Back to Journals » Therapeutics and Clinical Risk Management » Volume 16
The Association of Red Blood Cell Distribution Width to Platelet Count Ratio and 28-Day Mortality of Patients with Sepsis: A Retrospective Cohort Study
Authors Ge S, Lin S , Zhang L , Zeng M
Received 29 June 2020
Accepted for publication 11 September 2020
Published 19 October 2020 Volume 2020:16 Pages 999—1006
DOI https://doi.org/10.2147/TCRM.S268523
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Deyun Wang
Shanhui Ge, Shan Lin, Lishan Zhang, Mian Zeng
Department of Medical Intensive Care Unit, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, Guangdong 510080, China; Institute of Pulmonary Diseases, Sun Yat-sen University, Guangzhou, Guangdong, People’s Republic of China
Correspondence: Mian Zeng
Department of Medical Intensive Care Unit, The First Affiliated Hospital of Sun Yat-sen University, No. 58 Zhongshan Road 2, Guangzhou, Guangdong 510080, People’s Republic of China
Email [email protected]
Background: Sepsis is a life-threatening and inflammatory disease with high morbidity and mortality. Red blood cell distribution width to platelet count ratio (RPR) was known as an inflammatory biomarker and was related to poor outcomes of various diseases.
Aim: This study was intended to explore the association between RPR and mortality of sepsis patients.
Methods: A retrospective cohort study was undertaken in patients with sepsis, and the data were collected from a public database called Medical Information Mart for Intensive Care III (MIMIC-III). The primary outcome was 28-day mortality while the secondary outcomes were 90-day mortality and ICU mortality. Multivariable regression analyses, as well as interaction and stratified analyses, were conducted to investigate the relation between RPR and sepsis mortality.
Results: In total, we enrolled 7531 patients with 1316 deaths. RPR was independently correlated with 28-day mortality (per 0.1 increase: HR=1.04; 95% CI 1.02– 1.06), 90-day mortality (per 0.1 increase: HR=1.04; 95% CI 1.03– 1.06) and ICU mortality (per 0.1 increase: OR=1.06; 95% CI 1.02– 1.10). Twenty-eight-day survival was worse in the high RPR (≥ 0.134) group according to the Kaplan–Meier curve analyses (Log rank test, p< 0.001). In stratified analyses, Sequential Organ Failure Assessment (SOFA) score and length of ICU stay had interactive effects with the high RPR (≥ 0.134) group on 28-day mortality.
Conclusion: RPR is a novel biomarker that indicates poor prognosis of sepsis patients. Clinicians are required to pay more attention to those patients with high RPR.
Keywords: ICU, sepsis, prognosis, inflammatory marker, MIMIC-III
Introduction
Sepsis, a life-threatening condition that contributes to high mortality in intensive care units, is a substantial burden to public health worldwide.1 It is reported that global sepsis-related deaths were estimated up to 11.0 million in 2017, although the incidence and mortality of sepsis show a decreasing trend over the years.2 Caused by infections, sepsis manifests as systemic inflammation with organ dysfunction and immune disorder. Several common inflammation biomarkers including c-reactive protein, procalcitonin, and white blood cell count have shown prognostic values on sepsis.3,4 Early identification of those who are in danger of adverse outcomes might be conducive to prompt and adequate treatments of sepsis.5
Complete blood count (CBC) is one of the most common and inexpensive laboratory tests in the hospital. Derived from CBC, red blood cell distribution width (RDW) is a parameter that reflects the heterogeneity of red blood cell (RBC) volume, and classifies anemia. Elevated RDW is considered to be an inflammatory marker and predicts poor outcomes of several diseases including heart failure,6 acute kidney injury,7 sepsis,8 and cancers.9 Platelets play a major role in regulating inflammation and innate immunity. Platelets adhere to endothelium and mediate neutrophil chemotaxis, infiltration, and secretion of proinflammatory chemokines in the progress of acute inflammation.10 Platelet count decreases in severe diseases and is a predictor for mortality.11,12 Red blood cell distribution width to platelet count ratio (RPR) is a novel and simple indicator of inflammation. Studies have reported the value of RPR in detecting hepatic fibrosis13 and predicting poor prognosis of severe burn injury.14 However, few studies have investigated the correlation between RPR and sepsis. Herein, our study aimed to explore the relationship between RPR and sepsis mortality.
