Back to Journals » International Journal of Nanomedicine » Volume 15
The Advances of Ceria Nanoparticles for Biomedical Applications in Orthopaedics
Authors Li H , Xia P, Pan S, Qi Z, Fu C , Yu Z, Kong W, Chang Y, Wang K , Wu D, Yang X
Received 1 July 2020
Accepted for publication 10 August 2020
Published 29 September 2020 Volume 2020:15 Pages 7199—7214
DOI https://doi.org/10.2147/IJN.S270229
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yan Shen
Hongru Li, Peng Xia, Su Pan, Zhiping Qi, Chuan Fu, Ziyuan Yu, Weijian Kong, Yuxin Chang, Kai Wang, Dankai Wu, Xiaoyu Yang
Department of Orthopedic Surgery, The Second Hospital of Jilin University, Changchun 130041, People’s Republic of China
Correspondence: Dankai Wu; Xiaoyu Yang
Department of Orthopedic Surgery, The Second Hospital of Jilin University, Changchun, TX 130041, People’s Republic of China
Email [email protected]; [email protected]
Abstract: The ongoing biomedical nanotechnology has intrigued increasingly intense interests in cerium oxide nanoparticles, ceria nanoparticles or nano-ceria (CeO2-NPs). Their remarkable vacancy-oxygen defect (VO) facilitates the redox process and catalytic activity. The verification has illustrated that CeO2-NPs, a nanozyme based on inorganic nanoparticles, can achieve the anti-inflammatory effect, cancer resistance, and angiogenesis. Also, they can well complement other materials in tissue engineering (TE). Pertinent to the properties of CeO2-NPs and the pragmatic biosynthesis methods, this review will emphasize the recent application of CeO2-NPs to orthopedic biomedicine, in particular, the bone tissue engineering (BTE). The presentation, assessment, and outlook of the orthopedic potential and shortcomings of CeO2-NPs in this review expect to provide reference values for the future research and development of therapeutic agents based on CeO2-NPs.
Keywords: CeO2-NPs, green synthesis, ROS, bone tissue engineering, coating, orthopedic implants
Introduction
Cerium is the first element with 4f electron among the 17 rare earth elements or lanthanides. The peculiar 4f orbitals of equal energy endow cerium with characteristic physicochemical properties.1 Besides, cerium turns out the most abundant of the rare earth elements. Light, electricity, magnetism, and other fields have witnessed inordinately ample scope for the application of cerium.2–5 At present, efforts have gone into exploring the further application of cerium. CeO2-NPs are nanocrystalline derived from cerium. Cerium is mostly in the form of ceria with unique face-centered cubic fluorite lattice structure. The common knowledge believes that the fast and convenient oxidation state transition from Ce3+ to Ce4+ contributes to the high redox activity of CeO2.6 During the oxidation state transition, the alternating loss of oxygen and/or other electrons in CeO2 and CeO2−x (non-stoichiometric compounds) generates oxygen vacancies or defects in the lattice structures7 seen in Figure 1. The high oxygen storage capacity of the lattice and the high oxygen mobility in the lattice impel a broad application of ceria to biological effect relevant to redox reaction.8 Evidence suggests that a higher surface to volume ratio of CeO2-NPs makes the surface atomic lattices softer than those in bulk. Nanometer effect is significant to the catalytic activity of CeO2. Compared with the conventional block structure, nano-CeO2 can improve catalytic activity by two orders of magnitude.9 The reduced particle size and increased surface-to-volume ratio lead to the formation of more oxygen vacancies. Loss of even one oxygen atom after size reduction will result in a high lattice strain.10 The stronger the catalytic activity, the higher the effect of CeO2-NPs in biomedical applications.
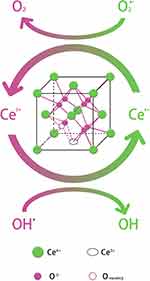 |
Figure 1 The structural representation of CeO2-NP, and its self-storage stability and self-regeneration capacity exerting antioxidant chemical reaction. |
The rapid development of nanotechnology has broadened the heuristic path for nanoezyme based on inorganic nanoparticles. The combination of computer simulation and theoretical calculation has solved the possible catalytic mechanism of these nano-enzymes. CeO2-NPs is a typical nano-enzyme, the Ce3+ to Ce4+ valence conversion of which is analogous to various redox enzymes’ mechanisms and can also catalyze the reversible redox in cells and tissues.11 The Ce4+ and low surface vacancy formation are essential for oxidation, while the Ce3+ and electron reshuffling in lattice oxygen vacancy provides the impetus for reduction. The specific catalytic mechanisms of the simulated enzymes are partly precise, such as automatic recovery after redox and substrate binding, but some are still under study.12,13 The ability to mimic the activity of multiple enzymes provides excellent convenience for biomedical applications that are primarily dependent on redox activity, such as anti-inflammation, anti-bacteria, angiogenesis, and others.
Excellent catalyst performance distinguishes CeO2-NPs’ multipurpose industrial application to photochemistry and electrochemistry, such as solid oxide battery,14 degradation of organic pollutants,15 high-performance catalyst,16 sensors,17 abrasive particles,18 coating material,19 and others. The application of CeO2-NPs to bioinformatics and computational biology has been receiving increasing attention. CeO2-NPs have played an essential role in tissue engineering and regenerative medicine, especially for orthopedic medical treatment,20 for their prospective oxidation resistance, antibacterial property, anti-inflammation, cancer resistance, nontoxicity, angiogenesis, drug/gene delivery, and others.21,22
CeO2-NPs’ Properties and Synthesis
CeO2-NPs’ Properties
The application of CeO2-NPs to nano-biomedical technology has been increasingly conventional. The increasing research on their atomic lattice model, lattice parameter, surface oxygen vacancy, and others by electron microscope and microscope provide the experimental basis for their robust catalytic mechanism.23,24 Huang et al25 further discovered the highest reactivity on the surface of CeO2 (100) through transmission electron microscopy and first-principles calculations. After the investigation of the electronic nano-structure of CeO2 and associated catalytic complexes through density functional theory (DFT), Bruix and Neyman8 elaborated on the reasons why CeO2-NPs in specific size represent a higher reactivity and the interaction between nanostructures and metal carriers. Size also affects CeO2-NPs’ enhanced electronic conductivity, pressure-induced phase transformation, size-induced lattice relaxation, and blue shift in ultraviolet absorption spectra.9,26 The size will limit or enhance the uptake of CEO2-NPs by cells, and affect the biological parameters such as biological half-life, diffusivity, immunogenicity, and others.27,28 Besides, size also affects the toxicity of CEO2-NPs in vivo and internal environment.29 Except for catalytic performance, for, the recommendation is to read on for more specific material physics and defect chemistry of ceria.9,30
In nanomedicine, inorganic enzyme mimic nanomaterials have become the latest research focus. Besides mimicking the structure and function of natural enzymes, enzyme mimic nanomaterials are more stable, more controllable, and more natural to prepare at a lower cost. CeO2-NPs have become one of the research proprieties because of their multiple enzyme activities,31,32 such as mimetic activities of superoxide dismutase,33 catalase,7 phosphatase,34,35 peroxidase,36 oxidase,37 and others. Although studies have illustrated that the mimic activities of phosphatase and catalase follow different chemical methods and involve different active sites,38 the more complex mechanisms warrant further study. Surprisingly, superoxide dismutase- and catalase-mimetic activities serve as the two primary methods to eliminate reactive oxygen species (ROS). Proverbially, ROS is the initiator of oxidative stress in many diseases. The adaption of various enzymes’ properties to 3D biomaterials in the treatment system achieves CeO2-NPs’ antibacterial, anti-inflammatory, anticancer effect directly or indirectly.21,39
CeO2-NPs’ Synthesis
The temperature, reactants’ concentration, pH, reaction environments, and stabilizers in the synthesis of CeO2-NPs affect CeO2-NPs’ physicochemical and biological properties.40 A well-designed synthetic method can fine-tune CeO2-NPs’ surface properties. Traditional chemical synthesis and maturing green synthesis are the primary synthetic methods for CeO2-NPs. General views believe that the green synthesis without the requirement for severe reaction conditions of high temperature and high pressure is more propitious to biological applications for avoiding potential chemical toxicity and maintaining higher biocompatibility.41 However, chemical synthesis’s advantages suggest it shall continue befittingly, for instance, the increasingly maturing ability to control the reaction conditions and reactant properties, achievable mass production, and diminishable chemical toxicity by altering end-capping reagent and other measures.42 A large number of recent research on synthetic methods for CeO2-NPs have emerged.21,43 The review recapitulated some of the recently reported synthetic approaches related to or potential for biomedical applications. The strong recommendation is to read on for synthetic methods and more detailed classifications.
