Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 10
The 24-hour skin hydration and barrier function effects of a hyaluronic 1%, glycerin 5%, and Centella asiatica stem cells extract moisturizing fluid: an intra-subject, randomized, assessor-blinded study
Authors Milani M , Sparavigna A
Received 16 June 2017
Accepted for publication 25 July 2017
Published 11 August 2017 Volume 2017:10 Pages 311—315
DOI https://doi.org/10.2147/CCID.S144180
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Massimo Milani,1 Adele Sparavigna2
1Difa Cooper, Caronno Pertusella, Varese, 2Dermatologic Institute Dermig Milan, Milan, Italy
Introduction: Moisturizing products are commonly used to improve hydration in skin dryness conditions. However, some topical hydrating products could have negative effects on skin barrier function. In addition, hydrating effects of moisturizers are not commonly evaluated up to 24 hours after a single application. Hyaluronic acid (HA) and glycerin are very well-known substances able to improve skin hydration. Centella asiatica extract (CAE) could exert lenitive, anti-inflammatory and reepithelialization actions. Furthermore, CAE could inhibit hyaluronidase enzyme activity, therefore prolonging the effect of HA. A fluid containing HA 1%, glycerin 5% and stem cells CAE has been recently developed (Jaluronius CS [JCS] fluid).
Study aim: To evaluate and compare the 24-hour effects of JCS fluid on skin hydration and on transepidermal water loss (TEWL) in healthy subjects in comparison with the control site.
Subjects and methods: Twenty healthy women, mean age 40 years, were enrolled in an intra-subject (right vs left), randomized, assessor-blinded, controlled, 1-day trial. The primary end points were the skin hydration and TEWL, evaluated at the volar surface of the forearm and in standardized conditions (temperature- and humidity-controlled room: 23°C and 30% of humidity) using a corneometer and a vapometer device at baseline, 1, 8 and 24 hours after JCS fluid application. Measurements were performed by an operator blinded for the treatments.
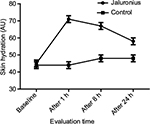
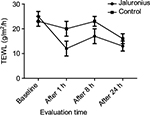
Results: Skin hydration after 24 hours was significantly higher (P=0.001; Mann–Whitney U test) in the JCS-treated area in comparison with the control site. JCS induced a significant (P=0.0001) increase in skin hydration at each evaluation time (+59% after 1 hour, +48% after 8 hours and +29% after 24 hours) in comparison with both baseline (P=0.0001) and non-treated control site (P=0.001). TEWL after 24 hours was significantly lower (P=0.049; Mann–Whitney U test) in the JCS-treated area in comparison with the control site (13±4 arbitrary units [AU] vs 16±6 AU). JCS fluid significantly reduced post-stripping TEWL in comparison with baseline after 1, 8 and 24 hours (–52%, –32% and –48%, respectively). In the control site, TEWL was not reduced in comparison with baseline values at each time point’s evaluation.
Conclusion: A single application of JCS significantly improves skin hydration for up to 24 hours at the same time as improving skin barrier function.
Keywords: moisturizing, hyaluronic acid, Centella asiatica stem cells, randomized controlled trial
Introduction
Hydration of the skin is an important indicator for the maintenance of a proper skin barrier function both in cosmetic field and in pathological skin diseases.1 Moisturizing products are commonly used to improve hydration in skin dryness conditions.2 However, some topical hydrating products could have paradoxical negative effects on skin barrier function because they contain ingredients that are harmful for the skin or since their activity is not based on physiological occlusion of skin surface.3 In addition, hydrating effects of moisturizer are not commonly evaluated up to 24 hours after a single application.4 Hyaluronic acid (HA) and glycerin are very well-known substances able to improve skin hydration.5 HA is an anionic, non-sulfated glycosaminoglycan distributed widely throughout connective, epithelial and neural tissues.6 HA is also a major component of skin, where it is involved in tissue repair.7 HA is abundant in extracellular matrices contributing to tissue hydrodynamics.8 Centella asiatica extract (CAE) is a rich source of amino acids, flavonoids, terpenoids, essential oils and alkaloids.9 CAE could exert lenitive, anti-inflammatory, re-epithelialization and cytoprotective actions.10 In addition, CAE could inhibit hyaluronidase enzyme activity, prolonging the effect of HA.11 Centella asiatica meristem culture (CAMC) is obtained from a cell culture of C. asiatica consisting of a population of undifferentiated stem cells originating from leaves.12 The cells are then filtered to remove the culture medium. A fluid containing HA 1%, glycerin and C. asiatica meristem cell culture and caprylic glycerides has been recently developed (Jaluronius CS [JCS] fluid; Difa Cooper, Caronno Pertusella, Italy).
