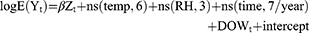Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 14
The Association Between Short-Term Air Pollution Exposure and Post-Adolescent Acne: The Evidence from a Time Series Analysis in Xi’an, China
Authors Li X, An SJ, Liu XL, Ji AL, Cao Y, Xiang Y, Ma XY, Hu Q, Yuan ZQ, Li YF, Lu YG , Cai TJ
Received 14 May 2021
Accepted for publication 9 June 2021
Published 25 June 2021 Volume 2021:14 Pages 723—731
DOI https://doi.org/10.2147/CCID.S320248
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Xiang Li,1,2,* Shu-Jie An,3,* Xiao-Ling Liu,1,* Ai-Ling Ji,4 Yi Cao,5 Ying Xiang,1 Xiang-Yu Ma,1 Qin Hu,1 Zhi-Quan Yuan,1 Ya-Fei Li,1 Yuan-Gang Lu,2 Tong-Jian Cai1
1Department of Epidemiology, College of Preventive Medicine, Army Medical University (Third Military Medical University), Chongqing, People’s Republic of China; 2Department of Plastic & Cosmetic Surgery, Daping Hospital, Army Medical University (Third Military Medical University), Chongqing, People’s Republic of China; 3Medical Department, Xijing Hospital, Air Force Medical University (Fourth Military Medical University), Xi’an, People’s Republic of China; 4Department of Preventive Medicine & Chongqing Engineering Research Center of Pharmaceutical Sciences, Chongqing Medical and Pharmaceutical College, Chongqing, People’s Republic of China; 5Department of Health Economics Management, Xijing Hospital, Air Force Medical University (Fourth Military Medical University), Xi’an, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yuan-Gang Lu
Department of Plastic & Cosmetic Surgery, Daping Hospital, Army Medical University, 10 Changjiang Branch Road, Daping, Chongqing, 400042, People’s Republic of China
Tel +86-23-68757597
Fax +86-23-68757596
Email [email protected]
Tong-Jian Cai Email [email protected]
Background: Post-adolescent acne is a common skin disease faced by adults. However, whether air pollution (AP) serves as a risk factor for post-adolescent acne remains elusive.
Aim: To determine the relationship between short-term AP exposure (within 7 days) and outpatient visits for post-adolescent acne.
Methods: Daily outpatient visit data for post-adolescent acne and routinely AP data between 2010 and 2013 were collected from Xi’an, China. A generalized additive regression model was used to analyze the relationship between outpatient visits for post-adolescent acne and short-term ambient AP exposure. The gender-specific analyses were conducted as well.
Results: Totally, 27,190 outpatient visits for post-adolescent acne were included. The results revealed that a 10 μg/m3 increase in PM10, SO2, and NO2 at lag 0– 7 day was associated with the increase of outpatient visits for post-adolescent acne at 0.84% (95% CI: 0.53%, 1.16%), 1.61% (95% CI: 0.12%, 3.10%), and 3.50% (95% CI: 1.60%, 5.40%), respectively. The significant positive associations of PM10, SO2, and NO2 were found at both single-lag models and moving average models. The gender-specific analyses showed that the effect estimates of PM10 was stronger for females than for males, while there was no observed gender difference in the effects of SO2 and NO2.
Conclusion: Short-term exposure to AP was associated with increased outpatient visits for post-adolescent acne, especially for females in the effects of PM10.
