Back to Journals » Therapeutics and Clinical Risk Management » Volume 14
Th17/Treg homeostasis, but not Th1/Th2 homeostasis, is implicated in exacerbation of human bronchial asthma
Authors Zou XL , Chen ZG, Zhang TT , Feng DY , Li HT, Yang HL
Received 25 April 2018
Accepted for publication 7 July 2018
Published 6 September 2018 Volume 2018:14 Pages 1627—1636
DOI https://doi.org/10.2147/TCRM.S172262
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Deyun Wang
Xiao-ling Zou,1,2,* Zhuang-gui Chen,2,3,* Tian-tuo Zhang,1,2 Ding-yun Feng,1,2 Hong-tao Li,1,2 Hai-ling Yang1,2
1Department of Pulmonary Diseases, The Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou, Guangdong Province, People’s Republic of China; 2Institute of Respiratory Disease of Sun Yat-Sen University, 3Department of Pediatrics Diseases, The Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou, Guangdong Province, People’s Republic of China
*These authors contributed equally to this work
Background: Th17 and regulatory T cell (Treg) play crucial roles in the pathogenesis of asthma. However, the association between Th17/Treg homeostasis and asthma exacerbation remains unclear.
Patients and methods: To investigate the role of Th17/Treg bias in asthma exacerbation, 49 asthma patients were enrolled in the current study, of whom 31 had acute asthma exacerbation (exacerbation group) and 18 did not (non-exacerbation group). Meanwhile, 17 healthy subjects were recruited as normal controls (control group). By measuring interleukin (IL)-4, IL-13, interferon (IFN)-γ, IL-10, and IL-17A levels in plasma using enzyme-linked immunosorbent assay (ELISA) and determining the mRNA expression of T-bet, GATA-3, forkhead/winged helix transcription factor (Foxp3), and receptor-related orphan receptor γt (RORγt) in peripheral blood mononuclear cells (PBMCs) by quantitative real-time PCR.
Results: We found that IL-17A/IL-10 and RORγt/Foxp3 ratios were significantly increased in the exacerbation group compared with that in the non-exacerbation group, while IL-4/IFN-γ and GATA-3/T-bet ratios remained unchanged. Moreover, IL-17A/IL-10 and RORγt/Foxp3 ratios, but not IL-4/IFN-γ or GATA-3/T-bet ratios, negatively correlated with forced expiratory volume in the first second (FEV1)/FEV1pred and Asthma Control Test Questionnaire (ACT) scores in both exacerbation group and non-exacerbation group. In contrast, the IL-4/IFN-γ ratio was negatively correlated with FEV1/FEV1pred and ACT scores only in the non-exacerbation group but not in the exacerbation group, while the ratio of GATA-3/T-bet was correlated with neither FEV1/FEV1pred nor ACT scores in both groups with asthma.
Conclusion: Our results suggest that the homeostasis of the Treg and Th17 cells is broken in asthma exacerbation and correlates with asthma severity and disease control status. The outcome has significant implication in understanding the progression of asthma and providing helpful information for physicians in the diagnosis and treatment of asthma patients.
Keywords: bronchial asthma, exacerbation, T cell, Th17/Treg
Introduction
Bronchial asthma is an allergic disease that is characterized by airway inflammation, airway wall remodeling, and airway hyperresponsiveness. It is caused by immune dysfunction, in which the helper T (Th) cells play a crucial role.1 Th2/Th1 skewing, which is regulated by T-bet and GATA binding protein 3 (GATA-3), the key transcription factors for naive T cell differentiation toward Th1 and Th2 cells, respectively, is believed to be the driving force for the pathogenesis of asthma.2,3 For example, the activated Th2 cells and their secreted cytokines, such as interleukin (IL)-4, IL-5, and IL-13, are key regulators to develop chronic inflammation and airway hyperresponsiveness, while the Th1 cytokines, eg, interferon (IFN)-γ, prevent this process in patients with mild to moderate asthma.4 Although supported by intensive research, this theory does not fully explain the development of asthma. Evidence has shown that reversing the Th1/Th2 imbalance cannot completely restore asthmatic symptoms, suggesting the presence of alternative immunoregulatory mechanisms underlying asthma progression.5
Besides the Th1/Th2 subsets, another two cell subsets with relatively independent function, Th17 cells and regulatory T cells (Tregs), have been proven to be also important in asthma pathogenesis in emerging research.6,7 For instance, as a minor fraction of approximately 5%–10% of CD4+ T cells, Tregs expressing the forkhead/winged helix transcription factor (Foxp3) take important roles in preventing autoimmunity and maintaining self-tolerance and exert their function partly by the secretion of anti-inflammatory cytokines, such as IL-10 and transforming growth factor (TGF)-β1.8 In comparison, Th17 cells expressing retinoic acid receptor-related orphan receptor γt (RORγt) play a critical role in the induction of autoimmune tissue injuries and inflammation by producing cytokines IL-17A.9 IL-6 is a key cytokine in controlling the balance between Th17 cells and Tregs. When exposed to both IL-6 and TGF-β1, naive CD4+ T cells differentiate into Th17 cells. In the absence of the last cytokine, IL-6 (plus IL-1) favors the degradation of FOXP3 and prevents the generation of Tregs.
