Back to Journals » Clinical Interventions in Aging » Volume 16
The Analysis of Oxidative Stress Markers May Increase the Accuracy of the Differential Diagnosis of Alzheimer’s Disease with and without Depression
Authors Polak-Szabela A, Dziembowska I, Bracha M, Pedrycz-Wieczorska A, Kedziora-Kornatowska K, Kozakiewicz M
Received 12 March 2021
Accepted for publication 14 May 2021
Published 16 June 2021 Volume 2021:16 Pages 1105—1117
DOI https://doi.org/10.2147/CIA.S310750
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Nandu Goswami
Anna Polak-Szabela,1 Inga Dziembowska,2 Marietta Bracha,3 Agnieszka Pedrycz-Wieczorska,4 Kornelia Kedziora-Kornatowska,1 Mariusz Kozakiewicz3
1Department of Geriatrics, L. Rydygier Collegium Medicum in Bydgoszcz, Nicolaus Copernicus University, Torun, Poland; 2Department of Pathophysiology, Collegium Medicum in Bydgoszcz, Nicolaus Copernicus University, Torun, Poland; 3Department of Geriatrics, Division of Biochemistry and Biogerontology, L. Rydygier Collegium Medicum in Bydgoszcz, Nicolaus Copernicus University, Torun, Poland; 4Department of Histology, Embryology and Cytology, Medical University, Lublin, Poland
Correspondence: Marietta Bracha Email [email protected]
Introduction: The aim of work is to assess the usefulness of oxidative stress parameters in the differential diagnosis of dementia of the Alzheimer’s type and dementia of the Alzheimer’s type with coexisting depression.
Methods: The study involved three groups of people: patients with Alzheimer’s disease (AD) (AD; N=27), patients with Alzheimer’s disease and depression (D) (AD+D; N=30), and a control group that consisted of people without dementia and without depression (C; N=24). The assessment of cognitive functioning was carried out using among alia, Auditory Verbal Learning Test and Verbal Fluency Test. Furthermore, we determined the activity of superoxide dismutase (SOD-1) and superoxide anion radical.
Results: Multiple models with different combinations of independent variables showed that SOD together with Rey delayed recall were the best significant predictors of AD with the area under curve (AUC) of 0.893 (p = 0.001) and superoxide anion radical (O2•−) together with verbal fluency – sharp objects were the best significant predictors of AD +D diagnosis with the AUC of 0.689 (p = 0.034).
Conclusion: This study confirmed the value of neuropsychological diagnosis and analysis of oxidative stress markers in the diagnosis of AD and major depressive disorder (MDD) in the course of AD. The combination of the use of biochemical markers and neuropsychological tests seems particularly important for differential diagnosis.
Keywords: Alzheimer’s disease, depression, oxidative stress, superoxide dismutase, superoxide anion radical, verbal fluency, Auditory Verbal Learning Test
Introduction
Alzheimer’s disease (AD) is the most prevalent neurodegenerative disorder related to age characterized by several neuropathological events including amyloid and tau deposition, neuronal loss, and synaptic dysfunction.1,2 These changes lead to deficits in the area of cognitive and behavioral functioning.3 Alzheimer’s disease is now considered the most common cause of dementia, accounting for about 70% of all dementias, not only to the high frequency of diagnosis of this disease but also to the relatively long survival of patients.4–7 The prevalence of this type of dementia increases with age.4–7 Depression is among the most common neuropsychiatric disorder affecting more than 50% of patients diagnosed with AD.8,9 The etiology of both conditions may be influenced by various factors: genetics, toxins (endogenous and exogenous), bacterial and viral infections.8,9 Growing evidence suggests that oxidative stress, the lack or limited ability of the body to inhibit uncontrolled free radical reactions, plays an extremely important role in the pathogenesis of both Alzheimer’s disease and depression.10,11
Free radicals are molecules containing at least one unpaired electron on the atomic orbital.12 They are very reactive because they seek to pair electrons by abstracting them from or donating them to other molecules.12 A significant proportion of free radicals (approximately 90%) generated in the body is formed by reactions occurring in the respiratory chain in the mitochondria of cells.12 The remainder (about 10%) is produced by physiological reactions occurring in different cell structures. Free radicals are also produced by affecting the external factor cell such as ultraviolet and ionizing radiation, ultrasound, elevated temperature or tobacco smoke, as well as in the metabolism processes of various exogenous chemicals.12
The presence of two unpaired electrons in separate atomic orbitals makes oxygen, which is an essential element for life, showing its “other side” as it is susceptible to reactive oxygen species (ROS), which can modify and damage cells by reacting with their components.12 Since ROS is one of the most active bactericidal compounds, the “aerobic explosion” has an antibacterial effect in the body, making it easier for macrophages to eliminate the pathogen. Phagocytic cells, such as granulocytes, monocytes and macrophages, use the phenomenon of “aerobic explosion” to eliminate pathogens.13 In addition, ROS contributes to the increased activation of T lymphocytes and the adhesion of leukocyte cells into the endothelial, which allows these cells to penetrate from the circulatory system to the site of the inflammatory reaction.13 Elevated intracellular ROS levels cause damage to lipids, proteins and DNA, leading to pathologies at the level of systems and organs.12
Beta-amyloid deposits in the brain in the intercellular space and the deposition of hyperphosphoric tau protein inside neurons have been associated with neuronal loss in the course of AD.14–17 In addition, other proteins with a pathological structure are also involved in the transformations leading to neuronal atrophy in neurodegenerative disorders: alpha-synuclein and nuclear TDP protein 43.18 The presence of proteins with a pathological structure results in the activation of glial cells in the brain (astroglial and microglial), triggering inflammatory processes, as free oxygen radicals, excitatory amino acids, interleukins, as well as nitric oxide are released.1,18 Additionally, these compounds contribute to the death of neurons and their connections.1,18 Furthermore, there is a loss of cholinergic neurons in the forebrain, there are also changes in the synapses of the cerebral cortex and hippocampus.1,18