Precipitation Method
Precipitation proves the easiest and most widely used synthetic method CeO2-NPs. The common precursor is cerium nitrate hexahydrate and the reaction environment is alkaline (seen in Table 1).44–48 Other precursors and capping agents are still in research and development.
 |
Table 1 CeO2-NPs Synthesized by Precipitation Method for Biological Applications |
Hydrothermal Method
The hydrothermal method is a common synthetic method for CeO2-NPs by heating water as the solvent in the autoclave. The synthetic process of the hydrothermal method can complete by the mediation of the surface-active agent. The hydrothermal method can produce multiple forms of CeO2-NPs. The solvents, stabilizers, and synthesis condition can influence CeO2-NPs’ biomedical-related performances (seen in Table 2).7,49–52
 |
Table 2 CeO2-NPs Synthesized by Hydrothermal Method for Biological Applications |
Green Synthesis
Green synthesis for CeO2-NPs has received increasing focus. Except for the elimination of the adverse effects of agents and chemical methods on the synthetic environment, green synthesis is not harsh on the reaction conditions, which is popular with the biological application, especially when the biocompatibility is enhanced. Nutrients, fungi, plants, bacteria, and biopolymers are the known material able to mediate the synthesis of CeO2-NPs (Seen in Table 3).27,43,53–59 For instance, primary and secondary metabolites in plant extracts can serve as capping or stabilizing agents.60 Most natural CeO2-NPs represent antioxidant, antibacterial, photocatalytic activity.61–64 When the reactants for synthesis combine with biocompatibility materials or other materials beneficial to biological applications will achieve better synthesizing effect,65–67 such as biological sensing property and others.
 |
Table 3 CeO2-NPs Synthesized by Green Synthesis for Biological Applications |
Alternate Methods of Synthesis
The synthetic methods determine the configuration, physical and chemical properties, surface groups, zeta potential, and others, thus determining the application behavior and therapeutic effect of CeO2-NPs. Recently, the solvothermal method, a microwave-mediated end-capping of alcohols such as ethylene glycol, could synthesize CeO2-NPs co-doped with Co2+ and La 3+, which presented a certain weak magnetism, different shapes, and controllable sizes.68,69 The study on the application of magnetic nanoparticles to the drug delivery system started early.70 Alzheimer’s disease model has witnessed the application of magnetic CeO2-NPs constitution to the magnetic separation of amyloid-beta (A-beta) peptides; Moreover, the magnetic CeO2-NPs constitution could enhance the magnetic resonance imaging (MRI) in the treatment for cerebral hemorrhage.71,72 This review believes that CeO2-NPs co-doped with Co2+ and La 3+ has potential in the future magnetic drug delivery field.
Spray coating has also recently been applied to the synthetic CeO2-NPs in cellular biology. Vassie et al73 first investigated the effect of particle size on uptake and intracellular transport of CeO2-NPs (d = 7 and 94 nm) synthesized by flame spray pyrolysis in human cancer cells. The findings demonstrated that larger CeO2-NPs presented a more robust clearance of intracellular ROS than the smaller ones, and the longer the therapy went, the more clearance of intracellular ROS. Simultaneously, folic acid-functionalized CeO2-NPs showed a greater regulation on ROS than the control group of CeO2-NPs in colon cancer cells.74 However, CeO2-NPs induced ROS in ovarian carcinoma cells, probably because of the increasing uptake of CeO2-NPs by ovarian carcinoma cells. The experimental results imply a prospect in the application of CeO2-NPs to anticarcinogen delivery.
The synthesis of CeO2-NPs via the sol-gel process is one of the classic popular methods. The microemulsion of reverse micelles acts as one of the generic assistive technologies in the sol-gel process, which is easy to control the superficial area, form, and other properties of nanoparticles. Torres-Romero et al synthesized Titania-Ceria composite at different sizes through titanium butoxide and cerium nitrate hexahydrate as the precursors.75 Three years later, Torres-Romero et al76 indicated that the Titania-Ceria composite presented excellent biocompatibility and delivery efficiency in the drug delivery systems (DDSs) with daunorubicin (DNR) against cancer. Another exciting piece of research demonstrated that CeO2-NPs synthesized by the sol-gel process reduced brain edema, microglia/macrophage recruitment around the hemorrhagic lesion, and inflammatory protein expression after the intravenous injection into mouse models of cerebral hemorrhage.77
Reverse-Phase has been applied in the first study in which liposomes acted as the carrier for cerium oxide nanoparticles.78 Such a system has the advantages of liposome targeting, protection from protein scavenging, static stability, as well as CeO2-NPs catalytic activity, and potent antioxidant capacity. Besides, the system presented excellent biocompatibility, tolerance, and uptake efficiency in fibroblasts. Except for the above methods, oxidation, ball grinding, thermal decomposition, acoustic chemistry, and others are outside the scope of this review for their little value in the biological application.
Orthopedic Biomedical Applications
Bone defects caused by congenital deformity, natural disasters, traffic accidents, and others are common orthopedic disease in clinical medicine. Traditional treatment methods, including autograft and allograft, have presented limitations.79 The development of new bone graft substitutes has been a hot research topic. Bone tissue engineering (BTE) provides a new solution to the above limitations.80 The selection of BTE materials is significant because the required properties are numerous and complex. The acknowledged properties do cover osteoconductivity, biocompatibility, degradability, mechanical properties, pore structure, and processability.81
Moreover, traditional treatment methods require regulatory capacity on the shape, imaging, infection, healing, immune response, and others.82 At present, the composite materials used for traditional treatment primarily include medical metal, bioceramics, and biopolymer materials.83 CeO2-NPs have begun to look for a favorable position in these materials. Further exploration has gone into CeO2-NPs’ potential in stem cells, scaffold materials, and growth factors, namely the three significant elements of BTE. The potentials cover CeO2-NPs’ capacity, properties to enhance or resist other material. Synchronously, as a component of the nano-drug delivery system, CeO2-NPs will act as a pro-oxidant or antioxidant according to different environments in the complex extracellular environment of cancer cells, to induce apoptosis of cancer cells by relying on oxidative stress.84,85 CeO2-NPs have also involved in the treatment of osteosarcoma. Hence, this review designs to summarize the primary data of CeO2-NPs in orthopedics.
Osteosarcoma
Anticancer therapy has not witnessed a broad application of CeO2-NPs. Interestingly enough, CeO2-NPs can exhibit antioxidant activity or oxidizing agents from different pH levels of subcellular localization.86 For example, in Colorectal Carcinoma Cells, CeO2-NPs can induce DNA fragmentation by increasing the production of ROS, resulting in cellular apoptosis through the p53-dependent mitochondrial signaling pathway.84 Yazici et al87,88 discovered positive effects to varying degrees on osteosarcoma when they studied the cytotoxicity of 0.1 M and 0.01 M dextran-coated CeO2-NPs based on dose and time dependence. After that, the results found that at pH 6.0, CeO2-NPs were the most damaging to bone cancer cells and the least damaging to healthy bone cells.89 Besides ROS and other mechanisms related to redox reactions, activated Cytotoxic CD8+ T cells (CTLs) treated by CeO2-NPs released more effector molecules and cytokines, including interleukin-2 (IL-2) and tumor necrosis factor-α (TNF-α), granzyme B and perforin, which could lead to better immunotherapy for cancer.90 The above research attested CeO2-NPs a type of promising nanoparticles for bone cancer treatment. Surface functionalization of CeO2-NPs and tumor microenvironment will affect the activity of antioxidants or pro-oxidant. Cell type or cell microenvironment may also pose different effects on cytotoxicity. However, the specific influencing mechanism needs further exploration.
Recombination with Hydroxyapatite (HA)
Hydroxyapatite (HA), the natural form of calcium apatite, is the primary mineral component of bones and teeth. HA can bind to tissue at the interface by chemical bonds to release ions and participate in metabolism.91 What is more remarkable, the new bone will regenerate along the surface of the direct bone-to-implant contact where HA serves as the implant.92 HA has found a broad application in the clinical and experimental field for biocompatibility, nontoxicity, and osteoconductivity. However, HA’s mechanical modulus and fracture toughness are not ideal.93 The two primary recombinations between CeO2 and HA are HA-based scaffolds, and surface coatings for other bone implants, namely the composite coating of CeO2 and HA.
Pandey et al have made a further study on hydroxyapatite reinforced with ceria and silver (HA-CeO2-Ag). After obtaining hydroxyapatite with 5 wt% CeO2 NPs and 2.5 wt% Ag NPs (HA-5C-2.5Ag) by spark plasma sintering (SPS), they first tried to make up the low mechanical and tribological properties of HA alone by HA-CeO2-Ag.94 Fretting and scratch tests testified the protective tribofilm and oxide protection in CeO2/Ag reinforced hydroxyapatite. HA-CeO2-Ag restricted the tribological damage effectively over multi-length scales. They further improved the tests on the bactericidal activity, inoxidizability, and bioactivity of HA-CeO2-Ag.95 The results showed that the count of human osteoblasts (hFOBs) in the experimental group increased by 6.7 times compared to the control group. The filopodial extensions (60–150 μm) and matrix-like deposition reflected the cell-substrate intimacy. The analysis believed that increased protein hydrophobicity might enhance the absorptivity of HA-CeO2-Ag to cells. They also believed that HA-CeO2-Ag could act as not only the independent porous scaffolds, surgically for internal fixation, to be a reliable substrate with effective load-bearing capacity in orthopedic applications, but also a type of antimicrobial bioactive coatings on the femur stem (during implant manufacturing) for total hip arthroplasty.