Methods
Database
Patient information was collected from the freely available Medical Information Mart for Intensive Care III (MIMIC-III) Database (v1.4) which contains clinical data of more than 40,000 patients admitted to the intensive care units of Beth Israel Deaconess Medical Center between 2001 and 2012.15 Access to the MIMIC-III database was approved by the institutional review boards of both the Beth Israel Deaconess Medical Center and Massachusetts Institute of Technology Affiliates after finishing the required training course, i.e., the CITI (Collaborative Institutional Training Initiative) “Data or Specimens Only Research” course (Record ID: 29459220). No informed consent was required as the private information of patients was de-identified in the database.
Study Design and Participants
We conducted the retrospective cohort study on adult patients (age≥18 years) with the diagnosis of sepsis. Patients who were included had a length of stay in ICU of more than one day and less than that of the hospitalization. Sepsis was defined using the criteria of the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) which defines sepsis as infections with two or more points of Sequential Organ Failure Assessment (SOFA) scores.16 Patients were excluded when: (1) Repeated hospital admissions were recorded; (2) RPR could not be calculated. Also, we only calculated the RPR of the first ICU admission if the patient had several ICU admissions in one hospitalization.
Data Collection
Our data were extracted from the database using Transact-SQL language and codes from the MIMIC Code Repository (https://github.com/MIT-LCP/mimic-code).17 Patients’ information consists of age, gender, mechanical ventilation on the first day, renal replacement therapy on the first day, ICU length of stay, Sequential Organ Failure Assessment (SOFA) scores, Elixhauser Comorbidity Index (SID30), comorbidities, and laboratory parameters which contained white blood cell, red blood cell distribution width, hemoglobin, and platelet count from the blood sample. The severity scores and laboratory parameters were both collected within 24 hours after ICU admission. The exposure factor RPR was equal to red blood cell distribution width divided by platelet count. The primary outcome was 28-day mortality while the secondary outcomes were 90-day mortality and ICU mortality.
Statistical Analysis
All analyses were conducted using EmpowerStats (www.empowerstats.com; X&Y Solutions Inc.) and statistical packages R (The R Foundation; http://www.r-project.org; version 3.4.3 2018–02-18). Qualitative variables were described with median and interquartile range while categorical variables were expressed as counts and percentages. Continuous and categorical variables were analyzed using Kruskal–Wallis and Chi-square (or Fisher’s exact) tests, respectively. We constructed univariate and multivariable Cox proportional hazards models for the association of RPR with 28-day and 90-day mortality and performed logistic regression models to analyze the correlation between RPR and ICU mortality, using two adjusted models to adjust potential confounding factors (details in Table 2). Smooth curve fitting based on a generalized additive model was utilized to identify the relation between RPR and 28-day mortality. We further performed a two-piecewise linear regression model to assess the threshold effect of RPR on 28-day mortality based on the smoothing plot. The threshold was determined according to the log-likelihood ratio test comparing the non-segmented model to the segmented regression model. Kaplan–Meier curve method was used to compare the survival probability between low RPR group and high RPR group if the threshold effect existed. To assess the robustness of our results, we conducted interaction and stratified analyses on subgroups which were classified according to gender, age, SOFA, length of ICU stay, mechanical ventilation or renal replacement therapy on the first day, and hematological disorder.