Depression of the elderly is characterized, inter alia, by anhedonia and the coexistence of various cognitive dysfunctions: in terms of executive function, memory, processing speed, attention and visual-spatial skills19 and it is associated with an increased risk of developing AD compared to healthy older adults.4–7 Pathomechanism of the development of major depressive disorder (MDD) is still associated with many unknowns, but it is believed to have a multi-factorial origin, involving dysfunction of many areas of the brain, such as the hippocampus, prefrontal cortex, nucleus and amygdala.20 In addition, MDD pathophysiology is associated with an inflammatory process due to microglial activation, increased cytokine release and increased oxidative stress, astrocyte atrophy and changes in glutamatergic regulation, which can lead to local damage.21 The activated microglial initiates the fission of precursor forms of interleukin-1β (IL-1β) into its active form.22,23 Exacerbation of the inflammatory process can result in a significant increase in the production and expression of pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and IL-1β, as well as reactive oxygen species (ROS) and nitric oxide, contributing to neurodegenerative processes associated with psychiatric disorders.22,23
The relationship between depression and Alzheimer’s disease is complex and not fully explained. Scientific research indicates that depression in the elderly may be one of the first signs of the dementia process. On the other hand, it is believed that brain damage caused by progressive Alzheimer’s disease can contribute to the development of depression. Changes in the brain in the same structures can be the biological cause of both depression and dementia.24
Due to the similar etiology and clinical picture of AD and MDD, it is necessary to look for comprehensive diagnostic methods that take into account both the biological and functional aspects of cognitive performance, especially at the prodromal stage. Another crucial aspect is the availability of the methods used and the relative ease of diagnosis. Due to the above mentioned AD structural and functional changes, neuropsychological tools to assess episodic memory and the ability to acquire new information are particularly useful.25,26 The development of a combination of high sensitivity and specificity markers is particularly urgent in the diagnosis of neurodegenerative dementia.26
Therefore, for the purposes of this work, tools classified as in-depth neuropsychological diagnosis were selected. Furthermore, they were relatively simple and not very expensive to perform, and they were available to most clinical psychologists. Due to the emerging new evidence supporting the role of oxidative stress in the pathogenesis of MDD and AD, they were combined with biochemical markers: superoxide dismutase SOD and superoxide anion radical (O2•−).
Materials and Methods
The research was conducted among patients of the Geriatric Clinics and Geriatric Outpatient Clinic in Bydgoszcz. Patient selection was based on the WHO definition of old age, which assumes that the onset of old age is at the age of 60.
The study involved three groups of participants: 27 patients with Alzheimer’s disease (AD), 30 patients with Alzheimer’s disease with coexisting major depressive disorder (AD+D), and a control group of 24 healthy participants without dementia and without depression.
The diagnosis of probable Alzheimer’s disease (prodromal stage) was based on the current criteria of the International Statistical Classification of Diseases and Health Problems ICD-10. The cognitive impairments appeared gradually (more than 6 months earlier) and slowly increased. They significantly disrupted the daily functioning of patients (this was confirmed by the collected interviews from the subjects and in many cases from objective caregivers). The deficits not only affected memory processes but also decreased levels of other mental functions, in relation to the pre-disease status of the subjects. The subjects were classified into groups based on the profile of cognitive impairment characteristic of dementia of the Alzheimer’s type and biochemical and CT tests. The following screening scales were used for the initial assessment of the severity of dementia: The Folstein’s Mini Mental State Examination (MMSE) and the Clock Drawing Test (CDT). As a part of the routine diagnosis, subjective and physical examinations were carried out, as well as the following laboratory tests: blood count, general urine test, ionogram, creatinine, AspAT, ALAT, lipid profile, EST, CRP, total protein, vitamin B12 and folic acid levels, TSH. The results of computed tomography (CT) of the head were collected, or this examination was performed as a part of a planned diagnosis.
The following exclusion criteria were used: the occurrence of another cause of dementia (vascular brain disease, HIV infection, Parkinson’s disease, Huntington’s disease, normotensive hydrocephalus, hypothyroidism, vitamin B12 or folic acid deficiency, hypercalcemia, alcohol or drug abuse).
The diagnosis of major depressive disorder was made on the basis of the criteria of the International Statistical Classification of Diseases and Health Problems ICD-10. The Geriatric Depression Scale (GDS) in its abbreviated version including 15 traits was used to study the severity of depressive symptoms. Qualified personnel collected blood from the basilic vein from patients who were included in the study. The clot and anticoagulant were taken within one blood draw during routine tests in the amount of about 7 mL. Blood was collected at 8 am to polyethylene tubes with lithium heparin and tubes without anticoagulant. The material was transported to the Department and Biochemistry Department of the Collegium Medicum in Bydgoszcz of Nicolaus Copernicus University in Torun. The tests were conducted on the same day, approximately 1 hour after the material was collected. Plasma was separated from the blood obtained from the morphotic elements and haemolysate was prepared from isolated red blood cells by the addition of 1:1, v/v bidestylated water).
