European incidence rates. Diabetologia. 2001;44(3):B9–B16. doi:10.1007/PL00002961
82. Pedersen AB, Mikkelsen EM, Cronin-Fenton D, et al. Missing data and multiple imputation in clinical epidemiological research. Clin Epidemiol. 2017;9:157–166. doi:10.2147/CLEP.S129785
83. Steck AK, Rewers MJ. Genetics of type 1 diabetes. Clin Chem. 2011;57(2):176–185. doi:10.1373/clinchem.2010.148221
84. Petrone A, Galgani A, Spoletini M, et al. Residual insulin secretion at diagnosis of type 1 diabetes is independently associated with both, age of onset and HLA genotype. Diabetes Metab Res Rev. 2005;21(3):271–275. doi:10.1002/dmrr.549
85. Lempainen J, Vaarala O, Mäkelä M, et al. Interplay between PTPN22 C1858T polymorphism and cow’s milk formula exposure in type 1 diabetes. J Autoimmun. 2009;33(2):155–164. doi:10.1016/j.jaut.2009.04.003
86. NIH: U.S. National Library of Medicine. Type 1 Diabetes Prediction and Prevention (DIPP) Study. Available from: https://clinicaltrials.gov/ct2/show/NCT03269084?id=NCT03269084&draw=2&rank=1&load=cart.
87. Bingley PJ, Wherrett DK, Shultz A, Rafkin LE, Atkinson MA, Greenbaum CJ. Type 1 diabetes TrialNet: a multifaceted approach to bringing disease-modifying therapy to clinical use in type 1 diabetes. Diabetes Care. 2018;41(4):653–661. doi:10.2337/dc17-0806
88. Barbara Davis Center for Diabetes. DAISY - The Diabetes Auto Immunity Study in the young. Available from: https://medschool.cuanschutz.edu/barbara-davis-center-for-diabetes/research/clinical-epidemiology/daisy-research.
89. Rewers M, Hyöty H, Lernmark Å, et al. The Environmental Determinants of Diabetes in the Young (TEDDY) Study: 2018 update. Curr Diab Rep. 2018;18(12):136. doi:10.1007/s11892-018-1113-2
90. Ministry of the Environment - Government of Japan. Japan Environment and Children’s Study. Available from: http://www.env.go.jp/chemi/ceh/en/index.html.
91. Institutionen för klinisk och experimentell medicin (IKE). ABIS (All Babies in Southeast Sweden). Available from: https://www.abis-studien.se/hem/english-11100423.
92. IDF - Institute of Diabetes Research. BABYDIAB & DiMelli. Available from: https://www.helmholtz-muenchen.de/en/idf/studies-and-consortia/studies/babydiab-dimelli/index.html.
93. Rewers M, Ludvigsson J. Environmental risk factors for type 1 diabetes. Lancet (London, England). 2016;387(10035):2340–2348. doi:10.1016/S0140-6736(16)30507-4
94. Virtanen SM, Nevalainen J, Kronberg-Kippilä C, et al. Food consumption and advanced β cell autoimmunity in young children with HLA-conferred susceptibility to type 1 diabetes: a nested case-control design. Am J Clin Nutr. 2012;95(2):471–478.
95. Funda DP, Kaas A, Bock T, Tlaskalová-Hogenová H, Buschard K. Gluten-free diet prevents diabetes in NOD mice. Diabetes Metab Res Rev. 1999;15(5):323–327. doi:10.1002/(SICI)1520-7560(199909/10)15:5<323::AID-DMRR53>3.0.CO;2-P
96. Hummel M, Bonifacio E, Naserke HE, Ziegler AG. Elimination of dietary gluten does not reduce titers of type 1 diabetes-associated autoantibodies in high-risk subjects. Diabetes Care. 2002;25(7):1111–1116. doi:10.2337/diacare.25.7.1111
97. Mariño E, Richards JL, McLeod KH, et al. Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nat Immunol. 2017;18(5):552–562. doi:10.1038/ni.3713
98. Norris JM, Barriga K, Klingensmith G, et al. Timing of initial cereal exposure in infancy and risk of islet autoimmunity. JAMA. 2003;290(13):1713–1720. doi:10.1001/jama.290.13.1713
99. Norris JM, Yin X, Lamb MM, et al. Omega-3 polyunsaturated fatty acid intake and islet autoimmunity in children at increased risk for type 1 diabetes. JAMA. 2007;298(12):1420–1428. doi:10.1001/jama.298.12.1420
100. Hathout EH, Beeson WL, Ischander M, Rao R, Mace JW. Air pollution and type 1 diabetes in children. Pediatr Diabetes. 2006;7(2):81–87. doi:10.1111/j.1399-543X.2006.00150.x
101. Hathout EH, Beeson WL, Nahab F, Rabadi A, Thomas W, Mace JW. Role of exposure to air pollutants in the development of type 1 diabetes before and after 5 yr of age. Pediatr Diabetes. 2002;3(4):184–188. doi:10.1034/j.1399-5448.2002.30403.x
102. El-Morsi DA, Rahman RH, Abou-Arab A. Pesticides residues in Egyptian diabetic children: a Preliminary Study. J Clinic Toxicol. 2012;2(6). doi:10.4172/2161-0495.1000138
103. Langer P, Tajtáková M, Guretzki H, et al. High prevalence of anti-glutamic acid decarboxylase (anti-GAD) antibodies in employees at a polychlorinated biphenyl production factory. Arch Environ Health. 2002;57(5):412–415. doi:10.1080/00039890209601429
104. Haynes A, Bower C, Bulsara MK, Finn J, Jones TW, Davis EA. Perinatal risk factors for childhood type 1 diabetes in Western Australia—a population-based study (1980–2002). Diabet Med. 2007;24(5):564–570. doi:10.1111/j.1464-5491.2007.02149.x
105. D’Angeli MA, Merzon E, Valbuena LF, Tirschwell D, Paris CA, Mueller BA. Environmental factors associated with childhood-onset type 1 diabetes mellitus: an exploration of the hygiene and overload hypotheses. Arch Pediatr Adolesc Med. 2010;164(8):732–738. doi:10.1001/archpediatrics.2010.115
106. Cardwell CR, Stene LC, Joner G, et al. Birthweight and the risk of childhood-onset type 1 diabetes: a meta-analysis of observational studies using individual patient data. Diabetologia. 2010;53(4):641–651. doi:10.1007/s00125-009-1648-5
107. Hyppönen E, Kenward MG, Virtanen SM, et al. Infant feeding, early weight gain, and risk of type 1 diabetes. Childhood Diabetes in Finland (DiMe) Study group. Diabetes Care. 1999;22(12):1961–1965. doi:10.2337/diacare.22.12.1961
108. Virk J, Li J, Vestergaard M, Obel C, Lu M, Olsen J. Early life disease programming during the preconception and prenatal period: making the link between stressful life events and type-1 diabetes. PLoS One. 2010;5(7):e11523.
109. Jin N, Wang Y, Crawford F, et al. N-terminal additions to the WE14 peptide of chromogranin A create strong autoantigen agonists in type 1 diabetes. Proc Natl Acad Sci U S A. 2015;112(43):13318–13323. doi:10.1073/pnas.1517862112
110. Delong T, Wiles TA, Baker RL, et al. Pathogenic CD4 T cells in type 1 diabetes recognize epitopes formed by peptide fusion. Science (New York, NY). 2016;351(6274):711–714. doi:10.1126/science.aad2791
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