Keywords: short-term, air pollution, post-adolescent acne, adult acne, time-series analysis
Introduction
Acne vulgaris, an inflammatory disease of the pilosebaceous follicles, has been traditionally considered a common skin disease for teenagers.1 In recent years, the number of adults affected by new-onset acne or persistent acne is gradually rising.2,3 Approximately 61.9% of populations in the United States4 and 41% of female individuals in France5 were found to be affected by acne during their adulthood. It results in with lower qualities of life and negative psychosocial conditions for patients.6,7
Especially, acne of adults over 25 years of age is generally defined as post-adolescent acne, which is considered to be a particular subtype of acne differing from adolescent acne.8 It mainly occurs in females with mild-to-moderate severity. In addition, compared with adolescent acne, post-adolescent acne has more inflammatory lesions and less comedones.9 Moreover, unlike adolescent acne, which always appears on the forehead, nose, and upper cheeks, post-adolescent acne predominantly occurs on the chin, jawline, and neck.9
Many factors have been thought to be associated with the risk of acne, such as smoking habits,10 body mass index (BMI),11 genetic factors,12,13 and cosmetics,14 etc. Recent studies stressed that air pollution (AP) has adverse impacts on people’s health, especially cardiovascular and pulmonary systems.15–22 Some studies also take AP’s effects into consideration of the induction and aggravation of acne, because AP could contribute to hyper-seborrhea and hyper-sebum, both of which have been found to be prevalent in acne lesions.23–28 However, given that the clinical features of post-adolescent acne, including the vulnerable population, the degree of inflammation, and the primary parts of bodies, were different from those of adolescent acne, the risk factors reported for acne are necessary to be reclassified and re-identified for both adults and teenagers.29 Attention should also be paid to the epidemiological evidence regarding the effect of AP on post-adolescent acne.
In this study, time-series analyses were conducted to investigate the association between outpatient visits for post-adolescent acne and daily AP in Xi’an, a northwestern Chinese city with relatively heavy AP. Subgroup analyses were also carried out to investigate the potential gender difference in such association.
Methods
Data for Outpatient Visits
Daily outpatient visit data for post-adolescent acne between 1 October 2010 and 31 December 2013 were obtained from Xijing Hospital in Xi’an, a northwestern Chinese city with an area of 10,108 km2 and a population of over 8 million in 2014.30 It has distinct climate fluctuations with four seasons. The medical services of Xijing Hospital are accessible seven days a week. The diagnosis of acne vulgaris (ICD-10: L70) was defined by dermatologists according to Andrews’ Diseases of the Skin-Clinical Dermatology (11th edition). Both new acne cases and the relapses of previous diagnosed patients whose residential areas were in Xi’an City and whose ages were over 25 years old were included. The informed consent of patients was not obtained because there was no individual interaction with patients. In addition, the Ethics Committee of Third Military Medical University (Army Military Medical University) approved this study, and the protocols complied with the Declaration of Helsinki.
Environmental Data
AP data between 1 October 2010 and 31 December 2013 were acquired from the Environmental Monitoring Center of Xi’an, including particulate matter less than 10 μm in aerodynamic diameter (PM10), sulfur dioxide (SO2), and nitrogen dioxide (NO2). The daily (24-h) mean concentrations were used as metrics of AP exposures, which were calculated by averaging hourly data across 13 monitoring stations covering Xi’an. The data of daily mean temperature and daily relative humidity during the study period were obtained from the local meteorological bureau.
Statistical Analysis
Firstly, the descriptive analyses were performed to characterize the basic information of outpatient visits, AP data, and meteorological data. The generalized additive model (GAM) was conducted to investigate the association between daily AP and outpatient visits for post-adolescent acne because daily outpatient visits followed quasi-Poisson distribution. In such models, the outpatient visits of post-adolescent acne were included as the dependent variable, while daily AP, temperature, and relative humidity were included as independent variables. The formula used for the analyses and the choosing of degrees of freedom (df) were described in detail in our previous studies,31,32 and the formula was as follows:
Gender-specific analyses were also conducted by Z-test. The estimated differences between gender subgroups were shown by the estimates of differences between groups and their 95% confidence intervals (95% CIs).
Analyses were performed using the “mgcv” package in R 3.6.0 (http://www.r-project.org). The results were presented as the percentage increase of outpatient visits for post-adolescent acne with a 10 μg/m3 increase of PM10, SO2, and NO2 per day. P-values less than 0.05 (P<0.05) were considered statistically significant.