The Bioethical Commission at Nicolaus Copernicus University in Torun Ludwik Rydygier Collegium Medicum in Bydgoszcz (KB 260/2007) agreed to carry out the research.
Cognitive Performance
The assessment of cognitive functioning was carried out, inter alia, with the help of the following neuropsychological tests.
Auditory Verbal Learning Test (AVLT) is characterized by a high diagnostic value in the diagnosis of Alzheimer’s disease. It evaluates the process of acquiring new information. The subjects memorized 15 words presented verbally in five consecutive attempts. After each presentation of the words by the researcher, the subjects mentioned all the memorized words in the sample (specifying the learning curve). Then, after a 20-minute break, they pointed memorized words from the list presented five times earlier (indicating the durability of the memory trace). The final task of the test was to recognize words learned in five attempts out of 30 different words on the list. The 2010 European Federation of Neurological Societies guidelines for the diagnosis and treatment of Alzheimer’s disease, in a psychological study, pay particular attention to the inclusion of post-deferral reminders and post-hint reminders in Alzheimer’s dementia diagnosis.27
Verbal fluency test (semantic fluency – animals, phonemic fluency – words beginning with the letter k (without proper names), fluency of subcategories – sharp objects with which one can cut oneself). The respondent’s task was to say as many words as possible according to the given criterion within one minute. It is a tool that mainly evaluates language functions (verbal production, ability to update names), as well as semantic memory and executive functions. Despite the lack of Polish test standards for verbal fluency assessment, the standard in the phonetic category is 12–16 words per minute, in the semantic category 18–20 words per minute, while in the subcategory of the fluency category 6–8 words per minute.
Biochemical Analyses
The Misra and Fridovich method was used to determine the activity of superoxide dismutase (SOD-1) in erythrocytes. SOD-1 activity is expressed in U/gHb. An amount of enzyme which inhibits adrenaline oxidation in 50% was taken as a unit of enzymatic activity (U).28 The amount of superoxide anion radical (O2•−) in whole blood was determined using the Bellavite et al. method, based on the reduction of cytochrome C in the presence of superoxide dismutase and 0.1 mL of whole blood. The extinction read at a light length of 550 nm from the supernatant to the air gave the value of the generation of superoxide anion radical. The stimulated value was obtained after stimulation of the sample with zymosan. The results were expressed in nmol/min/cell.29
Statistical Analysis
Statistical analysis of the results was carried out using STATISTICA v. 13.1 computer program from StatSoft (Cracow, Poland). When two categorical variables were analyzed, the differences in groups were studied using the Kruskal-Wallis Chi-squared test. Single-factor logistic regression analyses were performed with age, gender, SOD (O2•−), MMSE score, CDT score, verbal fluency test results and RAVLT results as independent variables, and clinical diagnosis as a dependent variable (AD or AD+D). These independent variables were also included in the multidimensional logistic regression analysis to evaluate their prognosis based on clinical diagnosis. Multiple models with different combinations of independent variables have been tested. The clinical diagnosis ROC curves were created in relation to psychological and biochemical markers to assess optimal cut-off values. Youden Index has been calculated to find optimal thresholds. DeLong method and a two-stage nonparametric test that uses a shifted area under the ROC curve (sAUC), along with AUCs were performed in order to detect differences between ROC curves for different models.
Results
Table 1 contains the group characteristics.
 |
Table 1 Group Characteristics |
The first univariate analysis showed that all examined parameters except for gender (p = 0.1178), years of education (p = 0.0511) and superoxide activity (p = 0.0610) were significant predictors of AD diagnosis (Table 2).
 |
Table 2 The Univariate Model for Alzheimer’s Disease Diagnosis |
The univariate analysis showed that all parameters except for age, gender, years of education, AVLT percent forgetting, AVLT percent false negative errors and SOD were significant predictors of AD + D diagnosis (Table 3).
 |
Table 3 The Univariate Model for Alzheimer’s Disease with Comorbid Depression (AD+D) Diagnosis |
Multiple models with different combinations of independent variables were tested in the multivariate analysis for AD diagnosis prediction. In the multivariate analysis of the selected prediction model, it was found that SOD together with the raw score of AVLT delayed recall were the best significant predictors of AD disease with the AUC of 0.893 (p = 0.0012). The adjusted odds ratios (aOR) are presented in Table 4. For the AVLT – delayed recall aOR was 0.448 (95% CI: 0.300–0.668, p = 0.0002), which was similar to OR in univariate model (0.448, 95% CI: 0.300–0.668, see Table 2). For the result of SOD activity aOR was 0.983 (95% CI: 0.973–0.974, p = 0.0034), which is also quite similar to OR in univariate model for this parameter (0.986, 95% CI: 0.980–0.993, see Table 2). These results indicate that both lower scores in AVLT – delayed recall and lower SOD levels are predictive for AD.
 |
Table 4 Multivariate Models for Alzheimer’s Disease without Depression (AD) and Alzheimer’s Disease with Comorbid Depression (AD+D) Diagnosis |
The univariate analysis for predictors of AD+D is presented in Table 3 and showed that, although SOD activity was not significant here (p = 0.0983), the activity of O2•− was negatively associated with AD+D (OR = 0.991, 95% CI: 0.985–0.998, p = 0.0140). Also, MMSE and CDT scores were negative predictors of AD=D (OR 0.851, p = 0.0001, and OR = 0.766, p – 0.0031, respectively). Out of AVLT indices sum of immediately recalled words, learning and delayed recall were negatively associated with AD+D (OR 0.910, p = 0.008; OR = 0.720, p = 0.0152 and OR = 0.632, p =0.002 respectively), whereas false-positive errors and the sum of all errors were positive predictors of AD +D (OR = 1.14, p = 0.0046 and OR – 1.16, p = 0.0016, respectively). Also, patients from AD+D group performed worse in all verbal fluency task, including phonemic verbal fluency task (OR = 0.863 for AD+D diagnosis, p = 0.0262), and both semantic tasks (OR = 0.894, p = 0.0183 for animals and OR = 0.667, p = 0.0012 for sharp objects).