 |
Table 1 Characteristics of the Patients with Sepsis |
 |
Table 2 Association of RPR with Clinical Outcomes |
Result
Patient Demographics
Finally, we enrolled 7531 patients who fulfilled the criteria of sepsis 3, of which 1316 patients (17.47%) died. The median age was 68.77 years (range 55.49–80.05) and the median length of ICU stay was 4.24 days (range 2.23–9.27). 56.09% of patients received mechanical ventilation within 24 hours after ICU admission while 5.21% of patients underwent renal replacement therapy. The most common comorbidities of sepsis patients were hypertension (52.52%), followed by fluid and electrolyte disorders (47.42%), congestive heart failure (35.63%), and cardiac arrhythmias (33.44%). As shown in Table 1, the following variables indicated statistically significant differences between survivors and non-survivors: age, mechanical ventilation on the first day, renal replacement therapy on the first day, SOFA scores, Elixhauser Comorbidity Index (SID30), platelet count, RDW, and RPR. As expected, non-survivors had higher RDW and RPR values compared with survivors.
Association of RPR with Clinical Outcomes
To analyze the association between sepsis mortality and RPR, we performed univariate and multivariable regression analysis. As shown in Table 2, after adjustment for potential confounding factors (model II), mortality increased by 4% in 28-day mortality per 0.1 increase of RPR (HR=1.04; 95% CI 1.02–1.06), 4% in 90-day mortality (HR=1.04; 95% CI 1.02–1.06), and 5% in ICU mortality (OR=1.05; 95% CI 1.02–1.09).
Threshold Effect Analysis
As shown in Figure 1, we observed a non-linear relationship between RPR and 28-day mortality after adjustment for model II (shown in Table 2). We further found a significant threshold of RPR (0.134) using the two-piecewise linear regression model (Table 3). As a significant p-value (0.047) was detected by Log-likelihood ratio test, the segmented regression model was more suitable to describe the correlation between RPR and 28-day mortality. In our segmented regression model, when RPR ≥ 0.134, 28-day mortality increased by 4% per 0.1 increase of RPR (adjusted HR=1.04, 95% CI 1.02~1.06, p<0.001), but RPR was not associated with 28-day mortality when it was less than 0.134 (per 0.1 adjusted HR=0.86, 95% CI 0.71~1.04, p=0.110).
 |
Table 3 Threshold Effect Analysis of RPR on 28-Day Mortality with Piecewise Linear Regression |
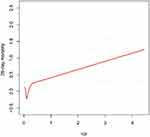 |
Figure 1 Smooth curve fitting for the association between RPR and 28-day mortality. A non-linear relationship was detected after adjusting for Model II in Table 2. |
Subgroup Analyses
We divided patients into high and low RPR groups according to the threshold of 0.134. Kaplan–Meier analysis (Figure 2) showed that 28-day survival was better for the low RPR group (Log rank test, p<0.001), and 28-day mortality increased by 16% in the high RPR group (adjusted HR=1.16, 95% CI 1.01~1.34). For the purpose of identifying the potential factors that influence the impact of RPR on 28-day mortality, we conducted subgroup analyses (Table 4, Figure 3). SOFA score had a significant interaction with RPR on 28-day mortality. Patients whose SOFA scores were not less than 6 points had a higher risk of 28-day mortality (HR=1.38, 95% CI 1.18~1.63). The association between RPR and 28-day mortality was similar for other subgroups with no statistically significant interactions (p for interaction >0.05 in the adjusted model).
 |
Table 4 Stratified Analyses of the Association Between RPR and 28-Day Mortality |
 |
Figure 2 Kaplan–Meier curve of 28-day survival in sepsis patients. Patients were divided into two groups according to RPR (RPR<0.134 and RPR ≥0.134). |
 |
Figure 3 Forest plot for stratified analyses. |
Discussion
In the present study, we confirmed that elevated RPR was correlated to increased risk of sepsis mortality after adjustment for potentially confounding factors. We further observed a non-linear association between RPR and 28-day mortality of sepsis patients and discovered a significant threshold of 0.134. What’s more, in light of our result, SOFA score was interactive with RPR on 28-day mortality.