Results
Table 1 shows demographic information of daily outpatient visits and environmental factors. Totally, 27,190 outpatient visits for post-adolescent acne were recorded, including 7,587 males and 19,603 females. The average daily outpatient visits were 22.9. During the period, the daily mean concentrations of PM10, SO2, NO2 were 142.6 µg/m3, 44.7 µg/m3, and 48.5 µg/m3, respectively. The average daily temperature was 14.3°C and relative humidity was 62.4%.
 |
Table 1 Descriptive Statistics for Daily Outpatient Visits, Concentrations of Air Pollutants, and Weather Conditions |
In Figure 1, the time series of outpatient visits, air pollutants, and meteorological factors were shown. The outpatient visits for post-adolescent acne were increasing over time, especially those for female patients. AP was more severe during winter, and there were minor peaks of outpatient visits in the same period. The temperature had a significant undulation period over the year, which was higher during summers. However, the relative humidity showed no apparent seasonal trend.
In Figure 2, the correlations between AP and daily post-adolescent acne outpatient visits in single-lag days (lag 0 to lag 7) and cumulative exposure days (lag 0–1 to lag 0–7) were shown. In single-lag models, the positive association was evidenced from lag 0 to lag 3 and at lag 6 for PM10, at lag 3 for SO2, and from lag 0 to lag 3 for NO2. In cumulative exposure models, both PM10 and NO2 showed significant positive associations with outpatient visits for post-adolescent acne from lag0–1 to lag 0–7, while SO2 had such associations from lag 0–4 to lag 0–7. To be specific, every 10 μg/m3 increase of PM10, SO2, and NO2 was associated with the increase of outpatient acne visits at 0.84% (95% CI: 0.53%, 1.16%), 1.61% (95% CI: 0.12%, 3.10%), and 3.50% (95% CI: 1.60%, 5.40%) at lag 0–7 day, respectively. In gender-specific analyses, the significant association between PM10 and female outpatient visits for post-adolescent acne was observed from lag 0 to lag 6 and from 0–1 to lag 0–7, but no significant association was found for males, suggesting that females were more susceptible than males. The results of Z-test also showed that the association of PM10 was stronger in females than in males at lag 0–6 and lag 0–7 (Table S1). For NO2, the significant positive association was evidenced in both males and females. As for SO2, the significant positive association was only observed at lag 1 for males and at lag 0–7 for females. Thus, PM10, SO2 and NO2 concentrations were positively associated with the outpatient visits for post-adolescent acne, and the estimate effects of PM10 were stronger in females.
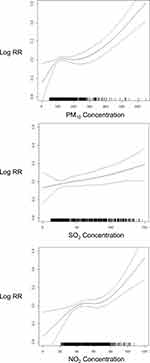
Figure 3 illustrates the exposure–response curves between air pollutants and outpatient-visits for post-adolescent acne at lag 0–7 day. The relationships of both PM10 and NO2 were inverse S-shaped in the curves. Specifically, the association of PM10 was linear when the concentrations were above 200 µg/m3 or below 100 µg/m3, with no observed lower- and upper-bounds. Interestingly, the relationship turned to be flat when PM10 levels ranged from 100 µg/m3 to 200 µg/m3. For NO2, the linear relationship was observed when the concentrations were above 70 µg/m3 or below 40 µg/m3, with no observed lower- and upper-bounds. The relationship turned to be flatter when the its levels ranged from 40 µg/m3 to 70 µg/m3. Compared with those of PM10 and NO2, the exposure–response curve of SO2 rose steadily in the entire range of pollutant concentrations, demonstrating its mild but still positive correlation with the risk of post-adolescent acne.
Table 2 reveals the effects of two-pollutant models (lag 0–7). After adjusting for PM10 or NO2, the effect of SO2 turned to be statistically insignificant. Moreover, the effect of NO2 remained robust and statistically significant after adjusting for SO2 (3.75%, 95% CI: 1.25%, 6.25%), but it turned to be insignificant after adjusting for PM10 (0.90%, 95% CI: −1.38%, 3.19%). Furthermore, the effect of PM10 increased slightly after adjusting for SO2 (0.86%, 95% CI: 0.51%, 1.21%), while its effect mildly decreased but still obvious after adjusting for NO2 (0.76%, 95% CI: 0.38%, 1.14%).
 |
Table 2 Percent Change (Mean and 95% CI) of Daily Outpatient Visits for Post-Adolescent Acne Associated with per 10 μg/m3 Increase of Pollutant Concentration at Lag 0–7 Day in Two-Pollutant Models |
Discussion
In this study, we provided novel evidence of the relationship between short-term AP exposure and outpatient visits for post-adolescent acne. Through time-series analyses, we found positive associations between outpatient visits for post-adolescent acne and short-term exposure to ambient PM10, SO2, and NO2. In gender subgroup analyses, the associations of PM10 appeared to be more significant in females than in males.