Study aim
We evaluated and compared the 24-hour effects of JCS fluid on skin hydration and on transepidermal water loss (TEWL) in healthy subjects in comparison with the control site (non-treated site).
Subjects and methods
Population
This was a single-center, randomized, controlled, intra-subject, assessor-blinded 1-day study. Data were generated, recorded and processed in accordance with the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines for good clinical practice.13 The study protocol was approved by the local investigator review board (Dermig Institute). A total of 20 healthy women, mean (standard deviation [SD]) age 40 (10) years, were enrolled, after their written informed consent. The inclusion criterion was a normal skin without acute skin diseases. The exclusion criteria were the presence of skin dryness (skin hydration <30 arbitrary units [AU]), a positive history of current smoking, major skin diseases, pregnancy or breastfeeding, systemic corticosteroid or cytostatic therapy within 30 days, any use of local drugs within 30 days that might influence the skin texture, any condition on the inner upper arms that could interfere with a clear-cut assessment of the skin and finally, current participation in any other clinical study. The primary outcome was to compare skin hydration, expressed in AU, and TEWL, expressed in g/m2/h, values after a single application of JCS fluid at hour 24 between treated and control sites. Skin hydration and TEWL were evaluated in standardized conditions (temperature- and humidity-controlled room 23°C and 30% of relative humidity and an acclimatization time of 40 minutes) and according to guidelines14 using a corneometer (Corneometer® CM 825; Courage + Khazaka Electronic GmbH, Cologne, Germany) and a vapometer (Tewameter TM 300; Courage + Khazaka Electronic GmbH) device at baseline, 1, 8 and 24 hours after JCS fluid application and expressed as AU. The skin area evaluated was the volar surface of the forearms. JCS was applied (0.4 mL) in the tested area to cover a surface of 5 × 15 cm. The opposite volar forearm surface was the control. The measurements were performed by an operator blinded for the treatments. Stripping procedures were performed according to Pinnagoda et al:14 the tested area was stripped with repeated applications of clear tape of 15 mm (Scotch®; 3M, Milan, Italy); for each skin-tested area, 40 times tape stripping were performed by the same specialized technician.
Ethics approval and consent to participate
This study was conducted in compliance with the ethical principles originating in or derived from the Declaration of Helsinki and in compliance with the guidelines of Good Clinical Practice. All subjects provided signed informed consent. The study protocol was approved by the local investigator review board (Dermig Institute) on January 2017.
Statistical analysis
Statistical analysis was performed using GraphPad statistical software version 13.0 (GraphPad Software, Inc., La Jolla, CA, USA). Continuous variables were expressed as mean±SD. The primary end points of the trial were the comparison between treated and control sites of skin hydration and TEWL values after 24 hours. Additional end points of the trial were the evolution of skin hydration and TEWL values at baseline and after 1, 8 and 24 hours after application in comparison with the control sites. The Wilcoxon and Mann–Whitney U tests were used for the analysis of the study outcomes. The Wilcoxon test was used to compare paired data (intra-sites) at each time point in comparison with baseline, and the Mann–Whitney U test was used to compare non-paired data (treated site vs not treated site after 24 hours). Sample size calculation was performed with a hypothesis to find a positive difference in skin hydration between treated and not treated site at the end of the treatment (24 hours) of at least 20%. With an effect size (Cohen’s d value) of 0.6, with an alpha value of 0.05 and with a power of 80%, a total of at least 20 subjects should be enrolled to detect this difference between the active treated site vs control. The sample size was calculated using G*Power statistical software version 3.9 (G*Power, Heinrich Heine University, Kiel, Germany). A P-value of <0.05 was considered as significant. The study was an intra-subject (right vs left) trial. Each subject was randomized to apply the test product on the right or left site (volar forearm skin) according to a computer-generated randomization list (blocks of four).
Results
Skin hydration after 24 hours was significantly higher (P=0.001; Mann–Whitney U test) in the JCS-treated area in comparison with the control site (57±8 AU vs 47±8 AU, representing a 21% higher skin hydration). In addition, JCS induced a significant (P=0.0001; Wilcoxon test) increase in skin hydration at each evaluation time point (+59% after 1 hour, +48% after 8 hours and +29% after 24 hours) in comparison with the baseline value (Figure 1). In control sites, no statistical modification of skin hydration was observed at each time-point evaluation in comparison with baseline. TEWL after 24 hours was significantly lower (P=0.049; Mann–Whitney U test) in the JCS-treated area in comparison with the control site (13±4 AU vs 16±6 AU). JCS fluid significantly (P=0.001) reduced post-stripping TEWL in comparison with baseline after 1, 8 and 24 hours (–52%, –32% and –48%, respectively). In the control site, TEWL was not reduced in comparison with baseline values at each time-point evaluation (Figure 2).