Experimental and clinical data have indicated that the homeostasis between Th17 and Tregs is important in maintaining lung immunity,10 and the imbalance of Th17/Treg is associated with the pathogenesis of inflammatory and autoimmune diseases such as allergic rhinitis (AR) and asthma.11,12 For example, elevated Th17 cell response coupled with the deficiency of Treg response were observed in bronchial asthma in both adults and children.13,14 This is supported by a significant increase in Th17 cell numbers in their peripheral blood and Th17 cytokines in lung tissue, sputum, and bronchoalveolar lavage fluid (BALF), as well as a corresponding decrease of Treg numbers, IL-10 release, and Foxp3 levels in the peripheral blood and BALF.12,14 Studies have showed that in allergic patients, Treg numbers decrease in peripheral blood and BALF and cannot suppress the cell proliferation and cytokine production of allergen-stimulated CD4+ T cells.15,16 In addition, several murine studies have demonstrated that the induction of Treg function reverses airway hyperresponsiveness and protects against experimentally induced asthma.17
The results of these previous studies implicated a skewing of Th17/Treg balance in asthmatic condition. Importantly, IL-17 was also reported to be correlated with airway hypersensitivity as well as asthma severity,18,19 indicating that Th17/Treg homeostasis may play a role in modulating asthma progression. However, whether Th17/Treg homeostasis could be adopted to predict asthma progression, such as asthma exacerbation, is still unclear and under investigation. Thus, we hypothesized that Th17/Treg homeostasis was involved in asthma exacerbation and correlated with disease severity.
In the current study, we analyzed the expression profile of Th17 and Tregs in patients with different asthma control status, with the purpose to find out whether the bias of Th17/Treg homeostasis is involved in asthma exacerbation and its relationship with lung function and the Asthma Control Test Questionnaire (ACT) scores. The outcome has significant implication in understanding the progression of asthma and providing helpful information for physicians in the diagnosis and treatment of asthma patients.
Patients and methods
Subjects
Patients with diagnosed asthma attending the Third-Affiliated Hospital of Sun Yat-sen University, China, were recruited in the study between December 2016 and May 2017 based on the diagnostic criteria of the Global Initiative for Asthma (GINA), 2016 guidelines. According to the asthma exacerbation situation, patients were divided into two groups. Patients with asthma exacerbation within 1 week were assigned to the exacerbation group, while patients with asthma remission within 3 months were assigned to the non-exacerbation group. The exclusion criteria were as follows: the chest X-ray showed pulmonary infection; treatment with systemic glucocorticoid within 1 month before the study or treatment with other immunosuppressive agents or desensitization therapy; complications related to severe heart, hepatic, renal disease, or malignancy; and pregnancy. In addition, 17 healthy subjects served as the control group, who were nonsmokers with normal pulmonary function, negative allergy tests, and free from upper respiratory tract infections in the 4 weeks preceding the study. This study was conducted respecting the Declaration of Helsinki of the World Medical Association 1964 (and subsequent ratifications) on ethical principles for medical research in humans and approved by the Research Ethics Board (No 2013229) of the Third-Affiliated Hospital of Sun Yet-sen University. Written informed consent was obtained from all individuals.
Sample preparation
Ten milliliters of heparinized peripheral venous blood was collected from each participant. Plasma, used to measure cytokine concentrations, was isolated from the peripheral blood and stored at −20°C for further use. Peripheral blood mononuclear cells (PBMCs) were obtained by Ficoll-Hypaque density centrifugation (1,200× g for 20 minutes at room temperature), and monocytes/macrophages were depleted from the PBMC suspension by using L-leucine methyl ester. The detailed steps were following the protocol introduced by Fuss et al,20 in 2009. Isolated PBMCs were stored at −80°C after addition of 1 mL Trizol solution for the reverse transcription polymerase chain reaction (RT-PCR) analysis.