Multiple models with different combinations of independent variables were tested in the multivariate analysis for AD+D diagnosis prediction. In the multivariate analysis of the selected prediction model, it was found that O2•−together with verbal fluency – sharp objects were the best significant predictors of AD +D diagnosis with the AUC of 0.689 (p = 0.0312). Adjusted odds ratios (aOR) are presented in Table 4. For the verbal fluency task – sharp objects and O2• aOR’s was even a bower than OR’s: 0.612, p = 0.0008 and 0.990, p = 0.0065, respectively. These results indicate that both lower scores in verbal fluency task – sharp objects together with SOD levels are most predictive for AD+D.
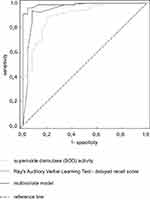
Furthermore, the comparison between receiver operating curves (ROC) with DeLong method showed that discrimination is superior when combining neuropsychological and oxidative stress markers than when using either of them alone either for AD (Figure 1) or for AD+D (Figure 2, Table 5). For AD diagnosis, the multivariate model increased all discrimination characteristics when compared to SOD (p = 0.007) and AVLT DELAY RECALL (p = 0.015; Table 5). As for AD+D diagnosis, the multivariate model also improved both sensitivity to 0.811 (p = 0.018 when compared to O2•− and p = 0.028 when compared to verbal fluency – sharp objects alone); however, the specificity remained still relatively as low as 0.589 (Table 5).
 |
Table 5 Comparsion of Univariate and Multivariate Areas Under Curve (AUC) and Predictive Values for Alzheimer’s Disease without Depression (AD) and Alzheimer’s Disease with Comorbid Depression (AD+D) |
To overcome the limitation of no non-AD depressed patients, and confirm the results, we performed also an analysis for all AD patients without control group (with non-depressed AD participants serving as controls). Results of this analysis are similar to the results of multivariate model for AD+D diagnosis, with area under ROC of 0.784 (SE=0.06, 95% CI 0.655–0.882, p = 0.0311), and presented in Table 6.
 |
Table 6 Multivariate Model for Alzheimer Disease with Comorbid Depression (AD+D) Diagnosis with Alzheimer’s Disease without Depression (AD) Group Serving as a Control Group Only (N = 57 Participants) |
Discussion
With regard to AD diagnostics, it was observed that the greatest diagnostic value was the combination of SOD and RAVLT activity level studies, in particular, the delayed recall.27,30
SOD superoxide dismutase belongs to the group of enzymes of the first line of defense against excess free radicals.31 It converts superoxide anion radical into hydrogen peroxide and oxygen molecule.31 It neutralizes quickly and efficiently any molecule that can develop into a free radical or any free radical, with the ability to induce the production of other radicals.31 One of the first studies involving oxidative stress in AD, conducted by Gsell et al., provided evidence of H2O2 accumulation in the brain with Alzheimer’s disease.32 These results were consistent in many post-mortem areas of the brain, from mild to strongly affected AD regions.33 A few years later, Thome et al. reported a decrease in mitochondrial SOD 2 levels and unchanged serum SOD1 levels in AD patients, compared to the control group selected according to the age and gender.33 Chang et al, in his well-crafted literature review, demonstrated, on the basis of a total of 80 cases, that AD is indeed associated with a significant increase in SOD levels.34 In addition, differences in SOD levels may reflect different stages of the disease: SOD levels appear to be elevated in the early stages of AD, but there is an exhaustion in the later stages of the disease progression.34 It therefore appears that SOD activity is a promising marker for enriching neuropsychological analysis of AD patients and, consequently, increasing sensitivity and diagnostic relevance.
The neuropsychological profile at the initial stage of Alzheimer’s disease is an increasingly well-described important predictor of further development of the disease.35,36 The early stages of AD are characterized by episodic memory deficits, which are caused by medial lobe atrophy and loss of neurons in the primary cholinergic system of the forebrain.35,36 The effect of this disorder is the disruption of the neural network, which is crucial for episodic memory function.35,36 Thus, the clinical feature of AD is a deficit in the ability to learn and remember new information. Patients with AD show a general episodic memory deficit: they do not benefit from the guidance or structure of the test; their ability to recognize is just as flawed as free play after deferral. These patients have a deficit in acquiring new information.37
The Rey Auditory Verbal Learning Test (AVLT) test is used to assess the process of acquiring new information and episodic auditory memory.27 Previous studies have shown that the deterioration in AVLT results well reflects the underlying pathology caused by Alzheimer’s disease (AD), making the indicators obtained in this test a good marker for early detection of AD among people reporting memory problems.27
A study estimating AVLT results based on gray matter density showed that the best predictors of the results obtained in the test were medial temporal lobe structures and amygdala to evaluate immediate responses. Whilst angular gyrus, hippocampus and amygdala were the most accurate to assess AVLT forgetting percentage. In addition, the conversion of MCI to AD within 3 years could be predicted from observed or estimated AVLT results with accuracy comparable to MRI-based biomarkers.27 Recent research suggests that there are both involvement of the widespread cortical network and the importance of its interactive roles in the memory process. In addition to the temporal lobe, prefrontal and parietal areas are associated with episodic memory.38–40 The structures involved in the memory formation process are the hippocampus and the structure surrounding the olfactory sulcus.38–40 It is in these areas that the degenerative process takes place at the early stages of Alzheimer’s disease. It is manifested as a deterioration in memory trace consolidation represented by the result in the attempt to freely reproduce after a 20-minute deferral in AVLT.38–40 Many researchers have previously confirmed that those were independent predictors of the progression of Alzheimer’s disease.41–46
Both AD and AD+D groups were characterized by a lack of memory trace of deferred auditory hearing memory of any form and a significant decrease in memory skills.