Discussion
Skin hydration is a crucial component in maintaining a healthy skin,15 and water is considered essential for the normal functions of the skin.16 Cosmetic ingredients aimed at restoring a physiological hydration while helping to decrease skin evaporation improve appearance and tactile properties of the skin.17 Glycerin and HA are considered two potent humectant moisturizing agents.18 Topical glycerin can normalize skin hydration in an aquaporin-deficient mouse skin model.19 HA is naturally present in both dermis and epidermis and binds to extracellular matrix through CD44 receptor, playing a role in epidermal barrier function and hydration.20 HA has an important role in the normal epidermis. HA can speed up reepithelialization process due to several of its properties.21 HA is an integral part of the extracellular matrix of basal keratinocytes. In addition, HA possesses free-radical scavenging function, and it could improve keratinocyte proliferation and migration.22 In normal skin, HA is found in relative high concentrations in the basal layer of the epidermis where proliferating keratinocytes are found.23 Maintaining the extracellular space and providing an open, as well as hydrated, structure for the passage of nutrients are the main functions of HA in epidermis.24 Epidermal HA also functions as a manipulator in the process of keratinocyte proliferation, which is essential in normal epidermal function and during reepithelialization in tissue repair.25 In the wound healing process, HA is expressed in the wound margin, in the connective tissue matrix, and in collocating with CD44 expression in migrating keratinocyte.26 Pavicic et al27 have demonstrated that an HA 0.1% cream improves skin hydration in women with chronoaging; however, no data regarding this effect up to 24 hours after a single application were reported. HA skin effects could be limited by enzymatic degradation by hyaluronidase.28 An interesting effect of CAE is that it can inhibit the enzymatic action of hyaluronidase: Nema et al29 have demonstrated that CAE has potent elastase, matrix metalloproteinase and hyaluronidase inhibitory effects. The hydrating serum we have evaluated in this trial contains glycerin, HA and CEA. Therefore, the hydrating action of HA in this formulation could be prolonged over the time, thanks to the inhibition activity of hyaluronidase present in the CAE explaining the long-lasting moisturizing effect we observed in this study. The main limitation of this study was that it was not a double-blinded study. However, we used an assessor-blinded approach to evaluate the primary outcome of the trial. In addition, the outcomes were determined in an objective manner by means of instrumental devices. Therefore, we consider that the internal validity of the results obtained could be judged high.
Conclusion
Our study demonstrates for the first time that a single daily application of JCS fluid containing HA, glycerin and CAE induced a long-lasting (up to 24 hours) hydrating and moisturizing effect, improving at the same time the skin barrier function in healthy women.
Acknowledgment
This study was supported by Difa Cooper SpA.
Author contributions
Both authors participated in study design, data collection, data interpretation, development, review, and final approval of the manuscript. Both authors contributed toward drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
MM is the medical director of Difa Cooper, an Industrial Farmaceutica Cantabria group company that commercializes the tested product. AS is the head of research of Dermig Institute. She received an unrestricted grant from Difa Cooper to conduct the trial. The authors report no other conflicts of interest in this work.