Although acne is typically recognized as an adolescent disorder, a series of studies have reported that the incidence of acne in adults over 25 years old has steadily grown over time.2,33,34 A consistent rise in the outpatient visits for post-adolescent acne also has been found in Xi’an, China during the study period. The reason for such growth was complex and might be attributed to that some lifestyle factors associated with post-adolescent acne such as smoking35 and western diet36,37 are gradually prevalent in adults.38,39 However, the outpatient visits for post-adolescent acne exhibited a season variation, peaking in winter and decreasing in summer. Such variation indicated that some factors vary with seasonal fluctuation, such as environmental factors, may also affect the incidence and the aggravation of post-adolescent acne. Especially, in the present study, the levels of air pollutants (PM10, SO2, NO2) also showed season variations, which seem to have the same fluctuations with the outpatient visits for post-adolescent acne. Therefore, it’s imperative to investigate the association between post-adolescent acne and ambient AP.
Our study observed an obvious relationship between short-term exposures to PM10, SO2, and NO2 and outpatient visits for post-adolescent acne, which was consistent with previous studies investigating the associations between AP and some inflammatory skin diseases. For example, Guo et al40 suggested that short-term exposure to AP, represented by PM, was significantly related to the outpatient visits for eczema and dermatitis. Li et al41 found that short-term elevations of PM10, SO2, and NO2 were associated with the increased outpatient visits for eczema. Moreover, Kim et al42 also indicated that the risk of atopic dermatitis symptoms in young children could be increased with short-term exposure to PM10, NO2, and ozone (O3). Such correlations were plausible from a biological perspective: skin, a barrier between internal and external environment, directly contacts air pollutants. Various airborne pollutants can permeate the epidermal barrier, and then trigger skin barrier dysfunction,43 immune dysregulation,44 and dermal inflammation.45 In such processes, the induction of inflammatory cascade, generation of free radicals, activation of AhR (aryl hydrocarbon receptor), and alterations of cutaneous microflora resulted from air pollutants all may be involved in the inflammatory processes and immune responses of post-adolescent acne.46,47
Furthermore, our data showed that the association between post-adolescent acne outpatient visits and PM10 was more evident in females than in males. One possible explanation was that exposure to PM could result in the imbalance of female hormones via interfering with estrogen-regulated pathways.48,49 Given that the severity of female acne could fluctuate with hormones in their lifespan, especially in the period of menstruation, pregnancy, and menopause, the irregular changes of hormones affected by AP, especially PM, may further enhance the possibility of the onset and aggravation of post-adolescent acne during female adulthood.3,50 This can also, at least partly, explain why post-adolescent acne is more prevalent in females than in males.34,51 In another perspective, males and females have physiological, chemical, and biophysical differences in skin.52 Such gender difference of skin characteristics in terms of stratum corneum hydration, transepidermal water loss, sebum production, and skin thickness may contribute to their dissimilar abilities against environmental stressors, including AP. From this viewpoint, this study indicated that male skin seems to have stronger properties to counteract or prevent against AP-induced skin damages.
Although it is unavoidable for skin to exposure to air pollutants, many anti-pollutant strategies can be taken into consideration to decrease the risk of post-adolescent acne. To begin with, moisturizer can be utilized to accelerate the recovery of skin barrier and prevent contaminants from adhering directly to the skin.53 Furthermore, oral or topical antioxidants such as vitamin E,54 and anti-inflammatory drugs such as adapalene and benzoyl peroxide can be conducive to relieve pollution-induced skin oxidation and inflammation.55 Lastly, governments should make specific policies to control AP and to reduce the exposure levels of air pollutants.56–59
This study has several major advantages. Firstly, this study was conducted with a large sample of post-adolescent acne outpatients. Secondly, we provided the first evidence that short-term exposure to AP may contribute to the increased risk of post-adolescent acne. Thirdly, this research was conducted in Xi’an, a major metropolis in northwestern China with a considerably large population and relatively heavy pollution.