With regard to AD+D diagnosis, it was observed that the greatest diagnostic value was the combination of superoxide anion radical and verbal fluency, in particular, the acute subject subcategory.
The results of our own work therefore indicate that in AD with coexisting MDD, the key roles are played not only by the body’s defense capabilities (represented by SOD) but also oxidative stress itself (represented by an anion radical). This therefore leads to the hypothesis that stress with unbalanced defense forces is associated with the development of MDD in the AD course.
Indeed, the research carried out in the recent years highlights the potential role of oxidative stress in the pathogenesis of the clinical picture of depression as one of the factors relevant to the development of this disease. Increased levels of reactive oxygen species (ROS) and reactive nitrogen species (RNS) were observed in depression, as well as altered antioxidant concentrations ie GSH in depressed patients.47
Patients with depression had lower antioxidant values such as vitamin E, zinc and serum coenzyme Q10, as well as lower antioxidant enzymes.48–50 Oxidative stress can also affect the pathogenesis of depression by interacting with neurogenesis and neuroplasticity processes, inflammatory processes in the brain and monoamine retake.
The effectiveness of depression treatment can consist of inhibiting the activity of pro-inflammatory cytokines and oxidative stress, tilting the balance in favor of antioxidant mechanisms.51,52
In the neuropsychological picture, people in the AD and AD+D groups revealed a decrease in the ability to create and speak words fluently according to the recommended criterion, as well as the ability to update vocabulary and systematically search semantic memory resources. In AD +D and AD groups, the sum of the words spoken in each category was significantly lower compared to the control group.
The Verbal Fluency Test is a tool that evaluates primarily language functions (verbal production, ability to update names), as well as semantic memory and executive functions.53 Verbal fluency refers to the ability to generate as many words as possible in a limited time, without repetition, and according to phonology (each word begins with a given letter) or a semantic rule (each word belongs to a given semantic category).53 Verbal knowledge requires not only access to and search for specific words in lexical memory but also response monitoring to avoid repetition and suppression of words irrelevant to an exercise, in order to stick to task rules.53–56 These tasks include language processing and elements of the executive function model.35,53,55,56 The standard evaluation of results is quantitative and includes the number of words according to the given criterion and errors – out-of-category responses and repetitions.35,53,55,56
Previous studies of verbal fluency in depressed patients confirmed the observations made in our study: patients with depression produced fewer words in the semantic category than the control group, in the absence of a change in the tasks involving phonemic fluency.53 It is worth to note that subcategory of sharp objects, which seemed in our analysis, to be predictive for depression in AD, reflects mainly the ability of abstractive thinking and executive functions.
The reduced ability to switch semantic subcategories was associated with a reduced ability to change mental attitudes, which supports the hypothesis that verbal fluency disorder reflects general executive problems in depression.53 The evidence from neuroimaging studies indicates the neurobiological basis of this compound, primarily the need for simultaneous involvement of the hippocampus and frontal lobe structures. Sejunaite et al found that executive function, which also acts as a mediator between neuropsychological domains and daily functioning, is the main area of cognitive impairment in depression.57–60 Impaired executive functions cause memory deficiencies in patients with depressive disorders and affect memory processes during coding, learning and search phases.61 The deficit in executive function in patients with depressive disorder was combined with functional disorders in the frontal lobes and impaired hippocampal function.61 The study of memory impairment focused mainly on omissions, ie false negative results. Memory omissions are due to the failure to recall or recognize information to which individuals have been exposed in everyday life or in laboratory conditions. Semantic fluency can also be particularly interesting in characterizing cognitive difficulties in MDD. The ability to perform well in verbal fluency requires integrating semantic search strategies and word generation.61 This may indicate that performance degradation may be due to a less integrated semantic network, leading to unorganized search processes, making it difficult to search for words in the same category.
The results obtained in this work indicate that semantic fluency tests may help in the differential diagnosis of patients with depression in patients with early-stage dementia of the Alzheimer’s type. This observation can be very useful clinically, due to the simplicity and low cost and low time effort, and thus causing a small burden on the patient, which is of particular importance in view of the ease of fatigue of this group of patients.
This study contains some limitations that can serve as a guidance for planning and implementing future research in this field. They concern, in particular, the methodological aspects of the analyses carried out.
Restrictive inclusion criteria have contributed not only to reducing the minimum test numbers needed to achieve adequate statistical strength of the tests but also to significant limitations in inference. It is also unclear how other disease units may affect the activity of antioxidant enzymes, which limits the possibility of reference of test results to people who do not have a disease with free radical etiology. In addition, the absence of people with depression but without dementia in the study group leads to difficulties in assessing the strength of the effect of depression itself on the obtained results. Another limitation is that other neurodegenerative disorders were not included. Thus, we cannot evaluate whether this oxidative stress biomarkers are specific of AD or might be present in other neurodegenerative disorders.