Li et al96–98 prepared CeO2-HA composite coatings by plasma spraying technique and conducted considerable research to testify the coatings’ application. Because of the antioxygenic property of CeO2, the increase of CeO2 content in the coatings can improve the cell viability and reduce the cell apoptosis, but decrease the chemical stability slightly. The up-regulation of Wnt/β-catenin signal transduction can better protect BMSC from H2O2-induced damage in osteoblasts differentiation. Besides, CeO2-HA composite coatings can protect H2O2-induced BMSC from generating osteoclasts, which is in reflection by the increased OPG/RANKL ratio. The above research results provide a theoretical basis for the material in osteoporosis bone regeneration.96 In the study on the inflammatory response, the results found that the increase of CeO2 content in HA coatings enhanced the osteogenic activity of BMSC through the Smad-dependent-BMP signaling pathway.97 The addition of CeO2 also endowed HA coatings with anti-inflammatory effect. HA-30Ce, by inducing a drift towards an M2 phenotype, presented an ideal effect on macrophage polarization. Such results suggested that the composite coatings had osteogenic and anti-inflammatory properties. Except for the above experiments, a group result found that a higher Ce4+ concentration up-regulated the expression of anti-inflammatory cytokines (IL-10 and IL-1RA) and osteoinductive molecules (BMP2 and TGF-1) by macrophages, implying that the regulation of cerium valence might be a valuable strategy for improving osteogenic properties and reducing inflammatory responses.99
Another application is that the CeO2 and HA composites attach to other materials with specific mechanical stability, such as AZ91 Mg alloy.100 Researchers developed manganese (Mn), and strontium (Sr) substituted hydroxyapatite (Mn, Sr-HAP) coatings on the CeO2 coated AZ91 Mg alloy, which enhanced the corrosion resistance of the whole material to facilitate the clinical application of AZ91 Mg alloy. Sanyal et al101 prepared ceria-stabilized zirconia (CSZ) in fluorohydroxyapatite (FHA) by the sol-gel method. As a hard material, CSZ has received verification on toughness and osteoconduction. Besides, the composite of HA-CNT- CeO2 -Ag, namely plasma-sprayed HA-coated Ti-6A1-4V,102 is 2.3 times, 1.6 times, and 3.1 times enhanced Vickers hardness, estimated modulus, and fracture toughness, respectively, than HA alone. Meanwhile, the composite of HA-CNT-CeO2-Ag also presented cell adhesion, bactericidal activity, and others. Similar effects were also in a composite of cerium-doped glass-reinforced hydroxyapatite (GR-HA).103
Ceria Doped in Mesoporous Bioactive Glasses
Mesoporous bioactive glasses (MBG) based on SiO2-CaO-P2O5 composition were prepared in 2004 by combining the sol-gel method and supramolecular chemistry method.104 Subsequently, Shruti et al105 synthesized mesoporous bioactive glass scaffolds (MBG_Scs), based on 80% SiO2-15% CaO-5% P2O5 (in molar ratio) mesoporous sol-gel glasses substituted with Ce2O3, Ga2O3, and ZnO. This composite contains super-pores suitable for vascularized interconnections for nutrient supply and normal cell growth, seen in Figure 2.
 |
Figure 2 Schematic representation of possible biological properties possessed by Ce3+, Ga3+, and Zn2+-substituted MBG_Scs prepared by rapid prototyping: 3-D printing. Notes: Reprinted from Acta Biomaterialia, 9(1), Shruti S, Salinas AJ, Lusvardi G, Malavasi G, Menabue L, Vallet-Regi M. Mesoporous bioactive scaffolds prepared with cerium-, gallium- and zinc-containing glasses. 4836–4844, Copyright 2013, with permission from Elsevier.105 |
Direct addition of ceria into HA will generate cerium phosphate, which affects the biocompatibility to some extent.106 Therefore, Nicolini et al107 dopped CeO2-NPs with Ce 3+ and Ce 4+ at different molar ratios into MBGs with 80%SiO 2–15%CaO-5% P2O5. These MBGs with a high superficial area would form HA after being immersed in simulated body fluid (SBF). The test illustrated that Ce-MGB could reduce catalase and superoxide dismutase mimic (SOD). The best catalase activity appeared in 45S5 bio-glass containing cerium, and the highest cerium content could achieve 5.3%. Both infrared spectroscopy and X-ray diffraction analysis verified the existence of HA in some type of Ce-MGB. The above tests, including bioactivity tests, indicated the feasibility of MBG with inoxidizability and synthetase mimic activity. Atkinson et al108 synthesized Ce-MBGs by evaporation-induced self-assembly (EISA) and conducted the above tests. They verified the biocompatibility by mouse fibroblasts. The antibacterial and biological activities of 50% SiO2-(45-x) % CaO-5% P2O5 MBG with molar content of 1, 5% CeO2-NPs have also been verified.109,110 Recently, 45S5 bio-glass with ceria has been developed with a polyhedral shape and large size, and the cellular uptake capacity, survivability, and proliferate ability have been demonstrated.111 CeO2-NPs doped in MBG with MgO could decrease the degradation rate and enhance chemical durability. Meanwhile, the tests verified that the “sol-gel” synthesis technique promoted hydroxyapatite growth rate over the conventional “melt quenching” route.106
The above experiments are the preliminary evidences for the application of CeO2-NPs doped in MBG to bone regeneration materials. Future research may focus on MBG based on 3D bioactive scaffolds and in vivo experiments. Recently, Lu et al112 constructed CeO2-NPs modified Ce-BG scaffolds using hollow Ce-BG microspheres with chitosan (CTS) via a freeze-drying procedure. The CeO2-NPs in the scaffolds could rapidly promote the proliferation and osteogenic differentiation of hBMSC, which was verified by the up-regulation of osteogenic markers of osteocalcin (OCN), alkaline phosphatase (ALP), type I collagen (COL-1), and others. The enhancement of Ce-MBG scaffolds’ osteoinduction primarily relates to activated ERK pathways and can be prevented by the addition of selective ERK1/2 inhibitors (SCH772984). In vivo experiments on rat skull defect models showed that Ce-MBG scaffolds could promote collagen deposition, osteoblastic formation, and bone regeneration compared with sole MBG scaffolds.
Stabilized Zirconium Oxide Coating
Metal-free dental zirconia implants have attracted much attention. Due to the excellent mechanical properties, stable physical and chemical properties, and excellent biocompatibility, metal-free dental zirconia implants can avoid the grey appearance of gums and potential hypersensitivity of titanium implants.113 The durability of traditional materials raises questions. Yttria stabilized zirconia (3Y-TZP) is aging or low-temperature degradation (LTD), which basically involves a phase transformation that leads to microcracking, resulting in catastrophic failures;114 High-purity alumina (Al2O3) is weak in toughness. Base material of novel zirconia is emerging to overcome the significant shortcomings of 3Y-TZP.115
At present, the mainstream uses tetragroconic zirconia polycrystal (CE-TZP) with stable cerium dioxide as the second phase to improve the toughness of alumina composites and finally forms the composite of CE-TZP/Al2O3. The macroscopic and microscopic mechanisms for increased toughness have been demonstrated.116 Scientists have previously attested that CE- CE-TZP/Al2O3 could promote HA formation and osteoblast proliferation and differentiation.117 The research compared the biomechanical and histological behaviors of Ce-TZP/Al2O3 and 3Y-TZP in rats.118 Ce-TZP/Al2O3 showed stronger shear strength but slightly lower average surface roughness. No significant difference appeared in the new bone thickness around the implant in the bone marrow region and bone-implant contact (BIC). Osteoclasts were not observed at any time in the experiment of CE-TZP/Al2O3, but in 3Y-TZP group, which indicated the better biocompatibility of Ce-TZP/Al2O3. The surface roughness could be treated with hydrofluoric acid, and the nanometer morphology could significantly enhance bone formation and bone integration in vivo.119 In addition to rats, the study in dogs also proved CE-TZP/Al2O3 an excellent dental implant, including excellent bone resorption and soft tissue attachment.120,121
Based on CE-TZP/Al2O3 to continue to solve hydrothermal aging, Altmann et al developed a new type of stable zirconia-alumina-aluminate composite ceramics (ZA 8 Sr 8-Ce11). The result found the most conspicuous long-term attachment of primary osteoblasts, and mineralized deposition of extracellular matrix (ECM). ZA8 Sr8-Ce11 with microporous morphology is one of the best materials for clinical application.122 The 3D-printed Ce-TZP/Al2O3 showed compression strength similar to that of leather bone, almost 200 MPa. Besides, the viability and differentiation ability of cultured cells are also powerful. In general, such composites present excellent aesthetic properties, chemical stability, and negligible corrosion and abrasion, as well as excellent mechanical and biological properties.