Enzyme-linked immunosorbent assays (ELISAs)
Concentrations of cytokines IFN-γ, IL-4, IL-17A, IL-10, and IL-13 in plasma were measured by ELISA (R&D Systems, Inc, Minneapolis, MN, USA), according to manufacturer’s instructions. Briefly, blood samples were added in duplicate to 96-well plates with 100 μL per well. The appropriate biotin-conjugated antibodies were added to each well. Samples were incubated at room temperature for 2 hours. Wells were then aspirated, and each well was washed five times. Substrate solutions were added to each well and were incubated for 30 minutes at room temperature in the dark. The optical density (OD) of each well was determined using a microplate reader (Bio-Rad Model 680; Bio-Rad Laboratories Inc., Hercules, CA, USA) set to 450 nm. A standard curve was created with the average of the OD duplicate readings. Concentrations of target cytokines were calculated by comparing the OD value with the standard cure.
Quantitative real-time RT-PCR analysis
Total RNA was extracted from PBMCs by using Trizol reagent (Thermo Fisher Scientific, Waltham, MA, USA) with the Qiagen RNeasy mini protocol and was converted to cDNA using oligo-dT and SuperScript RT II (Thermo Fisher Scientific). cDNA was diluted, and real-time PCR for Foxp3, RORγt, GATA-3, T-bet, and an endogenous reference gene (β-actin) was performed using an ABI 7500 Sequence Detection System (Thermo Fisher Scientific) with the SYBR Green Master Mix Kit (Takara, Kyoto, Japan). The following primers were used: β-actin, 5′-GTGGGGCGCCCCAGGCACCA-3′ (forward) and 5′-CTCCTTATGTCACGCACGATTTC-3′ (reverse), with amplified length of 239 bp; T-bet, 5′-AATGTGACCCAGATGATTGTGC-3′ (forward) and 5′-CTTGGAAAGTAAAGATATGCGTGTT-3′ (reverse), with amplified length of 130 bp; GATA-3, 5′-TGAAGGATGCCAAGAAGTT-3′ (forward) and 5′-TGAACAAATGATTCGCCTA-3′ (reverse), with amplified length of 203 bp; Foxp3, 5′-AGAACGCCATCCGCCACAACCTGA-3′ (forward) and 5′-GCCCCTGTTCGTCCATCCTCCTTT-3′ (reverse), with amplified length of 190 bp; and RORγt, 5′-GGCCATTCAGTACGTGGTGGAGTTCGC-3′ (forward) and 5′-CCGTGCGGTTGTCAGCATTGTAGGC-3′ (reverse), with amplified length of 169 bp. PCR program: 94°C for 60 seconds, 56°C for 40 seconds, 72°C for 60 seconds, 40 cycles, 25 μL volume system. The mRNA expression was normalized to the expression of the β-actin housekeeping gene and recorded as ΔCT (comparative threshold cycle, or CT), and then the ΔΔCT values were converted to 2−ΔΔCT for comparison.
Lung function and ACT questionnaire
Lung function test of forced expiratory volume in the first second (FEV1, % predicted) was performed in all individuals. Patients with asthma in addition completed the ACT. Five questions were included in the questionnaire, with a score of 1–5 for each question. The total ACT score was obtained by summation of individual question scores.
Statistical analyses
SPSS 16.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analyses. Homogeneity of variance in the three groups was tested. If the tested group showed homogeneity, one-way analysis of variance (ANOVA) followed by Student–Newman–Keuls test was performed. When heteroscedasticity was present in the test group, the data were analyzed using Mann–Whitney test and expressed as median (interquartile range). The relationship between the cytokines/transcript expression and the lung function as well as the ACT score was performed with Spearman test. P-value of less than 0.05 was considered statistically significant.
Results
General patient characteristics
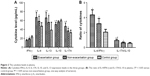
Forty-nine patients with bronchial asthma were enrolled and divided into two subgroups: 31 with asthma exacerbation and 18 without disease exacerbation. There were no significant differences in terms of age (P = 0.119) or gender (P = 0.211) among three study groups (Table 1). FEV1 (% predicted) was significantly lower in patients with asthma exacerbation than in those without asthma exacerbation (mean = 76.1 ± 8.1 vs 87.6 ± 6.6, respectively; P = 0.013), while mean ACT scores were lower in patients with asthma exacerbation than in those without exacerbation (P = 0.021; Figure 1).
  | Table 1 Patient characteristics processed data |
Cytokine and transcription factor levels in plasma
To study the response of Th cell subsets in patients with asthma, we measured the concentrations of IL-4, IL-13, IL-17A, IL-10, and IFN-γ in the plasma collected from all subjects. The results show that IL-4, IL-13, and IL-17A levels were increased significantly in asthma groups compared with the control subjects, with the exacerbation group higher than the non-exacerbation group. In contrast, IFN-γ levels were markedly reduced in asthma groups with a further decline in asthma exacerbation, and IL-10 levels were reduced only in the exacerbation group, but not in the non-exacerbation group (Figure 2A).