Furthermore, we did not use any AD biomarkers such as tau levels in CSF or FDG-PET results. In fact, all these markers are helpful in scientific research, but usually not available in routine diagnostics.
These limitations continue to inspire further research into the issues raised in this work.
Conclusions
This study confirms the value of neuropsychological diagnosis and analysis of oxidative stress markers in the diagnosis of AD and MDD in the course of AD. The combination of the use of biochemical markers and neuropsychological tests seems particularly important for differential diagnosis. For AD diagnostics, the greatest diagnostic value was observed in a combination of SOD and RAVLT, in particular, delayed recall. For MDD diagnosis in the course of AD, the greatest diagnostic value was observed in the combination of the examination of the anion radical concentration and a verbal fluency test.
Data Sharing Statement
The data presented in this study are available on request from the corresponding author.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board Bioethical Commission at Nicolaus Copernicus University in Torun Ludwik Rydygier Collegium Medicum in Bydgoszcz (KB 260/2007).
Acknowledgments
The authors would like to thank Katarzyna Porzych, Multispecialist Clinic “Bartodzieje”, Bydgoszcz, Poland, for her involvement and participation in the investigation.
The authors wish to express their appreciation to Jordi A Matias-Guiu, Department of Neurology, Hospital Clinico San Carlos, Madrid, Spain, for his insightful review and comments.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research received no external funding.
Disclosure
The authors declare no conflict of interest.
References
1. Firdaus Z, Singh TD. An insight in pathophysiological mechanism of Alzheimer’s disease and its management using plant natural products. Mini Rev Med Chem. 2020.
2. Uddin MS, Kabir MT, Rahman MS, et al. Revisiting the amyloid cascade hypothesis: from anti-Aβ therapeutics to auspicious new ways for Alzheimer’s disease. InternatIonal Journal of Molecular ScIences. 2020;21(16):1–34. doi:10.3390/ijms21165858
3. Dindelegan CM, Faur D, Purza L, et al. Distress in neurocognitive disorders due to Alzheimer’s disease and stroke. Exp Ther Med. 2020;20(3):2501–2509. doi:10.3892/etm.2020.8806
4. Andreu-Reinón ME, Huerta JM, Gavrila D, et al. Incidence of dementia and associated factors in the EPIC-Spain dementia cohort. J Alzheimers Dis. 2020;78(2):543–555. doi:10.3233/JAD-200774
5. Rabin LA, Wang C, Mogle JA, Lipton RB, Derby CA, Katz MJ. An approach to classifying subjective cognitive decline in community-dwelling elders. Alzheimers Dement (Amst). 2020;12(1):e12103.
6. Vlachos GS, Kosmidis MH, Yannakoulia M, et al. Dementia incidence in the elderly population of Greece: results from the HELIAD Study. Alzheimer Dis Assoc Disord. 2020;34(2):156–162. doi:10.1097/WAD.0000000000000361
7. Dubal DB. Sex difference in Alzheimer’s disease: an updated, balanced and emerging perspective on differing vulnerabilities. Handb Clin Neurol. 2020;175:261–273.
8. Chan CK, Soldan A, Pettigrew C, Wang J, Albert M, Rosenberg PB; BIOCARD Research Team. Depressive symptoms and CSF Alzheimer’s disease biomarkers in relation to clinical symptom onset of mild cognitive impairment. Alzheimers Dement (Amst). 2020;12(1):e12106.
9. Chan C, Rosenberg PB. Depression synergy with amyloid and increased risk of cognitive decline in preclinical Alzheimer disease. JAMA Netw Open. 2019;2(8):e198970. doi:10.1001/jamanetworkopen.2019.8970
10. Al-Kuraishy HM, Abdulhadi M, Hussien N, et al. Involvement of orexinergic system in psychiatric and neurodegenerative disorders: a scoping review. Brain Circ. 2020;6(2):70–80. doi:10.4103/bc.bc_42_19
11. Lévy E, El Banna N, Baïlle D, et al. Causative links between protein aggregation and oxidative stress: a review. Int J Mol Sci. 2019;20(16):3896. doi:10.3390/ijms20163896
12. Pisoschi AM, Pop A, Iordache F, Stanca L, Predoi G, Serban AI. Oxidative stress mitigation by antioxidants - an overview on their chemistry and influences on health status. Eur J Med Chem. 2020;209:112891.
13. Olakkaran S, Kizhakke Purayil A, Antony A, et al. Oxidative stress-mediated genotoxicity of malathion in human lymphocytes. Mutat Res. 2020;849:503138. doi:10.1016/j.mrgentox.2020.503138
14. Koychev I, Hofer M, Friedman N. Correlation of Alzheimer disease neuropathologic staging with amyloid and tau scintigraphic imaging biomarkers. J Nucl Med. 2020;61(10):1413–1418. doi:10.2967/jnumed.119.230458
15. Sun H-L, Chen S-H, Yu Z-Y, et al. Blood cell-produced amyloid-β induces cerebral Alzheimer-type pathologies and behavioral deficits. Mol Psychiatry. 2020. doi:10.1038/s41380-020-0842-1
16. Greenberg SM, Bacskai BJ, Hernandez-Guillamon M, et al. Cerebral amyloid angiopathy and Alzheimer disease - one peptide, two pathways. Nat Rev Neurol. 2020;16(1):30–42. doi:10.1038/s41582-019-0281-2
17. Gourmaud S, Shou H, Irwin DJ, et al. Alzheimer-like amyloid and tau alterations associated with cognitive deficit in temporal lobe epilepsy. Brain. 2020;143(1):191–209. doi:10.1093/brain/awz381
18. Mavroudis I, Petridis F, Chatzikonstantinou S, et al. Alpha-synuclein levels in the differential diagnosis of Lewy bodies dementia and other neurodegenerative disorders: a meta-analysis. Alzheimer Dis Assoc Disord. 2020;34(3):220–224. doi:10.1097/WAD.0000000000000381
19. Banning LCP, Ramakers IHGB, Deckers K, et al. Affective symptoms and AT(N) biomarkers in mild cognitive impairment and Alzheimer’s disease: a systematic literature review. Neurosci Biobehav Rev. 2019;107:346–359. doi:10.1016/j.neubiorev.2019.09.014
20. Kaufmann FN, Costa AP, Ghisleni G, et al. NLRP3 inflammasome-driven pathways in depression: clinical and preclinical findings. Brain Behav Immun. 2017;64:367–383.