Hematopoietic dysfunction is common in sepsis. Systemic infection and inflammation contributed to increased rupture of peripheral erythrocytes and decreased iron bioavailability, which correlated with the incidence of sepsis-associated anemia.18,19 In the meantime, inflammatory cytokines inhibited RBC maturation and nucleated red blood cell counts increased.20,21 These hematopoietic changes might lead to increased erythrocytes heterogeneity and then elevated RDW. Platelets are known to be the intersection of immune response and coagulation reaction in infectious diseases.22 Sepsis caused the transcriptional and translational changes in platelets and thrombocytopenia was detected in sepsis patients.23 Increased RDW8 and decreased platelets24 were independently related to the mortality of sepsis, respectively. A previous study reported that a simple scoring system including RDW, platelet count, and delta neutrophil index was a significant predictor for 28-day mortality of patients with severe sepsis and septic shock.25 Red blood cell distribution width to platelet count ratio (RPR) might be an index which reflects the status of red blood cells and platelets, simultaneously.
RPR, as an inflammation marker, was widely studied in predicting hepatic fibrosis and cirrhosis with cutoff points varying from 0.06 to 0.14 and good diagnostic accuracy.13,26–29 Recent researches have explored the potential diagnostic value of RPR for other diseases. Bilgin et al.30 revealed that RPR was useful for predicting the overall survival of patients with right-sided advanced colorectal cancer. Celik et al.31 reported that admission RPR dependently correlated with the no-reflow phenomenon in patients who were diagnosed with myocardial infarction and had undergone a primary percutaneous coronary intervention. Besides, RPR showed good predictive ability for mortality of acute pancreatitis and severe burn injury,14,32 which are common causes of sepsis. Therefore, we hypothesized RPR also related to sepsis. Two studies have reported the correlation between RPR and sepsis in children. Wang et al.33 revealed that RPR had high diagnostic utility for the prognosis of pediatric sepsis with an AUROC of 0.937. Similarly, the work of Karabulut and Arcagok showed that RPR was an available biomarker for the prediction of early-onset neonatal sepsis with an AUROC of 0.816.34 However, no study has investigated the relationship between RPR and adult sepsis. So, our study was the first to explore the underlying connection between RPR and adult sepsis. Our results indicated that RPR was an independent risk factor for 28-day mortality, 90-day mortality, and ICU mortality of adult sepsis patients. In our further stratified analyses, gender, age, mechanical ventilation or renal replacement therapy on the first day, length of ICU stay, and hematological disorder showed no interaction effect. Interestingly, SOFA (≥ 6) exhibited significant interaction with RPR (cutoff point 0.134) on 28-day mortality, which indicated that RPR might be a more useful biomarker in severe patients.
Some limitations of the present study need to be discussed. First of all, as a retrospective observational study, it is inevitable to have potential selection bias and confounding bias. We could only analyze the hazard ratio or odds ratio instead of relative risk. Further, the present study only collected the value of RPR within 24 hours of ICU admission, with a lack of analysis of baseline level and continuous fluctuations in RPR value. Finally, our data were collected from a single central electronic database and not representative enough for the general population. Our results need to be further verified in multi-center studies.
Conclusion
In short, we confirmed that RPR was a simple and effective biomarker independently related to mortality of adult sepsis patients in the present study. Patients with RPR value not less than 0.134 ought to be paid more attention as they are more likely to have an adverse prognosis. Further studies with multi-center design should be conducted to validate our results.
Funding
The study was supported by National Natural Science Foundation of China (Grant number 81670066); Guangdong Basic and Applied Basic Research Foundation (Grant number 2019A1515011198); Major Science and Technology Planning Project of Guangdong Province, China (Grant number 2016A020216009); and Critical Care Research Funding of the Aesculap Academy (2017).