Cerium Oxide Coating for Titanium-Based Implants
Titanium and its alloys have found a broad application in orthopedic and dental implants.123 The three critical factors for long-term clinical success of implants are antimicrobial, anti-inflammatory, and stability of osseointegration, which have been addressed in many ways.124,125 CeO2-NPs would undoubtedly be a suitable potential material. Li et al126 developed a novel Ti surface modified with different shapes of CeO2-NPs (nanorod, nanocube, and nano-octahedron). They also tested the antimicrobial and anti-inflammatory responses of the composite of different CeO2-NPs deposited into Ti. The results showed that the three types of CeO2-modified Ti showed the same strong antibacterial properties. Nano-octahedron CeO2 modified Ti had the best anti-inflammatory effect (Figure 3). Zhao et al127 deposited TiO2 coating doped with different percentages of CeO2 on the cp-TI substrate through APS. The results demonstrated that the dose dependence of CeO2 determined the corrosion resistance, cellular compatibility, and antibacterial properties. Less than 20% of doping would not affect the crystal structure.
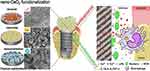 |
Figure 3 Schematic illustration of implant surface modified by CeO2-NPs (rod- CeO2, cube-CeO2, octa-CeO2) for antibacterial and anti-inflammatory properties.Notes: Reprinted from Acta Biomaterialia, 94, Li X, Qi M, Sun X, et al, Surface treatments on titanium implants via nanostructured ceria for antibacterial and anti-inflammatory capabilities. 627–643. Copyright 2019, with permission from Elsevier.126 |
As with the recombination with HA, the effect of CeO2-NPs as a coating for titanium-based implants on the cerium valence state was also investigated. The results showed significantly up-regulated expression of osteogenic genes and proteins at a high Ce4+ concentration, and high expression of the polarization of macrophages to the M2 phenotype. The increase in M2 percentage could increase the production of anti-inflammatory cytokines.128 Also, the high Ce4+ presented a higher catalase activity, but a lower peroxidase activity. Results of protein adsorption and conformation indicated that the exposed cell-binding sites of fibronectin and subsequent cell morphology were associated with the Ce valence state.80 Overall, regulation on cerium valence may be a good strategy for designing orthopedic/dental implant coatings with beneficial immune responses.
Other Applications
To further understand the biomaterial potential of CeO2, Ball et al129 fabricated porous ceria via direct foaming. The tests of cytotoxicity, inflammatory response, and reactive oxygen species found CeO2 similar to commercially available bio-glass. Another study found that cultured HMSCs increased osteogenic differentiation and collagen production when CeO2-NPs were doped to 3D nanocomposite scaffolds.130 Evidence suggested that ceria promoted the migration of bone marrow stromal cells and osteogenic differentiation through the Smad1/5/8 signaling pathway.131 An essential finding illustrated that CeO2-NPs could induce stem cells’ growth in PLGA scaffolds. The formed PLGA/nano-CeO2-NPs scaffolds could regulate roughness, thus improving cells’ sensitivity to host surface characteristics.132
Insufficient angiogenesis hinders the clinical application of bone tissue engineering materials. The current mainstream solution is that bone tissue materials carry endogenous angiogenic factors to promote the proliferation, migration, differentiation, and angiogenesis of endothelial cells (EC) and/or endothelial progenitor cells (EPC).133,134 Nethi et al135 demonstrated the angiogenic property of functional nanoconjugates of organosilane functionalized CeO2-NPs (nanospheres), and suggested that the expression of p38 MAPK/HIF-1α may be a reasonable signal transduction mechanism for its angiogenic property. In another research, CNPs accelerated the process of endochondral ossification by promoting sufficient hypertrophic differentiation of BMSCs via activation of the DHX15–p38 MAPK signaling pathway, which could better overcome the lack of vascularization and relevant hypoxia at the initial stage of implantation.136 CeO2-NPs can also activate calcium channels in mesenchymal stem cells and ultimately lead to EPC proliferation, migration, and differentiation through chain reaction137 (seen in Figure 4).
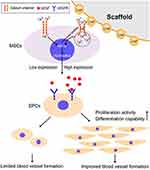 |
Figure 4 Scheme illustrates the mechanism of CNPs enhancing the blood vessel formation of EPCs. Notes: Reprinted with permission from Xiang J, Li J, He J, et al Cerium Oxide Nanoparticle Modified Scaffold Interface Enhances Vascularization of Bone Grafts by Activating Calcium Channel of Mesenchymal Stem Cells. ACS Applied Materials & Interfaces. 2016;8(7):4489–4499. Copyright © 2016 American Chemical Society.137 |
Challenges
The research on CeO2-NPs as a nanozyme based on inorganic nanoparticles is just unfolding. At present, the activity and specificity of nano-enzyme are still lower than that of a natural enzyme, and the influence of pH value is crucial.31,32 Significantly, in vivo biocompatibility is assessed by protein corona, and the presence of hard and soft protein corona will affect the bioactive interface, including usability, stability, and ecotoxicity. Haptens formed by adsorptive proteins also caused abnormalities in immune homeostasis.138 Antioxidant and pro-oxidant conversion is a double-edged sword, which needs total control on the external environmental conditions to ensure that the conversion can achieve the desired effect rather than the opposite effect. For instance, the pro-angiogenic and anti-angiogenic characteristics of CeO2-NPs are affected by the microenvironmental parameters, including pH, production of reactive oxygen species, and intracellular oxygen concentration.134 The same conversion issues that have led to conflicting toxicological reports pose challenges for future regulatory and environmental risk assessments for CeO2-NPs applications.29 Besides, Ce3+ enhances the expression and activity of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 1 (Nox1) in bone metabolism, which increases ROS levels, thus activating the RANKL-dependent osteoclast differentiation pathway and generating osteoclasts, which may cause abnormal bone resorption.139 The latest study showed that the genotoxicity of CeO2-NPs was a function of concentration and particle diameter in vitro.140 While in vivo experiments showed that short-term exposure of rats to uncoated CeO2-NPs could induce pulmonary inflammation and non-dose-dependent DNA damage,141 which increased the difficulty in modification and synthesis. In conclusion, the establishment of long-term clinical safety and ecological environmental safety assessment still needs a long process of research.
Discussion
CeO2-NPs has found a broad potential in the biomedical field. This review discussed the latest developments of CeO2-NPs’ orthopedic biomedical applications. The green synthetic method using biocompatible stabilizers grows in importance in the production of CeO2-NPs and its orthopedic biomedical applications. The surface chemistry, particle diameter, physical and chemical properties of CeO2-NPs need reasonable control. CeO2-NPs doped in the substitutes of metallic elements, like hot materials of graphene, PLGA, and others, are making progress in the application to bone implant materials. Besides, in terms of imaging, such as reducing the harmful effects of Gd, enhancing the contrast of MRI is very attractive;28 Regarding drug carrier, CeO2-NPs are applied as carrier encapsulated in liposome or pegylated to combine with other materials; magnetic nanoconjugate delivery system with core-shell structure is in research.27,142 In clinical application, the ability of CeO2-NPs to promote angiogenesis and development turns out critical. Despite the challenges mentioned above, it is expected that in the future, CeO2-NPs will overcome the limitations to work well in 3D tissue-engineered materials and flourish in interdisciplinary nanomedicine.