21. Kohman RA, Rhodes JS. The contribution of adult hippocampal neurogenesis to the progression of psychiatric disorders. Mod Trends Pharmacopsychiatry. 2017;31:124–151.
22. Singhal G, Jaehne EJ, Corrigan F, et al. Inflammasomes in neuroinflammation and changes in brain function: a focused review. Front Neurosci. 2014;8:315. doi:10.3389/fnins.2014.00315
23. Singhal G, Baune BT. Microglia: an interface between the loss of neuroplasticity and depression. Front Cell Neurosci. 2017;11:270. doi:10.3389/fncel.2017.00270
24. Fiske A, Loebach Wetherell J, Gatz M. Depression in older adults. Annu Rev Clin Psychol. 2009;5(1):363–389. doi:10.1146/annurev.clinpsy.032408.153621
25. Mohn C, Rund BR. Neurocognitive profile in major depressive disorders: relationship to symptom level and subjective memory complaints. BMC Psychiatry. 2016;16(1):108. doi:10.1186/s12888-016-0815-8
26. Ramirez-Gomez L, Zheng L, Reed B, et al. Neuropsychological profiles differentiate Alzheimer disease from subcortical ischemic vascular dementia in an autopsy-defined cohort. Dement Geriatr Cogn Disord. 2017;44(1–2):1–11. doi:10.1159/000477344
27. Moradi E, Hallikainen I, Hänninen T, et al.; Alzheimer’s Disease Neuroimaging Initiative. Rey’s Auditory Verbal Learning Test scores can be predicted from whole brain MRI in Alzheimer’s disease. Neuroimage Clin. 2017;13:415–427. doi:10.1016/j.nicl.2016.12.011
28. Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase.. The Journal of Biological Chemistry. 1972;247(10):3170–3175.
29. Bellavite P, Dri P, Della Bianca V, et al. The measurement of superoxide anion production by granulocytes In whole blood. A clinical test for the evaluation of phagocyte function and serum opsonic capacity. Eur J Clin Invest. 1983;13(4):363–368. doi:10.1111/j.1365-2362.1983.tb00114.x
30. Ighodaro O, Akinloye OA. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alexandria J Med. 2017.
31. Borgstahl GEO, Oberley-Deegan RE. Superoxide dismutases (SODs) and SOD mimetics. Antioxidants (Basel). 2018;7(11).
32. Gsell W, Conrad R, Hickethier M, et al. Decreased catalase activity but unchanged superoxide dismutase activity in brains of patients with dementia of Alzheimer type. J Neurochem. 1995;64(3):1216–1223. doi:10.1046/j.1471-4159.1995.64031216.x
33. Thome J, Gsell W, Rösier M, et al. Oxidative-stress associated parameters (lactoferrin, superoxide dismutases) in serum of patients with Alzheimer’s disease. Life Sci. 1997;60(1):13–19. doi:10.1016/S0024-3205(96)00583-8
34. Chang YT, Chang W-N, Tsai N-W, et al. The roles of biomarkers of oxidative stress and antioxidant in Alzheimer’s disease: a systematic review. Biomed Res Int. 2014;2014:182303. doi:10.1155/2014/182303
35. Jutten RJ, Sikkes SA, Amariglio RE, et al. Identifying sensitive measures of cognitive decline at different clinical stages of alzheimer’s disease. J Int Neuropsychol Soc. 2020;1–13.