To further investigate T helper cell differentiation in asthmatic patients, we examined the mRNA expression of transcription factors T-bet, GATA-3, Foxp3, and RORγt in PBMCs of all participants. Levels of GATA-3 and RORγt mRNA are significantly higher in patients with asthma exacerbation than the other two groups. In comparison, T-bet mRNA expression was markedly reduced in asthma groups but was unchanged in asthma exacerbation when compared with non-exacerbation asthma. In addition, patients with asthma exacerbation had decreased Foxp3 mRNA expression when compared with patients in the other two groups (P = 0.045), but the differences between the control and non-exacerbation groups were not statistically significant (P = 0.417; Figure 3A).
Correlation analysis between Th17/Treg homeostasis and exacerbation of asthma
We compared the expression ratios of IL-4/IFN-γ and IL-17A/IL-10 among the different groups and found that both IL-4/IFN-γ and IL-17A/IL-10 ratios were markedly increased in asthma patients compared with the healthy controls. Nevertheless, only IL-17A/IL-10 ratio (P = 0.036), but not IL-4/IFN-γ ratio (P = 0.328), was increased in the exacerbation group compared with the non-exacerbation group (Figure 2B).
To assess the correlation of Th17/Treg and Th1/Th2 homeostasis with different asthma status at the transcription level, we also compared the mRNA ratios of GATA-3/T-bet and RORγt/Foxp3 among the three groups. The results suggest that both GATA-3/T-bet and RORγt/Foxp3 ratios were significantly higher in PBMCs collected from two asthma groups than the control group. The GATA-3/T-bet ratio showed no significant difference between the two asthma groups, while RORγt/Foxp3 ratio increased in the exacerbation group compared with the non exacerbation group (Figure 3B).
Correlation analysis between Th17/Treg and Th2/Th1 homeostasis in patients with asthma
To determine whether Th17/Treg homeostasis correlates with Th1/Th2 homeostasis, a correlation analysis was conducted between IL-4/IFN-γ and IL-17A/IL-10 ratios in patients with or without asthma exacerbation. The results suggest that there was no significant correlation between the two ratios. In parallel, no correlation was observed between the ratio of GATA-3/T-bet and RORγt/Foxp3 in two asthma groups as well (Figure 4).
Correlation analysis between Th17/Treg homeostasis and lung function, ACT scores
To identify if Th17/Treg or Th1/Th2 homeostasis correlates with the severity of asthma, we performed correlation analysis between ratios of cytokines levels and FEV1/FEV1pred/ACT scores. Strikingly, in both asthma groups with or without exacerbation, the ratio of IL-17A/IL-10 was negatively correlated with FEV1/FEV1pred (r = −0.769, P = 0.001 for exacerbation group and r = −0.965, P = 0.003 for non-exacerbation group) and ACT scores (r = −0.487, P = 0.016 for exacerbation group and r = −0.667, P = 0.029 for non-exacerbation group). In comparison, the negative correlation between the IL-4/IFN-γ ratio and FEV1/FEV1pred/ACT scores was observed only in the non-exacerbation group (r = −0.842, P = 0.004 for FEV1/FEV1pred; r = −0.467, P = 0.044 for ACT scores), but not in asthma exacerbation group (P = 0.093 for FEV1/FEV1pred; P = 0.0075 for ACT scores; Figures 5 and 6). These observations suggest that IL-17A/IL-10 ratio may be more related to asthma severity than IL-4/IFN-γ.
The same analysis was also performed at transcriptional levels. As what we observed in cytokine expression levels, there is a negative correlation between RORγt/Foxp3 mRNA ratio and FEV1/FEV1pred in asthma patients with (r = −0.725, P = 0.016) or without exacerbation (r = −0.758, P = 0.028). As for ACT scores, correspondingly negative correlation was observed in exacerbation group (r = −0.632, P = 0.015), but not in non-exacerbation group (P = 0.086). In comparison, the GATA-3/T-bet ratio was not correlated with either FEV1/FEV1pred or ACT scores in both asthma groups (Figures 7 and 8).