Acknowledgments
We would like to thank all authors for their help in writing and revising the manuscript. The present work was funded by Jilin Province Science and Technology Development Plan Project, 20180101308JC.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Smiles DE, Batista ER, Booth CH, et al. The duality of electron localization and covalency in lanthanide and actinide metallocenes. Chem Sci. 2020;11(10):2796–2809. doi:10.1039/C9SC06114B
2. Bazhukova IN, Sokovnin SY, Ilves VG, et al. Luminescence and optical properties of cerium oxide nanoparticles. Opt Mater (Amst). 2019;92:136–142. doi:10.1016/j.optmat.2019.04.021
3. Naderi H, Sobati H, Sobhani-Nasab A, et al. Synthesis and supercapacitor application of cerium tungstate nanostructure. Chemistryselect. 2019;4(10):2862–2867. doi:10.1002/slct.201803753
4. Jung HK, Kim CH, Hong AR, et al. Luminescent and magnetic properties of cerium-doped yttrium aluminum garnet and yttrium iron garnet composites. Ceram Int. 2019;45(8):9846–9851. doi:10.1016/j.ceramint.2019.02.023
5. Bhuvanesh N, Suresh S, Velmurugan K, Thamilselvan A, Nandhakumar R. Quinoline based probes: large blue shifted fluorescent and electrochemical sensing of cerium ion and its biological applications. J Photochem Photobiol A-Chem. 2020;386:112103. doi:10.1016/j.jphotochem.2019.112103
6. Mohd Fadzil NA, Ab Rahim MH, Pragas Maniam G. Brief review of ceria and modified ceria: synthesis and application. Mater Res Express. 2018;5(8):085019. doi:10.1088/2053-1591/aad2b5
7. Wu L, Liu G, Wang W, et al. Cyclodextrin-modified CeO2 nanoparticles as a multifunctional nanozyme for combinational therapy of psoriasis. Int J Nanomedicine. 2020;15:2515–2527. doi:10.2147/IJN.S246783
8. Bruix A, Neyman KM. Modeling ceria-based nanomaterials for catalysis and related applications. Catal Letters. 2016;146(10):2053–2080. doi:10.1007/s10562-016-1799-1
9. Sun C, Li H, Chen L. Nanostructured ceria-based materials: synthesis, properties, and applications. Energy Environ Sci. 2012;5(9):8475–8505. doi:10.1039/c2ee22310d
10. Deshpande S, Patil S, Kuchibhatla SVNT, Seal S. Size dependency variation in lattice parameter and valency states in nanocrystalline cerium oxide. Appl Phys Lett. 2005;87(13):133113. doi:10.1063/1.2061873
11. Celardo I, Pedersen JZ, Traversa E, Ghibelli L. Pharmacological potential of cerium oxide nanoparticles. Nanoscale. 2011;3(4):1411–1420. doi:10.1039/c0nr00875c
12. Wei H, Wang E. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes. Chem Soc Rev. 2013;42(14):6060–6093. doi:10.1039/c3cs35486e
13. Pirmohamed T, Dowding JM, Singh S, et al. Nanoceria exhibit redox state-dependent catalase mimetic activity. Chem Commun. 2010;46(16):2736–2738. doi:10.1039/b922024k
14. Vibhu V, Flura A, Rougier A, et al. Electrochemical ageing study of mixed lanthanum/praseodymium nickelates La2-xPrxNiO4+delta as oxygen electrodes for solid oxide fuel or electrolysis cells. J Energy Chem. 2020;46:62–70. doi:10.1016/j.jechem.2019.10.012
15. Khan SA, Khan N, Irum U, et al. Cellulose acetate-Ce/Zr@Cu-0 catalyst for the degradation of organic pollutant. Int J Biol Macromol. 2020;153:806–816. doi:10.1016/j.ijbiomac.2020.03.013
16. Li S, Zhang Y, Wang Z, Du W, Zhu G. Morphological Effect of CeO2 catalysts on their catalytic performance in lean methane combustion. Chem Lett. 2020;49(5):461–464. doi:10.1246/cl.200049
17. Zito CA, Perfecto TM, Dippel A-C, Volanti DP, Koziej D. Low-temperature carbon dioxide gas sensor based on yolk-shell ceria nanospheres. ACS Appl Mater Interfaces. 2020;12(15):17757–17763. doi:10.1021/acsami.0c01641
18. Chen Y, Mu Z, Wang W, Chen A. Development of mesoporous SiO2/CeO2 core/shell nanoparticles with tunable structures for non-damage and efficient polishing. Ceram Int. 2020;46(4):4670–4678. doi:10.1016/j.ceramint.2019.10.198
19. Wen J, Song C, Liu TK, et al. Fabrication of dense gadolinia-doped ceria coatings via very-low-pressure plasma spray and plasma spray-physical vapor deposition process. Coatings. 2019;9(11):7. doi:10.3390/coatings9110717
20. Xu C, Qu X. Cerium oxide nanoparticle: a remarkably versatile rare earth nanomaterial for biological applications. NPG Asia Mater. 2014;6(3):e90. doi:10.1038/am.2013.88
21. Thakur N, Manna P, Das J. Synthesis and biomedical applications of nanoceria, a redox active nanoparticle. J Nanobiotechnology. 2019;17(1):84. doi:10.1186/s12951-019-0516-9
22. Kargozar S, Baino F, Hoseini SJ, et al. Biomedical applications of nanoceria: new roles for an old player. Nanomedicine. 2018;13(23):3051–3069. doi:10.2217/nnm-2018-0189
23. Sinitsyn AV, Gracheva ME. Atomistic model of a ceria nanoparticle with Ce3+ and Ce4+ atoms. Nanotechnology. 2020;31(31):315708. doi:10.1088/1361-6528/ab87c8
24. Passacantando M, Santucci S. Surface electronic and structural properties of CeO2 nanoparticles: a study by core-level photoemission and peak diffraction. J Nanopart Res. 2013;15(8):1785. doi:10.1007/s11051-013-1785-0
25. Huang J, Yu Y, Zhu J, Yu R. Oxygen adatoms and vacancies on the (110) surface of CeO2. Sci China Technol Sci. 2018;61(1):135–139. doi:10.1007/s11431-017-9154-9
26. Castano CE, O’Keefe MJ, Fahrenholtz WG. Cerium-based oxide coatings. Curr Opin Solid State Mater Sci. 2015;19(2):69–76. doi:10.1016/j.cossms.2014.11.005
27. Singh S, Ly A, Das S, Sakthivel TS, Barkam S, Seal S. Cerium oxide nanoparticles at the nano-bio interface: size-dependent cellular uptake. Artif Cells Nanomed Biotechnol. 2018;46(sup3):S956–S963. doi:10.1080/21691401.2018.1521818
28. Eriksson P, Tal AA, Skallberg A, et al. Cerium oxide nanoparticles with antioxidant capabilities and gadolinium integration for MRI contrast enhancement. Sci Rep. 2018;8(1):6999. doi:10.1038/s41598-018-25390-z
29. Dahle JT, Arai Y. Environmental geochemistry of cerium: applications and toxicology of cerium oxide nanoparticles. Int J Environ Res Public Health. 2015;12(2):1253–1278. doi:10.3390/ijerph120201253
30. Wu K, Sun L-D, Yan C-H. Recent progress in well-controlled synthesis of ceria-based nanocatalysts towards enhanced catalytic performance. Adv Energy Mater. 2016;6(17):1600501. doi:10.1002/aenm.201600501
31. Ragg R, Tahir MN, Tremel W. Solids go bio: inorganic nanoparticles as enzyme mimics. Eur J Inorg Chem. 2016;2016(13‐14):1906–1915. doi:10.1002/ejic.201501237
32. Kang T, Kim YG, Kim D, Hyeon T. Inorganic nanoparticles with enzyme-mimetic activities for biomedical applications. Coord Chem Rev. 2020;403:213092. doi:10.1016/j.ccr.2019.213092
33. Sozarukova MM, Shestakova MA, Teplonogova MA, Izmailov DY, Proskurnina EV, Ivanov VK. Quantification of free radical scavenging properties and SOD-like activity of cerium dioxide nanoparticles in biochemical models. Russ J Inorg Chem. 2020;65(4):597–605. doi:10.1134/S0036023620040208
34. Yao T, Tian Z, Zhang Y, Qu Y. Phosphatase-like activity of porous nanorods of CeO2 for the highly stabilized dephosphorylation under interferences. ACS Appl Mater Interfaces. 2019;11(1):195–201. doi:10.1021/acsami.8b17086
35. Liu H, Liu J. Self-limited Phosphatase-mimicking CeO2 Nanozymes. Chemnanomat. 2020.
36. Yang W, Li J, Yang J, et al. Biomass-derived hierarchically porous CoFe-LDH/CeO2 hybrid with peroxidase-like activity for colorimetric sensing of H2O2 and glucose. J Alloys Compd. 2020;815:152276. doi:10.1016/j.jallcom.2019.152276
37. Estevez AY, Ganesana M, Trentini JF, et al. Antioxidant enzyme-mimetic activity and neuroprotective effects of cerium oxide nanoparticles stabilized with various ratios of citric acid and EDTA. Biomolecules. 2019;9(10):562. doi:10.3390/biom9100562
38. Dhall A, Burns A, Dowding J, Das S, Seal S, Self W. Characterizing the phosphatase mimetic activity of cerium oxide nanoparticles and distinguishing its active site from that for catalase mimetic activity using anionic inhibitors. Environ Sci Nano. 2017;4(8):1742–1749.