Nonetheless, this study has several limitations. Firstly, like other similar ecological studies, air pollutant levels from fixed site monitoring stations instead of individual exposure levels were included into the main analysis models, which can lead to exposure errors known as “ecological fallacy”. Secondly, the data of other pollutants were not available, including particulate matter less than 2.5 μm in aerodynamic diameter (PM2.5), O3, and carbon monoxide (CO). Thirdly, also owing to the limitation of the availability, more confounders which may have potential effects on the relationship between AP and post-adolescent acne were not included for analyses, such as stress status, body mass index, and drug history.60,61 Fourthly, our data were only collected from a single hospital, which may make our results less representative of the whole city. Therefore, more work is needed to confirm our results in the future.
Conclusions
In conclusion, short-term air pollution (PM10, SO2, and NO2) exposure was associated with the increased risk of outpatient visits for post-adolescent acne. More interestingly, females were more sensitive to PM10 than men. This study provided novel evidence regarding the relationship between short-term ambient AP exposure and post-adolescence acne, which may have an important bearing on the intervention and prevention of this disease.
Abbreviation
AP, air pollution; CO, carbon monoxide; DOW, day of the week; df, degree of freedom; GAM, generalized additive model; NO2, nitrogen dioxide; O3, ozone; PACF, partial autocorrelation function; PM10, particulate matter less than 10 μm in aerodynamic diameter; PM2.5, particulate matter less than 2.5 μm in aerodynamic diameter; SO2, sulfur dioxide.
Acknowledgments
We would like to acknowledge the financial supports from the Outstanding Talent Support Program of Army Medical University, Innovative Training Program Foundation for the Talents of the Daping Hospital [2019CXJSC034], and Research and innovation projects of graduate students in Chongqing [CYS20367].
Disclosure
No potential conflict of interest was reported by the authors.
References
1. Tan JK, Bhate K. A global perspective on the epidemiology of acne. Br J Dermatol. 2015;172(Suppl 1):3–12. doi:10.1111/bjd.13462
2. Shen Y, Wang T, Zhou C, et al. Prevalence of acne vulgaris in Chinese adolescents and adults: a community-based study of 17,345 subjects in six cities. Acta Derm Venereol. 2012;92(1):40–44. doi:10.2340/00015555-1164
3. Perkins AC, Maglione J, Hillebrand GG, Miyamoto K, Kimball AB. Acne vulgaris in women: prevalence across the life span. J Womens Health. 2012;21(2):223–230. doi:10.1089/jwh.2010.2722
4. Yentzer BA, Hick J, Reese EL, Uhas A, Feldman SR, Balkrishnan R. Acne vulgaris in the United States: a descriptive epidemiology. Cutis. 2010;86(2):94–99.
5. Poli F, Dreno B, Verschoore M. An epidemiological study of acne in female adults: results of a survey conducted in France. J Eur Acad Dermatol Venereol. 2001;15(6):541–545. doi:10.1046/j.1468-3083.2001.00357.x
6. Zeichner JA, Baldwin HE, Cook-Bolden FE, Eichenfield LF, Fallon-Friedlander S, Rodriguez DA. Emerging issues in adult female acne. J Clin Aesthet Dermatol. 2017;10(1):37–46.