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Rhee C, Dantes R, Epstein L, et al. Incidence and trends of sepsis in US Hospitals using clinical vs claims data, 2009-2014. JAMA. 2017;318(13):1241–1249. doi:10.1001/jama.2017.13836
2. Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395(10219):200–211.
3. Cui N, Zhang H, Chen Z, et al. Prognostic significance of PCT and CRP evaluation for adult ICU patients with sepsis and septic shock: retrospective analysis of 59 cases. J Int Med Res. 2019;47(4):1573–1579. doi:10.1177/0300060518822404
4. Magrini L, Gagliano G, Travaglino F, et al. Comparison between white blood cell count, procalcitonin and C reactive protein as diagnostic and prognostic biomarkers of infection or sepsis in patients presenting to emergency department. Clin Chem Lab Med. 2014;52(10):1465–1472. doi:10.1515/cclm-2014-0210
5. Cawcutt KA, Peters SG. Severe sepsis and septic shock: clinical overview and update on management. Mayo Clin Proc. 2014;89(11):1572–1578. doi:10.1016/j.mayocp.2014.07.009
6. Xanthopoulos A, Papamichalis M, Zajichek A, et al. In-hospital red blood cell distribution width change in patients with heart failure. Eur J Heart Fail. 2019;21(12):1659–1661. doi:10.1002/ejhf.1546
7. Wang B, Lu H, Gong Y, et al. The association between red blood cell distribution width and mortality in critically Ill Patients with acute kidney injury. Biomed Res Int. 2018;2018:9658216.
8. Kim CH, Park JT, Kim EJ, et al. An increase in red blood cell distribution width from baseline predicts mortality in patients with severe sepsis or septic shock. Crit Care. 2013;17(6):R282. doi:10.1186/cc13145
9. Han F, Liu Y, Cheng S, et al. Diagnosis and survival values of neutrophil-lymphocyte ratio (NLR) and red blood cell distribution width (RDW) in esophageal cancer. Clin Chim Acta. 2019;488:150–158. doi:10.1016/j.cca.2018.10.042
10. Herter JM, Rossaint J, Zarbock A. Platelets in inflammation and immunity. J Thromb Haemost. 2014;12(11):1764–1775. doi:10.1111/jth.12730
11. Fawzy A, Anderson JA, Cowans NJ, et al. Association of platelet count with all-cause mortality and risk of cardiovascular and respiratory morbidity in stable COPD. Respir Res. 2019;20(1):86. doi:10.1186/s12931-019-1059-1
12. Wu M, Luan YY, Lu JF, et al. Platelet count as a new biomarker for acute kidney injury induced by hemorrhagic shock. Platelets. 2020;31(1):94–102. doi:10.1080/09537104.2019.1581921
13. Lee HW, Kang W, Kim BK, et al. Red cell volume distribution width-to-platelet ratio in assessment of liver fibrosis in patients with chronic hepatitis B. Liver Int. 2016;36(1):24–30. doi:10.1111/liv.12868
14. Qiu L, Chen C, Li SJ, et al. Prognostic values of red blood cell distribution width, platelet count, and red cell distribution width-to-platelet ratio for severe burn injury. Sci Rep. 2017;7(1):13720. doi:10.1038/s41598-017-13151-3
15. Johnson AE, Pollard TJ, Shen L, et al. MIMIC-III, a freely accessible critical care database. Sci Data. 2016;3:160035. doi:10.1038/sdata.2016.35
16. Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287
17. Johnson AE, Stone DJ, Celi LA, et al. The MIMIC code repository: enabling reproducibility in critical care research. J Am Med Inform Assoc. 2018;25(1):32–39. doi:10.1093/jamia/ocx084
18. Bateman RM, Sharpe MD, Singer M, et al. The effect of sepsis on the erythrocyte. Int J Mol Sci. 2017;18:9. doi:10.3390/ijms18091932
19. Ekregbesi P, Shankar-Hari M, Bottomley C, et al. Relationship between anaemia, haemolysis, inflammation and haem oxygenase-1 at admission with sepsis: a pilot study. Sci Rep. 2018;8(1):11198. doi:10.1038/s41598-018-29558-5
20. Toth J, Debreceni IB, Berhes M, et al. Red blood cell and platelet parameters are sepsis predictors in an Escherichia coli induced lethal porcine model. Clin Hemorheol Microcirc. 2017;66(3):249–259. doi:10.3233/CH-170271
21. Pierce CN, Larson DF. Inflammatory cytokine inhibition of erythropoiesis in patients implanted with a mechanical circulatory assist device. Perfusion. 2005;20(2):83–90. doi:10.1191/0267659105pf793oa
22. Manne BK, Xiang SC, Rondina MT. Platelet secretion in inflammatory and infectious diseases. Platelets. 2017;28(2):155–164. doi:10.1080/09537104.2016.1240766
23. Middleton EA, Rowley JW, Campbell RA, et al. Sepsis alters the transcriptional and translational landscape of human and murine platelets. Blood. 2019;134(12):911–923.