36. Hammers DB, Kucera A, Spencer RJ, et al. Examining the relationship between a verbal incidental learning measure from the WAIS-IV and neuroimaging biomarkers for Alzheimer’s pathology. Dev Neuropsychol. 2020;45(3):95–109. doi:10.1080/87565641.2020.1762602
37. Terrera GM, Harrison JE, Ritchie CW, et al. Cognitive functions as predictors of Alzheimer’s disease biomarker status in the European prevention of Alzheimer’s dementia cohort. J Alzheimers Dis. 2020;74(4):1203–1210. doi:10.3233/JAD-191108
38. Snytte J, Elshiekh A, Subramaniapillai S, et al. The ratio of posterior-anterior medial temporal lobe volumes predicts source memory performance in healthy young adults. Hippocampus. 2020;30(11):1209–1227. doi:10.1002/hipo.23251
39. Schwab S, Afyouni S, Chen Y, et al. Functional connectivity alterations of the temporal lobe and hippocampus in semantic dementia and Alzheimer’s disease. J Alzheimers Dis. 2020;76(4):1461–1475. doi:10.3233/JAD-191113
40. Tang L, Pruitt PJ, Yu Q, et al. Differential functional connectivity in anterior and posterior hippocampus supporting the development of memory formation. Front Hum Neurosci. 2020;14:204. doi:10.3389/fnhum.2020.00204
41. Hao L, Xing Y, Li X, et al. Risk factors and neuropsychological assessments of subjective cognitive decline. Front Neurosci. 2019;13:846. doi:10.3389/fnins.2019.00846
42. Xu Y, Chen K, Zhao Q, et al. Short-term delayed recall of auditory verbal learning test provides equivalent value to long-term delayed recall in predicting MCI clinical outcomes: a Longitudinal Follow-Up Study. Appl Neuropsychol Adult. 2020;27(1):73–81. doi:10.1080/23279095.2018.1481067
43. Hong X, Zhang Z-X, Wu L-Y, et al. Validity of auditory verbal learning test in diagnosis of Alzheimer’s disease. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2012;34(3):262–266. doi:10.3881/j.issn.1000-503X.2012.03.014
44. Zhao Q, Guo Q, Liang X, et al. Auditory verbal learning test is superior to rey-osterrieth complex figure memory for predicting mild cognitive impairment to Alzheimer’s disease. Curr Alzheimer Res. 2015;12(6):520–526. doi:10.2174/1567205012666150530202729
45. Rizk-Jackson A, Insel P, Petersen R, et al. Early indications of future cognitive decline: stable versus declining controls. PLoS One. 2013;8(9):e74062. doi:10.1371/journal.pone.0074062
46. Loring DW, Goldstein FC, Chen C, et al. False-positive error rates for reliable digit span and auditory verbal learning test performance validity measures in amnestic mild cognitive impairment and early Alzheimer disease. Arch Clin Neuropsychol. 2016;31(4):313–331. doi:10.1093/arclin/acw014
47. Shao A, Lin D, Wang L, et al. Oxidative stress at the crossroads of aging, stroke and depression. Aging Dis. 2020;11(6):1537–1566. doi:10.14336/AD.2020.0225
48. Manosso LM, Camargo A, Dafre AL, et al. Vitamin E for the management of major depressive disorder: possible role of the anti-inflammatory and antioxidant systems. Nutr Neurosci. 2020;1–15. doi:10.1080/1028415X.2020.1853417
49. Farhadnejad H, Neshatbini Tehrani A, Salehpour A, et al. Antioxidant vitamin intakes and risk of depression, anxiety and stress among female adolescents. Clin Nutr ESPEN. 2020;40:257–262. doi:10.1016/j.clnesp.2020.09.010
50. Tanvir S, Asif N, Qayyum R, et al. Trace metal profiling in patients with depression in Pakistani population. J Pak Med Assoc. 2020;70(11):1883–1886. doi:10.5455/JPMA.6154
51. Behr GA, Moreira JC, Frey BN. Preclinical and clinical evidence of antioxidant effects of antidepressant agents: implications for the pathophysiology of major depressive disorder. Oxid Med Cell Longiv. 2012;609421.
52. Kotan VO, Sarandol E, Kirhan EIW, Ozkaya G, Kirli S. Effects of long-term antidepressant treatment on oxidative status in major depressive disorder: a 24-Week Follow-Up Study. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(5):1284–1290. doi:10.1016/j.pnpbp.2011.03.021
53. Amunts J, Camilleri JA, Eickhoff SB, et al. Executive functions predict verbal fluency scores in healthy participants. Sci Rep. 2020;10(1):11141. doi:10.1038/s41598-020-65525-9
54. Guzzetti S, Mancini F, Caporali A, et al. The association of cognitive reserve with motor and cognitive functions for different stages of Parkinson’s disease. Exp Gerontol. 2019;115:79–87. doi:10.1016/j.exger.2018.11.020
55. Mougias A, Christidi F, Synetou M, et al. Differential effect of demographics, processing speed, and depression on cognitive function in 755 non-demented community-dwelling elderly individuals. Cogn Behav Neurol. 2019;32(4):236–246. doi:10.1097/WNN.0000000000000211
56. Olabarrieta-Landa L, Benito-Sánchez I, Alegret M, et al. Letter verbal fluency in Spanish-, Basque-, and Catalan-speaking individuals: does the selection of the letters influence the outcome? J Speech Lang Hear Res. 2019;62(7):2400–2410. doi:10.1044/2019_JSLHR-L-18-0365
57. Sejunaite K, Lanza C, Riepe MW. Everyday memory in healthy aging: porous but not distorted. Front Aging Neurosci. 2019;11:153. doi:10.3389/fnagi.2019.00153
58. Sejunaite K, Lanza C, Riepe MW. Everyday false memories in older persons with depressive disorder. Psychiatry Res. 2018;261:456–463. doi:10.1016/j.psychres.2018.01.030
59. Sejunaite K, Lanza C, Riepe MW. Everyday memory in patients with Alzheimer’s disease: fragmentary and distorted. J Alzheimers Dis. 2017;60(4):1489–1498. doi:10.3233/JAD-170493
60. Shao Z, Janse E, Visser K, et al. What do verbal fluency tasks measure? Predictors of verbal fluency performance in older adults. Front Psychol. 2014;5:772. doi:10.3389/fpsyg.2014.00772
61. Zhang FF, Peng W, Sweeney JA, et al. Brain structure alterations in depression: psychoradiological evidence. CNS Neurosci Ther. 2018;24(11):994–1003. doi:10.1111/cns.12835
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.