7. Chilicka K, Maj J, Panaszek B. General quality of life of patients with acne vulgaris before and after performing selected cosmetological treatments. Patient Prefer Adherence. 2017;11:1357–1361. doi:10.2147/PPA.S131184
8. Williams C, Layton AM. Persistent acne in women: implications for the patient and for therapy. Am J Clin Dermatol. 2006;7(5):281–290. doi:10.2165/00128071-200607050-00002
9. Holzmann R, Shakery K. Postadolescent acne in females. Skin Pharmacol Physiol. 2014;27(Suppl 1):3–8. doi:10.1159/000354887
10. Capitanio B, Sinagra JL, Ottaviani M, Bordignon V, Amantea A, Picardo M. Acne and smoking. Dermato-Endocrinology. 2009;1(3):129–135. doi:10.4161/derm.1.3.9638
11. Lu PH, Hsu CH. Body mass index is negatively associated with acne lesion counts in Taiwanese women with post-adolescent acne. J Eur Acad Dermatol Venereol. 2015;29(10):2046–2050. doi:10.1111/jdv.12754
12. Yang T, Wu WJ, Tian LM, et al. The associations of androgen-related genes CYP21A2 and CYP19A1 with severe acne vulgaris in patients from Southwest China. Clin Cosmet Investig Dermatol. 2021;14:313–331. doi:10.2147/CCID.S293171
13. Tian LM, Ke D. Acne vulgaris is associated with the human beta-defensin 1-gene polymorphisms in Han Chinese ethnic group patients. Clin Cosmet Investig Dermatol. 2021;14:123–128. doi:10.2147/CCID.S292797
14. Monfrecola G, Cacciapuoti S, Capasso C, Delfino M, Fabbrocini G. Tolerability and camouflaging effect of corrective makeup for acne: results of a clinical study of a novel face compact cream. Clin Cosmet Investig Dermatol. 2016;9:307–313. doi:10.2147/CCID.S115192
15. Shahi AM, Omraninava A, Goli M, Soheilarezoomand HR, Mirzaei N. The effects of air pollution on cardiovascular and respiratory causes of emergency admission. Emergency. 2014;2(3):107–114.
16. Burgess JL, Fleming JE, Mulenga EM, et al. Acute changes in sputum IL-10 following underground exposure to diesel exhaust. Clin Toxicol. 2007;45(3):255–260. doi:10.1080/15563650601072142
17. Zhou Z, Shao T, Qin M, et al. The effects of autophagy on vascular endothelial cells induced by airborne PM2.5. J Environ Sci. 2018;66:182–187. doi:10.1016/j.jes.2017.05.019
18. Xiao H, Zhang H. Skin inflammation and psoriasis may be linked to exposure of ultrafine carbon particles. J Environ Sci. 2020;96:206–208. doi:10.1016/j.jes.2020.06.028
19. Chi R, Li H, Wang Q, et al. Association of emergency room visits for respiratory diseases with sources of ambient PM2.5. J Environ Sci. 2019;86:154–163. doi:10.1016/j.jes.2019.05.015
20. Cheng Z, Liang X, Liang S, Yin N, Faiola F. A human embryonic stem cell-based in vitro model revealed that ultrafine carbon particles may cause skin inflammation and psoriasis. J Environ Sci. 2020;87:194–204. doi:10.1016/j.jes.2019.06.016
21. Sun H, Chen H, Yao L, et al. Sources and health risks of PM2.5-bound polychlorinated biphenyls (PCBs) and organochlorine pesticides (OCPs) in a North China rural area. J Environ Sci. 2020;95:240–247. doi:10.1016/j.jes.2020.03.051
22. Wang D, Li X, Zhang X, et al. Spatial distribution of health risks for residents located close to solvent-consuming industrial VOC emission sources. J Environ Sci. 2021;107:38–48. doi:10.1016/j.jes.2021.01.014
23. Kim KE, Cho D, Park HJ. Air pollution and skin diseases: adverse effects of airborne particulate matter on various skin diseases. Life Sci. 2016;152:126–134. doi:10.1016/j.lfs.2016.03.039
24. Liu W, Pan X, Vierkotter A, et al. A time-series study of the effect of air pollution on outpatient visits for acne vulgaris in Beijing. Skin Pharmacol Physiol. 2018;31(2):107–113. doi:10.1159/000484482