24. Cato LD, Wearn CM, Bishop JRB, et al. Platelet count: a predictor of sepsis and mortality in severe burns. Burns. 2018;44(2):288–297.
25. Kim YC, Song JE, Kim EJ, et al. A simple scoring system using the red blood cell distribution width, delta neutrophil index, and platelet count to predict mortality in patients with severe sepsis and septic shock. J Intensive Care Med. 2018;885066618787448.
26. Cai Y, Liu D, Cui J, et al. Diagnostic accuracy of red blood cell distribution width to platelet ratio for predicting staging liver fibrosis in chronic liver disease patients: a systematic review and meta-analysis. Medicine (Baltimore). 2019;98(14):e15096. doi:10.1097/MD.0000000000015096
27. Yuyun D, Zhihua T, Haijun W, et al. Predictive value of the red blood cell distribution width-to-platelet ratio for hepatic fibrosis. Scand J Gastroenterol. 2019;54(1):81–86. doi:10.1080/00365521.2018.1558786
28. Zhou W-J, Yang J, Zhang G, et al. Association between red cell distribution width-to-platelet ratio and hepatic fibrosis in nonalcoholic fatty liver disease. Medicine. 2019;98:30.
29. Karagoz E, Ulcay A, Tanoglu A, et al. Clinical usefulness of mean platelet volume and red blood cell distribution width to platelet ratio for predicting the severity of hepatic fibrosis in chronic hepatitis B virus patients. Eur J Gastroenterol Hepatol. 2014;26(12):1320–1324.
30. Bilgin B, Sendur MAN, Hizal M, et al. Prognostic effect of red cell distribution width-to-platelet ratio in colorectal cancer according to tumor stage and localization. J Cancer Res Ther. 2019;15(1):54–60.
31. Celik T, Balta S, Demir M, et al. Predictive value of admission red cell distribution width-platelet ratio for no-reflow phenomenon in acute ST segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Cardiol J. 2016;23(1):84–92. doi:10.5603/CJ.a2015.0070
32. İlhan M, İlhan G, Gök AFK, et al. Evaluation of neutrophil–lymphocyte ratio, platelet–lymphocyte ratio and red blood cell distribution width–platelet ratio as early predictor of acute pancreatitis in pregnancy. J Maternal-Fetal Neonatal Med. 2015;29(9):1476–1480. doi:10.3109/14767058.2015.1051026
33. Wang L, Cai Q. [Value of red blood cell distribution width-to-platelet count ratio in predicting the prognosis of children with sepsis]. Zhongguo Dang Dai Er Ke Za Zhi. 2019;21(11):1079–1083.
34. Karabulut B, Arcagok BC. New diagnostic possibilities for early onset neonatal sepsis: red cell distribution width to platelet ratio. Fetal Pediatr Pathol. 2019;1–10. doi:10.1080/15513815.2019.1686786
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