39. Jeong H-G, Cha BG, Kang D-W, et al. Ceria nanoparticles fabricated with 6-aminohexanoic acid that overcome systemic inflammatory response syndrome. Adv Healthcare Mater. 2019;8(9):1801548. doi:10.1002/adhm.201801548
40. Dhall A, Self W. Cerium oxide nanoparticles: a brief review of their synthesis methods and biomedical applications. Antioxidants. 2018;7(8):97. doi:10.3390/antiox7080097
41. Charbgoo F, Bin Ahmad M, Darroudi M. Cerium oxide nanoparticles: green synthesis and biological applications. Int J Nanomedicine. 2017;12:1401–1413. doi:10.2147/IJN.S124855
42. Ge J, Zhong L, Zhuo L, Tang B, Song W. Hierarchically nanoporous ceria nanoparticles with a high-surface area: synthesis, characterization, and their catalytic activity. J Nanosci Nanotechnol. 2011;11(1):125–130. doi:10.1166/jnn.2011.2999
43. Nyoka M, Choonara YE, Kumar P, Kondiah PPD, Pillay V. Synthesis of cerium oxide nanoparticles using various methods: implications for biomedical applications. Nanomaterials (Basel). 2020;10(2):242. doi:10.3390/nano10020242
44. Morlando A, Chaki Borrás M, Rehman Y, et al. Development of CeO2 nanodot encrusted TiO2 nanoparticles with reduced photocatalytic activity and increased biocompatibility towards a human keratinocyte cell line. J Mater Chem B. 2020;8(18):4016–4028. doi:10.1039/D0TB00629G
45. Lin Y-H, Shen L-J, Chou T-H, Shih Y-H. Synthesis, stability, and cytotoxicity of novel cerium oxide nanoparticles for biomedical applications. J Cluster Sci. 2020. doi:10.1007/s10876-020-01798-4
46. Jyothi PSP, Anitha B, Smitha S, Vibitha BV, Krishna PGA, Tharayil NJ. DNA-assisted synthesis of nanoceria, its size dependent structural and optical properties for optoelectronic applications. Bull Mater Sci. 2020;43(1):119. doi:10.1007/s12034-020-02102-w
47. Zhao X, Suo H, Zhang Z, Guo C. Upconverting CeO2: yb3+/Tm3+ hollow nanospheres for photo-thermal sterilization and deep-tissue imaging in the first biological window. Ceram Int. 2019;45(17, Part A):21910–21916. doi:10.1016/j.ceramint.2019.07.202
48. Rahdar A, Aliahmad M, Hajinezhad MR, Samani M. Xanthan gum-stabilized nano-ceria: green chemistry based synthesis, characterization, study of biochemical alterations induced by intraperitoneal doses of nanoparticles in rat. J Mol Struct. 2018;1173:166–172. doi:10.1016/j.molstruc.2018.06.092
49. Pujar MS, Hunagund SM, Barretto DA, et al. Synthesis of cerium-oxide NPs and their surface morphology effect on biological activities. Bull Mater Sci. 2020;43(1). doi:10.1007/s12034-019-1962-6.
50. Balaji S, Mandal BK, Vinod Kumar Reddy L, Sen D. Biogenic ceria nanoparticles (CeO2 NPs) for effective photocatalytic and cytotoxic activity. Bioengineering. 2020;7(1):26. doi:10.3390/bioengineering7010026
51. Nithya P, Sundrarajan M. Ionic liquid functionalized biogenic synthesis of Ag-Au bimetal doped CeO2 nanoparticles from Justicia adhatoda for pharmaceutical applications: antibacterial and anti-cancer activities. J Photochem Photobiol B-Biol. 2020;202:111706. doi:10.1016/j.jphotobiol.2019.111706
52. Ahamed M, Akhtar MJ, Khan MAM, Alaizeri ZM, Alhadlaq HA. Evaluation of the cytotoxicity and oxidative stress response of CeO2-RGO nanocomposites in human lung epithelial A549 cells. Nanomaterials. 2019;9(12):1709. doi:10.3390/nano9121709
53. Ayodhya D, Veerabhadram G. Green synthesis of garlic extract stabilized Ag@CeO2 composites for photocatalytic and sonocatalytic degradation of mixed dyes and antimicrobial studies. J Mol Struct. 2020;1205:127611. doi:10.1016/j.molstruc.2019.127611
54. Miri A, Akbarpour Birjandi S, Sarani M. Survey of cytotoxic and UV protection effects of biosynthesized cerium oxide nanoparticles. J Biochem Mol Toxicol. 2020;34(6):e22475. doi:10.1002/jbt.22475
55. Kaygusuz H, Erim FB. Biopolymer-assisted green synthesis of functional cerium oxide nanoparticles. Chem Pap. 2020;74(7):2357–2363. doi:10.1007/s11696-020-01084-7
56. Miri A, Darroudi M, Sarani M. Biosynthesis of cerium oxide nanoparticles and its cytotoxicity survey against colon cancer cell line. Appl Organomet Chem. 2020;34(1). doi:10.1002/aoc.5308
57. Elahi B, Mirzaee M, Darroudi M, Sadri K, Oskuee RK. Bio-based synthesis of nano-ceria and evaluation of its bio-distribution and biological properties. Colloids Surf B Biointerfaces. 2019;181:830–836. doi:10.1016/j.colsurfb.2019.06.045
58. Kargar H, Ghazavi H, Darroudi M. Size-controlled and bio-directed synthesis of ceria nanopowders and their in vitro cytotoxicity effects. Ceram Int. 2015;41(3):4123–4128. doi:10.1016/j.ceramint.2014.11.108
59. Patil SN, Paradeshi JS, Chaudhari PB, Mishra SJ, Chaudhari BL. Bio-therapeutic potential and cytotoxicity assessment of pectin-mediated synthesized nanostructured cerium oxide. Appl Biochem Biotechnol. 2016;180(4):638–654. doi:10.1007/s12010-016-2121-9
60. Senthilkumar RP, Bhuvaneshwari V, Malayaman V, et al. Biogenic method of cerium oxide nanoparticles synthesis using wireweed (Sida acuta Burm.f.) and its antibacterial activity against Escherichia coli. Mater Res Express. 2019;6(10):105026. doi:10.1088/2053-1591/ab37b9
61. Fereydouni N, Sadeghnia HR, Mobarhan MG, et al. Nanoceria: polyphenol-based green synthesis, mechanism of formation, and evaluation of their cytotoxicity on L929 and HFFF2 cells. J Mol Struct. 2019;1186:23–30.
62. Gunasekaran S, Dinesh A, Silambarasu A, Thirumurugan V, Shankar S. Rare earth element (REE) Nd3+ doped CeO2 nanoparticles using aloe vera leaf extract: structural, optical and antimicrobial activity. J Nanosci Nanotechnol. 2019;19(7):3964–3970. doi:10.1166/jnn.2019.16307
63. Irshad MS, Aziz MH, Fatima M, et al. Green synthesis, cytotoxicity, antioxidant and photocatalytic activity of CeO2 nanoparticles mediated via orange peel extract (OPE). Mater Res Express. 2019;6(9):0950a4. doi:10.1088/2053-1591/ab3326
64. Sharmila G, Muthukumaran C, Saraswathi H, Sangeetha E, Soundarya S, Kumar NM. Green synthesis, characterization and biological activities of nanoceria. Ceram Int. 2019;45(9):12382–12386. doi:10.1016/j.ceramint.2019.03.164
65. Du X, Jiang D, Chen S, et al. CeO2 nanocrystallines ensemble-on-nitrogen-doped graphene nanocomposites: one-pot, rapid synthesis and excellent electrocatalytic activity for enzymatic biosensing. Biosens Bioelectron. 2017;89:681–688. doi:10.1016/j.bios.2015.11.054
66. Senthilkumar RP, Bhuvaneshwari V, Ranjithkumar R, Sathiyavimal S, Malayaman V, Chandarshekar B. Synthesis, characterization and antibacterial activity of hybrid chitosan-cerium oxide nanoparticles: as a bionanomaterials. Int J Biol Macromol. 2017;104:1746–1752. doi:10.1016/j.ijbiomac.2017.03.139
67. Shen C, Pang K, Du L, Luo G. Green synthesis and enhanced photocatalytic activity of Ce-doped TiO2 nanoparticles supported on porous glass. Particuology. 2017;34:103–109.
68. Yang Z, Yin Z, Zhao Z, et al. Morphologies and magnetic properties of La-doped CeO2 nanoparticles by the solvothermal method in a low magnetic field. Mater Chem Phys. 2020;240:122148. doi:10.1016/j.matchemphys.2019.122148
69. Yang Z, Zhao Z, Yu J, et al. Effect of Co substitution and magnetic field on the morphologies and magnetic properties of CeO2 nanoparticles. Ceram Int. 2019;45(9):11927–11933. doi:10.1016/j.ceramint.2019.03.081
70. Byrappa K, Ohara S, Adschiri T. Nanoparticles synthesis using supercritical fluid technology - towards biomedical applications. Adv Drug Deliv Rev. 2008;60(3):299–327. doi:10.1016/j.addr.2007.09.001
71. Cha BG, Jeong H-G, Kang D-W, et al. Customized lipid-coated magnetic mesoporous silica nanoparticle doped with ceria nanoparticles for theragnosis of intracerebral hemorrhage. Nano Res. 2018;11(7):3582–3592. doi:10.1007/s12274-017-1924-5
72. Kim D, Kwon HJ, Hyeon T. Magnetite/Ceria nanoparticle assemblies for extracorporeal cleansing of amyloid-beta in alzheimer’s disease. Adv Mater. 2019;31(19):1807965. doi:10.1002/adma.201807965
73. Vassie JA, Whitelock JM, Lord MS. Endocytosis of cerium oxide nanoparticles and modulation of reactive oxygen species in human ovarian and colon cancer cells. Acta Biomater. 2017;50:127–141. doi:10.1016/j.actbio.2016.12.010
74. Vassie JA, Whitelock JM, Lord MS. Targeted delivery and redox activity of folic acid-functionalized nanoceria in tumor cells. Mol Pharm. 2018;15(3):994–1004. doi:10.1021/acs.molpharmaceut.7b00920
75. Torres-Romero A, Cajero-Juarez M, Contreras-Garcia ME. Titania-Ceria surfactant assisted sol-gel synthesis and characterization. Epitoanyag J Silicate Based Comp Mater. 2017;69(1):8–11.