References
Yosipovitch G, Xiong GL, Haus E, Sackett-Lundeen L, Ashkenazi I, Maibach HI. Time-dependent variations of the skin barrier function in humans: transepidermal water loss, stratum corneum hydration, skin surface pH, and skin temperature. J Invest Dermatol. 1998;110(1):20–23. | ||
Rawlings AV, Harding CR. Moisturization and skin barrier function. Dermatol Ther. 2004;17(s1):43–48. | ||
Anderson PC, Dinulos JG. Are the new moisturizers more effective? Curr Opin Pediatr. 2009;21(4):486–490. | ||
Wiren K, Nohlgård C, Nyberg F, et al. Treatment with a barrier-strengthening moisturizing cream delays relapse of atopic dermatitis: a prospective and randomized controlled clinical trial. J Eur Acad Dermatol Venereol. 2009;23(11):1267–1272. | ||
Kraft JN, Lynde CW. Moisturizers: what they are and a practical approach to product selection. Skin Therapy Lett. 2005;10(5):1–8. | ||
Tammi R, Ripellino JA, Margolis RU, Tammi M. Localization of epidermal hyaluronic acid using the hyaluronate binding region of cartilage proteoglycan as a specific probe. J Invest Dermatol. 1988;90(3):412–414. | ||
Wang TW, Sun JS, Wu HC, Tsuang YH, Wang WH, Lin FH. The effect of gelatin–chondroitin sulfate–hyaluronic acid skin substitute on wound healing in SCID mice. Biomaterials. 2006;27(33):5689–5697. | ||
Weindl G, Schaller M, Schäfer-Korting M, Korting HC. Hyaluronic acid in the treatment and prevention of skin diseases: molecular biological, pharmaceutical and clinical aspects. Skin Pharmacol Physiol. 2004;17(5):207–213. | ||
Inamdar PK, Yeole RD, Ghogare AB, De Souza NJ. Determination of biologically active constituents in Centella asiatica. J Chromatogr A. 1996;742(1–2):127–130. | ||
Tenni R, Zanaboni G, De Agostini MP, Rossi A, Bendotti C, Cetta G. Effect of the triterpenoid fraction of Centella asiatica on macromolecules of the connective matrix in human skin fibroblast cultures. Ital J Biochem. 1987;37(2):69–77. | ||
Kanlayavattanakul M, Lourith N. An update on cutaneous aging treatment using herbs. J Cosmet Laser Ther. 2015;17(6):343–352. | ||
Mangas S, Moyano E, Hernandez-Vazquez L, Bonfill M. Centella asiatica (L) Urban: An Updated Approach. Plant Secondary Terpenoids. Trivandrum: Research Signpost; 2009:55–74. | ||
International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH harmonized tripartite guideline: guideline for good clinical practice. J Postgrad Med. 2001;47(1):45–50. | ||
Pinnagoda J, Tupker RA, Agner T, Serup J. Guidelines for transepidermal water loss (TEWL) measurement. A report from the Standardization Group of the European Society of Contact Dermatitis. Contact Dermatitis. 1990;22(3):164–178. | ||
Verdier-Sévrain S, Bonté F. Skin hydration: a review on its molecular mechanisms. J Cosmet Dermatol. 2007;6(2):75–82. | ||
Lee SH, Jeong SK, Ahn SK. An update of the defensive barrier function of skin. Yonsei Med J. 2006;47(3):293–306. | ||
Harding CR, Watkinson A, Rawlings AV, Scott IR. Dry skin, moisturization and corneodesmolysis. Int J Cosmet Sci. 2000;22(1):21–52. | ||
Kim H, Kim JT, Barua S, et al. Seeking better topical delivery technologies of moisturizing agents for enhanced skin moisturization. Expert Opin Drug Deliv. 2017;20:1–5. | ||
Hara M, Verkman AS. Glycerol replacement corrects defective skin hydration, elasticity, and barrier function in aquaporin-3-deficient mice. Proc Natl Acad Sci U S A. 2003;100(12):7360–7365. | ||
Wertz PW. Stratum corneum lipids and water. Exog Dermatol. 2004;3(2):53–56. | ||
Chen WJ, Abatangelo G. Functions of hyaluronan in wound repair. Wound Repair Regen. 1999;7(2):79–89. | ||
Sato H, Takahashi T, Ide H, et al. Antioxidant activity of synovial fluid, hyaluronic acid, and two subcomponents of hyaluronic acid. Synovial fluid scavenging effect is enhanced in rheumatoid arthritis patients. Arthritis Rheum. 1988;31(1):63–71. | ||
Manuskiatti W, Maibach HI. Hyaluronic acid and skin: wound healing and aging. Int J Dermatol. 1996;35(8):539–544. | ||
Ghersetich I, Lotti T, Campanile G, Grappone C, Dini G. Hyaluronic acid in cutaneous intrinsic aging. Int J Dermatol. 1994;33(2):119–122. | ||
Papakonstantinou E, Roth M, Karakiulakis G. Hyaluronic acid: a key molecule in skin aging. Dermatoendocrinol. 2012;4(3):253–258. | ||
Peach RJ, Hollenbaugh D, Stamenkovic I, Aruffo A. Identification of hyaluronic acid binding sites in the extracellular domain of CD44. J Cell Biol. 1993;122(1):257–264. | ||
Pavicic T, Gauglitz GG, Lersch P, et al. Efficacy of cream-based novel formulations of hyaluronic acid of different molecular weights in anti-wrinkle treatment. J Drugs Dermatol. 2011;10(9):990–1000. | ||
Harada H, Takahashi M. CD44-dependent intracellular and extracellular catabolism of hyaluronic acid by hyaluronidase-1 and-2. J Biol Chem. 2007;282(8):5597–5607. | ||
Nema NK, Maity N, Sarkar BK, Mukherjee PK. Matrix metalloproteinase, hyaluronidase and elastase inhibitory potential of standardized extract of Centella asiatica. Pharm Biol. 2013;51(9):1182–1187. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.