25. Lefebvre MA, Pham DM, Boussouira B, Bernard D, Camus C, Nguyen QL. Evaluation of the impact of urban pollution on the quality of skin: a multicentre study in Mexico. Int J Cosmet Sci. 2015;37(3):329–338. doi:10.1111/ics.12203
26. Lefebvre MA, Pham DM, Boussouira B, et al. Consequences of urban pollution upon skin status. A controlled study in Shanghai area. Int J Cosmet Sci. 2016;38(3):217–223. doi:10.1111/ics.12270
27. Nouveau-Richard S, Zhu W, Li YH, et al. Oily skin: specific features in Chinese women. Skin Res Technol. 2007;13(1):43–48. doi:10.1111/j.1600-0846.2006.00185.x
28. Abolhasani R, Araghi F, Tabary M, Aryannejad A, Mashinchi B, Robati RM. The impact of air pollution on skin and related disorders: a comprehensive review. Dermatol Ther. 2021;34(2):e14840. doi:10.1111/dth.14840
29. Khunger N, Kumar C. A clinico-epidemiological study of adult acne: is it different from adolescent acne? Indian J Dermatol Venereol Leprol. 2012;78(3):335–341. doi:10.4103/0378-6323.95450
30. Yang Y, Jia Y, Ling S, Yao C. Urban natural resource accounting based on the system of environmental economic accounting in Northwest China: a case study of Xi’an. Ecosyst Serv. 2021;47:101233. doi:10.1016/j.ecoser.2020.101233
31. Liang Z, Xu C, Fan Y-N, et al. Association between air pollution and menstrual disorder outpatient visits: a time-series analysis. Ecotoxicol Environ Saf. 2020;192:110283. doi:10.1016/j.ecoenv.2020.110283
32. Xu C, Fan YN, Liang Z, et al. Unexpected association between increased levels of ambient carbon monoxide and reduced daily outpatient visits for vaginitis: a hospital-based study. Sci Total Environ. 2020;723:137923. doi:10.1016/j.scitotenv.2020.137923
33. Skroza N, Tolino E, Mambrin A, et al. Adult acne versus adolescent acne: a Retrospective Study of 1167 patients. J Clin Aesthet Dermatol. 2018;11(1):21–25.
34. Han XD, Oon HH, Goh CL. Epidemiology of post-adolescence acne and adolescence acne in Singapore: a 10-year retrospective and comparative study. J Eur Acad Dermatol Venereol. 2016;30(10):1790–1793. doi:10.1111/jdv.13743
35. Yang YS, Lim HK, Hong KK, et al. Cigarette smoke-induced interleukin-1 alpha may be involved in the pathogenesis of adult acne. Ann Dermatol. 2014;26(1):11–16. doi:10.5021/ad.2014.26.1.11
36. Romanska-Gocka K, Wozniak M, Kaczmarek-Skamira E, Zegarska B. The possible role of diet in the pathogenesis of adult female acne. Postepy Dermatol Alergol. 2016;33(6):416–420. doi:10.5114/ada.2016.63880
37. Alhetheli G, Elneam AIA, Alsenaid A, Al-Dhubaibi M. Vitamin D levels in patients with and without acne and its relation to acne severity: a Case-Control Study. Clin Cosmet Investig Dermatol. 2020;13:759–765. doi:10.2147/CCID.S271500
38. Mendez D, Tam J, Giovino GA, Tsodikov A, Warner KE. Has smoking cessation increased? An examination of the US adult smoking cessation rate 1990–2014. Nicotine Tob Res. 2017;19(12):1418–1424. doi:10.1093/ntr/ntw239
39. Cordain L, Eaton SB, Sebastian A, et al. Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr. 2005;81(2):341–354. doi:10.1093/ajcn.81.2.341
40. Guo Q, Liang F, Tian L, Schikowski T, Liu W, Pan X. Ambient air pollution and the hospital outpatient visits for eczema and dermatitis in Beijing: a time-stratified case-crossover analysis. Environ Sci Process Impacts. 2019;21(1):163–173. doi:10.1039/c8em00494c
41. Li Q, Yang Y, Chen R, et al. Ambient air pollution, meteorological factors and outpatient visits for eczema in Shanghai, China: a time-series analysis. Int J Environ Res Public Health. 2016;13(11):1106. doi:10.3390/ijerph13111106
42. Kim YM, Kim J, Han Y, Jeon BH, Cheong HK, Ahn K. Short-term effects of weather and air pollution on atopic dermatitis symptoms in children: a panel study in Korea. PLoS One. 2017;12(4):e0175229. doi:10.1371/journal.pone.0175229