76. Torres-Romero A, Cajero-Juarez M, Nunez-Anita RE, Contreras-Garcia ME. Ceria-doped titania nanoparticles as drug delivery system. J Nanosci Nanotechnol. 2020;20(7):3971–3980. doi:10.1166/jnn.2020.17206
77. Kang D-W, Kim CK, Jeong H-G, et al. Biocompatible custom ceria nanoparticles against reactive oxygen species resolve acute inflammatory reaction after intracerebral hemorrhage. Nano Res. 2017;10(8):2743–2760.
78. Grillone A, Li T, Battaglini M, et al. Preparation, characterization, and preliminary in vitro testing of nanoceria-loaded liposomes. Nanomaterials. 2017;7(9):276. doi:10.3390/nano7090276
79. Pryjmakova J, Kaimlova M, Hubacek T, Svorcik V, Siegel J. Nanostructured materials for artificial tissue replacements. Int J Mol Sci. 2020;21(7).
80. Shao D, Li K, You M, et al. Macrophage polarization by plasma sprayed ceria coatings on titanium-based implants: cerium valence state matters. Appl Surf Sci. 2020;504:144070.
81. Li HR, Pan S, Xia P, et al. Advances in the application of gold nanoparticles in bone tissue engineering. J Biol Eng. 2020;14(1). doi:10.1186/s13036-020-00236-3.
82. Crisan L, Crisan BV, Bran S, et al. Carbon-based nanomaterials as scaffolds in bone regeneration. Part Sci Technol. 2019:1–10. doi:10.1080/02726351.2019.1637382
83. Bow A, Anderson DE, Dhar M. Commercially available bone graft substitutes: the impact of origin and processing on graft functionality. Drug Metab Rev. 2019;51(4):533–544. doi:10.1080/03602532.2019.1671860
84. Datta A, Mishra S, Manna K, Das Saha K, Mukherjee S, Roy S. Pro-oxidant therapeutic activities of cerium oxide nanoparticles in colorectal carcinoma cells. Acs Omega. 2020;5(17):9714–9723. doi:10.1021/acsomega.9b04006
85. Abid SA, Taha AA, Ismail RA, Mohsin MH. Antibacterial and cytotoxic activities of cerium oxide nanoparticles prepared by laser ablation in liquid. Environ Sci Pollut Res Int. 2020;27(24):30479–30489. doi:10.1007/s11356-020-09332-9
86. De Marzi L, Monaco A, De Lapuente J, et al. Cytotoxicity and genotoxicity of ceria nanoparticles on different cell lines in vitro. Int J Mol Sci. 2013;14(2):3065–3077.
87. Alpaslan E, Yazici H, Golshan N, Ziemer KS, Webster TJ. Dextran coated cerium oxide nanoparticles for inhibiting bone cancer cell functions. In: Narayan R, Bose S, Bandyopadhyay A, editors. Biomaterials Science: Processing, Properties, and Applications V. Vol. 254; 2015:187–196.
88. Yazici H, Alpaslan E, Webster TJ. The role of dextran coatings on the cytotoxicity properties of ceria nanoparticles toward bone cancer cells. Jom. 2015;67(4):804–810. doi:10.1007/s11837-015-1336-5
89. Alpaslan E, Yazici H, Golshan NH, Ziemer KS, Webster TJ. pH-dependent activity of dextran-coated cerium oxide nanoparticles on prohibiting osteosarcoma cell proliferation. Acs Biomater Sci Eng. 2015;1(11):1096–1103. doi:10.1021/acsbiomaterials.5b00194
90. Tang S, Zhou L, Liu Z, et al. Ceria nanoparticles promoted the cytotoxic activity of CD8(+) T cells by activating NF-kappa B signaling. Biomater Sci. 2019;7(6):2533–2544. doi:10.1039/C9BM00113A
91. Wong T-W, Behl M, Yusoff NISM, et al. Bio-based composites from plant based precursors and hydroxyapatite with shape-memory capability. Compos Sci Technol. 2020;194:108138. doi:10.1016/j.compscitech.2020.108138
92. Han SH, Lee J, Lee KM, et al. Enhanced healing of rat calvarial defects with 3D printed calcium-deficient hydroxyapatite/collagen/bone morphogenetic protein 2 scaffolds. J Mech Behav Biomed Mater. 2020;108:103782. doi:10.1016/j.jmbbm.2020.103782
93. Avila JD, Alrawahi Z, Bose S, Bandyopadhyay A. Additively manufactured Ti6Al4V-Si-Hydroxyapatite composites for articulating surfaces of load-bearing implants. Addit Manuf. 2020;34:101241. doi:10.1016/j.addma.2020.101241
94. Pandey A, Nigam VK, Balani K. Multi-length scale tribology of hydroxyapatite reinforced with ceria and silver. Wear. 2018;404–405:12–21. doi:10.1016/j.wear.2018.01.006
95. Pandey A, Midha S, Sharma RK, et al. Antioxidant and antibacterial hydroxyapatite-based biocomposite for orthopedic applications. Mater Sci Eng C. 2018;88:13–24. doi:10.1016/j.msec.2018.02.014
96. Li K, Shen Q, Xie Y, You M, Huang L, Zheng X. Incorporation of cerium oxide into hydroxyapatite coating protects bone marrow stromal cells against H2O2-induced inhibition of osteogenic differentiation. Biol Trace Elem Res. 2018;182(1):91–104. doi:10.1007/s12011-017-1066-3
97. Li K, Shen Q, Xie Y, You M, Huang L, Zheng X. Incorporation of cerium oxide into hydroxyapatite coating regulates osteogenic activity of mesenchymal stem cell and macrophage polarization. J Biomater Appl. 2017;31(7):1062–1076. doi:10.1177/0885328216682362
98. Li K, Xie Y, You M, Huang L, Zheng X. Plasma sprayed cerium oxide coating inhibits H2O2-induced oxidative stress and supports cell viability. J Mater Sci Mater Med. 2016;27(6):100.
99. You M, Li K, Xie Y, Huang L, Zheng X. The effects of cerium valence states at cerium oxide coatings on the responses of bone mesenchymal stem cells and macrophages. Biol Trace Elem Res. 2017;179(2):259–270. doi:10.1007/s12011-017-0968-4
100. Gopi D, Murugan N, Ramya S, Shinyjoy E, Kavitha L. Ball flower like manganese, strontium substituted hydroxyapatite/cerium oxide dual coatings on the AZ91 Mg alloy with improved bioactive and corrosion resistance properties for implant applications. RSC Adv. 2015;5(35):27402–27411. doi:10.1039/C5RA03432A
101. Sanyal V, Kesavamoorthi R, Ramachandra Raja C. Investigation on mechanical behavior and bioactivity of fluorohydroxyapatite toughened by zirconium–cerium ions additions. Mater Today Proc. 2016;3(6):1923–1932.
102. Pandey A, Patel AK, Kumar V, et al. Enhanced tribological and bacterial resistance of carbon nanotube with ceria- and silver-incorporated hydroxyapatite biocoating. Nanomaterials. 2018;8(6):363. doi:10.3390/nano8060363
103. Morais DS, Fernandes S, Gomes PS, et al. Novel cerium doped glass-reinforced hydroxyapatite with antibacterial and osteoconductive properties for bone tissue regeneration. Biomed Mater. 2015;10(5):055008. doi:10.1088/1748-6041/10/5/055008
104. Yan X, Yu C, Zhou X, Tang J, Zhao D. Highly ordered mesoporous bioactive glasses with superior in vitro bone-forming bioactivities. Angew Chem Int Ed. 2004;43(44):5980–5984. doi:10.1002/anie.200460598
105. Shruti S, Salinas AJ, Lusvardi G, Malavasi G, Menabue L, Vallet-Regi M. Mesoporous bioactive scaffolds prepared with cerium-, gallium- and zinc-containing glasses. Acta Biomater. 2013;9(1):4836–4844. doi:10.1016/j.actbio.2012.09.024
106. Kaur P, Singh KJ, Yadav AK, Kaur S, Kaur R, Kaur S. Growth of bone like hydroxyapatite and cell viability studies on CeO2 doped CaO–P2O5–MgO–SiO2 bioceramics. Mater Chem Phys. 2020;243:122352. doi:10.1016/j.matchemphys.2019.122352
107. Nicolini V, Malavasi G, Lusvardi G, et al. Mesoporous bioactive glasses doped with cerium: investigation over enzymatic-like mimetic activities and bioactivity. Ceram Int. 2019;45(16):20910–20920. doi:10.1016/j.ceramint.2019.07.080
108. Atkinson I, Anghel EM, Petrescu S, et al. Cerium-containing mesoporous bioactive glasses: material characterization, in vitro bioactivity, biocompatibility and cytotoxicity evaluation. Micropor Mesopor Mater. 2019;276:76–88. doi:10.1016/j.micromeso.2018.09.029
109. Goh Y-F, Alshemary AZ, Akram M, Abdul Kadir MR, Hussain R. In-vitro characterization of antibacterial bioactive glass containing ceria. Ceram Int. 2014;40(1, Part A):729–737. doi:10.1016/j.ceramint.2013.06.062
110. Youness RA, Taha MA, El-Kheshen AA, El-Faramawy N, Ibrahim M. In vitro bioactivity evaluation, antimicrobial behavior and mechanical properties of cerium-containing phosphate glasses. Mater Res Express. 2019;6(7):075212. doi:10.1088/2053-1591/ab15b5
111. Anesi A, Malavasi G, Chiarini L, Salvatori R, Lusvardi G. Cell proliferation to evaluate preliminarily the presence of enduring self-regenerative antioxidant activity in cerium doped bioactive glasses. Materials. 2020;13(10):2297. doi:10.3390/ma13102297
112. Lu B, Zhu D-Y, Yin J-H, et al. Incorporation of cerium oxide in hollow mesoporous bioglass scaffolds for enhanced bone regeneration by activating the ERK signaling pathway. Biofabrication. 2019;11(2):025012. doi:10.1088/1758-5090/ab0676
113. Takemoto M, Fujibayashi S, Neo M, Suzuki J, Kokubo T, Nakamura T. Bone-bonding ability of a hydroxyapatite coated zirconia–alumina nanocomposite with a microporous surface. J Biomed Mater Res A. 2006;78A(4):693–701. doi:10.1002/jbm.a.30748
114. Sivaraman K, Chopra A, Narayan AI, Balakrishnan D. Is zirconia a viable alternative to titanium for oral implant? A critical review. J Prosthodont Res. 2018;62(2):121–133. doi:10.1016/j.jpor.2017.07.003
115. Apratim A, Eachempati P, Krishnappa Salian KK, Singh V, Chhabra S, Shah S. Zirconia in dental implantology: A review. J Int Soc Prev Community Dent. 2015;5(3):147–156. doi:10.4103/2231-0762.158014
116. Nawa M, Yamada K, Pezzotti G. Microscopic mechanisms behind the toughening behavior of ceria stabilized tetragonal zirconia/alumina nanocomposite for biomedical applications. Key Eng Mater. 2008;361–363:813–816. doi:10.4028/www.scientific.net/KEM.361-363.813
117. Pandey AK, Pati F, Mandal D, Dhara S, Biswas K. In vitro evaluation of osteoconductivity and cellular response of zirconia and alumina based ceramics. Mater Sci Eng C. 2013;33(7):3923–3930. doi:10.1016/j.msec.2013.05.032
118. Han J-M, Hong G, Lin H, et al. Biomechanical and histological evaluation of the osseointegration capacity of two types of zirconia implant. Int J Nanomedicine. 2016;11:6507–6516. doi:10.2147/IJN.S119519
119. Oshima Y, Iwasa F, Tachi K, Baba K. Effect of nanofeatured topography on ceria-stabilized zirconia/alumina nanocomposite on osteogenesis and osseointegration. Int J Oral Maxillofac Implants. 2017;32(1):81–91. doi:10.11607/jomi.4366
120. Lopez-Píriz R, Fernández A, Goyos-Ball L, et al. Performance of a new Al2O3/Ce-TZP ceramic nanocomposite dental implant: a pilot study in dogs. Materials (Basel). 2017;10(6):614. doi:10.3390/ma10060614
121. Igarashi K, Nakahara K, Haga-Tsujimura M, Kobayashi E, Watanabe F. Hard and soft tissue responses to three different implant materials in a dog model. Dent Mater J. 2015;34(5):692–701. doi:10.4012/dmj.2014-361
122. Altmann B, Karygianni L, Al-Ahmad A, et al. Assessment of novel long-lasting ceria-stabilized zirconia-based ceramics with different surface topographies as implant materials. Adv Funct Mater. 2017;27(40):1702512.
123. Putra NE, Mirzaali MJ, Apachitei I, Zhou J, Zadpoor AA. Multi-material additive manufacturing technologies for Ti-, Mg-, and Fe-based biomaterials for bone substitution. Acta Biomater. 2020;109:1–20. doi:10.1016/j.actbio.2020.03.037
124. Ding Y, Yuan Z, Liu P, Cai K, Liu R. Fabrication of strontium-incorporated protein supramolecular nanofilm on titanium substrates for promoting osteogenesis. Mat Sci Eng C-Mater Biol Appl. 2020;111:110851. doi:10.1016/j.msec.2020.110851
125. Prakash C, Singh S, Ramakrishna S, Krolczyk G, Le CH. Microwave sintering of porous Ti-Nb-HA composite with high strength and enhanced bioactivity for implant applications. J Alloys Compd. 2020;824.
126. Li X, Qi M, Sun X, et al. Surface treatments on titanium implants via nanostructured ceria for antibacterial and anti-inflammatory capabilities. Acta Biomater. 2019;94:627–643. doi:10.1016/j.actbio.2019.06.023
127. Zhao X, Liu G, Zheng H, Cao H, Liu X. Dose-dependent effects of CeO2 on microstructure and antibacterial property of plasma-sprayed TiO2 coatings for orthopedic application. J Therm Spray Technol. 2015;24(3):401–409. doi:10.1007/s11666-014-0179-x
128. Li J, Wen J, Li B, et al. Valence state manipulation of cerium oxide nanoparticles on a titanium surface for modulating cell fate and bone formation. Adv Sci. 2018;5(2):1700678. doi:10.1002/advs.201700678
129. Ball JP, Mound BA, Monsalve AG, Nino JC, Allen JB. Biocompatibility evaluation of porous ceria foams for orthopedic tissue engineering. J Biomed Mater Res A. 2015;103(1):8–15. doi:10.1002/jbm.a.35137
130. Karakoti AS, Tsigkou O, Yue S, et al. Rare earth oxides as nanoadditives in 3-D nanocomposite scaffolds for bone regeneration. J Mater Chem. 2010;20(40):8912–8919. doi:10.1039/c0jm01072c
131. Hu Y, Du Y, Jiang H, Jiang G-S. Cerium promotes bone marrow stromal cells migration and osteogenic differentiation via Smad1/5/8 signaling pathway. Int J Clin Exp Pathol. 2014;7(8):5369–5378.
132. Mandoli C, Pagliari F, Pagliari S, et al. Stem cell aligned growth induced by CeO2 nanoparticles in PLGA scaffolds with improved bioactivity for regenerative medicine. Adv Funct Mater. 2010;20(10):1617–1624. doi:10.1002/adfm.200902363
133. Bal Z, Kushioka J, Kodama J, et al. BMP and TGFSS use and release in bone regeneration. Turk J Med Sci. 2020.
134. Chen M, Zhang Y, Zhang W, Li J. Polyhedral oligomeric silsesquioxane-incorporated gelatin hydrogel promotes angiogenesis during vascularized bone regeneration. ACS Appl Mater Interfaces. 2020;12(20):22410–22425. doi:10.1021/acsami.0c00714
135. Nethi SK, Nanda HS, Steele TWJ, Patra CR. Functionalized nanoceria exhibit improved angiogenic properties. J Mater Chem B. 2017;5(47):9371–9383. doi:10.1039/C7TB01957B
136. Li J, Kang F, Gong X, et al. Ceria nanoparticles enhance endochondral ossification–based critical-sized bone defect regeneration by promoting the hypertrophic differentiation of BMSCs via DHX15 activation. FASEB J. 2019;33(5):6378–6389. doi:10.1096/fj.201802187R
137. Xiang J, Li J, He J, et al. Cerium oxide nanoparticle modified scaffold interface enhances vascularization of bone grafts by activating calcium channel of mesenchymal stem cells. ACS Appl Mater Interfaces. 2016;8(7):4489–4499. doi:10.1021/acsami.6b00158
138. Walkey C, Das S, Seal S, et al. Catalytic properties and biomedical applications of cerium oxide nanoparticles. Environ Sci Nano. 2015;2(1):33–53. doi:10.1039/C4EN00138A
139. Zhou L, Tang S, Yang L, et al. Cerium ion promotes the osteoclastogenesis through the induction of reactive oxygen species. J Trace Elem Med Biol. 2019;52:126–135. doi:10.1016/j.jtemb.2018.12.006
140. Arslan K, Akbaba GB. In vitro genotoxicity assessment and comparison of cerium (IV) oxide micro- and nanoparticles. Toxicol Ind Health. 2020;36(2):76–83. doi:10.1177/0748233720913349
141. Aimonen K, Catalan J, Suhonen S, et al. In vivo genotoxicity and inflammatory effects of uncoated and coated CeO2 NPs in mice. Toxicol Lett. 2016;258:S276. doi:10.1016/j.toxlet.2016.06.1965
142. Turin-Moleavin I-A, Fifere A, Lungoci A-L, et al. In vitro and in vivo antioxidant activity of the new magnetic-cerium oxide nanoconjugates. Nanomaterials. 2019;9(11):1565. doi:10.3390/nano9111565
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