43. Karki P, Meliton A, Shah A, et al. Role of truncated oxidized phospholipids in acute endothelial barrier dysfunction caused by particulate matter. PLoS One. 2018;13(11):e0206251. doi:10.1371/journal.pone.0206251
44. Zhu TH, Zhu TR, Tran KA, Sivamani RK, Shi VY. Epithelial barrier dysfunctions in atopic dermatitis: a skin-gut-lung model linking microbiome alteration and immune dysregulation. Br J Dermatol. 2018;179(3):570–581. doi:10.1111/bjd.16734
45. Verdin A, Cazier F, Fitoussi R, et al. An in vitro model to evaluate the impact of environmental fine particles (PM0.3–2.5) on skin damage. Toxicol Lett. 2019;305:94–102. doi:10.1016/j.toxlet.2019.01.016
46. Mancebo SE, Wang SQ. Recognizing the impact of ambient air pollution on skin health. J Eur Acad Dermatol Venereol. 2015;29(12):2326–2332. doi:10.1111/jdv.13250
47. Jusuf NK, Putra IB, Sari L. Differences of microbiomes found in non-inflammatory and inflammatory lesions of acne vulgaris. Clin Cosmet Investig Dermatol. 2020;13:773–780. doi:10.2147/CCID.S272334
48. Wenger D, Gerecke AC, Heeb NV, et al. In vitro estrogenicity of ambient particulate matter: contribution of hydroxylated polycyclic aromatic hydrocarbons. J Appl Toxicol. 2009;29(3):223–232. doi:10.1002/jat.1400
49. Clougherty JE. A growing role for gender analysis in air pollution epidemiology. Environ Health Perspect. 2010;118(2):167–176. doi:10.1289/ehp.0900994
50. Khunger N, Mehrotra K. Menopausal acne - challenges and solutions. Int J Womens Health. 2019;11:555–567. doi:10.2147/IJWH.S174292
51. Dreno B, Thiboutot D, Layton AM, et al. Large-scale international study enhances understanding of an emerging acne population: adult females. J Eur Acad Dermatol Venereol. 2015;29(6):1096–1106. doi:10.1111/jdv.12757
52. Rahrovan S, Fanian F, Mehryan P, Humbert P, Firooz A. Male versus female skin: what dermatologists and cosmeticians should know. Int J Womens Dermatol. 2018;4(3):122–130. doi:10.1016/j.ijwd.2018.03.002
53. Lebwohl M, Herrmann LG. Impaired skin barrier function in dermatologic disease and repair with moisturization. Cutis. 2005;76(6 Suppl):7–12.
54. Chan H, Chan G, Santos J, Dee K, Co JK. A randomized, double-blind, placebo-controlled trial to determine the efficacy and safety of lactoferrin with vitamin E and zinc as an oral therapy for mild to moderate acne vulgaris. Int J Dermatol. 2017;56(6):686–690. doi:10.1111/ijd.13607
55. Kircik LH. Anti-inflammatory dose doxycycline plus adapalene 0.3% and benzoyl peroxide 2.5% gel for severe acne. J Drugs Dermatol. 2019;18(9):924–927.
56. Wang Q, Fang J, Shi W, Dong X. Distribution characteristics and policy-related improvements of PM2.5 and its components in six Chinese cities. Environ Pollut. 2020;266(Pt3):115299. doi:10.1016/j.envpol.2020.115299
57. Huang X, Tang G, Zhang J, et al. Characteristics of PM2.5 pollution in Beijing after the improvement of air quality. J Environ Sci. 2021;100:1–10. doi:10.1016/j.jes.2020.06.004
58. Wang S, Xing J, Zhao B, Jang C, Hao J. Effectiveness of national air pollution control policies on the air quality in metropolitan areas of China. J Environ Sci. 2014;26(1):13–22. doi:10.1016/s1001-0742(13)60381-2
59. Bai H, Gao W, Zhang Y, Wang L. Assessment of health benefit of PM2.5 reduction during COVID-19 lockdown in China and separating contributions from anthropogenic emissions and meteorology. J Environ Sci. 2021. doi:10.1016/j.jes.2021.01.022
60. Goodman G. Acne--natural history, facts and myths. Aust Fam Physician. 2006;35(8):613–616.
61. Jusuf NK, Putra IB, Sutrisno AR. Correlation between stress scale and serum substance P level in acne vulgaris. Int J Gen Med. 2021;14:681–686. doi:10.2147/IJGM.S294509
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.