Discussion
In the current study, we showed that both the expression of Th17-related cytokine IL-17A and Th17-expressing transcription factor RORγt mRNA was increased in patients with or without asthma exacerbation, accompanied by correspondingly decreasing Treg-related cytokine IL-10 levels and transcription factor Foxp3 mRNA expression, when compared to control group. This finding is consistent with the results showed in previous studies,14,19 suggesting that the imbalance of Th17/Treg is involved in the pathogenesis of asthma.
Animal studies have also recently demonstrated the central roles of Th17 cells in allergic diseases such as asthma. In the study by Zhang et al,21 they found that by downregulating Th17 cell differentiation, acute airway inflammation of allergic asthma was inhibited in mice, while other researchers showed that Th17 cells could induce airway remodeling independent of the Th2 response in mice with genetic disruption of gp130 in T cells. They also found that all-trans retinoic acid administration ameliorated Th17-mediated disease and increased Treg activity, while dexamethasone inhibited eosinophilia but not neutrophilia and enhanced Th17 development in vitro. The result of their study established an important role for Th17 cells in airway remodeling and chronic neutrophilia, independent of allergic inflammation, which suggested that targeting the Th17/Treg axis might be therapeutic in neutrophilic and glucocorticoid-refractory asthma.22
To find out the relationship between Th17/Treg homeostasis and asthma exacerbation and to know whether Th17/Treg homeostasis could be adopted to predict asthma progression, such as asthma exacerbation, we compared the levels of cytokines and transcript factor mRNA specifically related to Th17 and Tregs, with the results showing that there is a greater Th17 (RORγt)/Treg (Foxp3) proportion (as well as the corresponding cytokines IL-17A/IL-10) during acute exacerbations of asthma (asthma attack), compared to the non-exacerbation group. By analyzing the correlations between Th17/Treg homeostasis and FEV1/FEV1pred, the important marker for evaluation of lung function, which is linked to asthma severity, and ACT scores, a well-known indicator for asthma control status,23–25 we found that a shift of Th17/Treg homeostasis to the Th17 direction might contribute to the disease severity of asthma. However, unlike Th17/Treg homeostasis, a shift of Th1/Th2 homeostasis to the Th2 responses has no significant correlation to lung function or ACT scores.
Since our results showed that the homeostasis of Th17/Treg, but not Th1/Th2 homeostasis, played a crucial role in asthma exacerbation and the disease progression, the former might be a potential therapeutic target for asthma exacerbation. Agents acting on Th17/Treg axis might stop asthma exacerbation by modulating the balance. In recent years, several studies have targeted Th17 cells for therapy in allergic diseases. In the research conducted by Zhao et al, they found that anti-IL-17 Ab treatment might protect against AR by giving anti-IL-17 Abs intranasally during the re-challenge of BALB/c mice with ovalbumin (OVA)-induced AR, with a concomitant increase in Treg response.26 Adenosine A2A receptor (A2AR) is considered as the main anti-inflammatory receptor by increasing Foxp3 expression and inhibiting Th17 cells generation. A recent study found that A2AR mRNA in PBMCs was associated with asthma severity, and A2AR agonist CGS-21680 could promote Foxp3 mRNA expression, TGF-β, and improve lung function while inhibiting RORγt mRNA expression, IL-17, and the infiltration of lung inflammation cells. Thus, they look forward that A2AR could be used to control asthma exacerbation by modulating the balance.27
Conclusion
The present study showed that Th17/Treg homeostasis, but not Th1/Th2 homeostasis, is related to asthma exacerbation and disease severity, which might provide novel strategies to treat asthma exacerbation by taking Th17/Treg homeostasis as a target. Moreover, further studies are needed to clarify the mechanism of Th17/Treg imbalance leading to the asthma exacerbation and affecting disease severity.
Acknowledgments
The authors would like to thank Dr Yan Yan for proofreading the manuscript. This study was supported by National Natural Science Foundation of China (grant numbers 81370114 and 81470219), Science and Technology Projects Foundation of Guangdong Province (number 2014A020212120), and the Natural Science Foundation of Guangdong Province, China (grant number 2017A030313525). Xiao-ling Zou and Zhuang-gui Chen are co authors of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
Iwata A, Kawashima S, Kobayashi M, et al. Th2-type inflammation instructs inflammatory dendritic cells to induce airway hyperreactivity. Int Immunol. 2014;26(2):103–114. | ||
Hirose K, Iwata A, Tamachi T, Nakajima H. Allergic airway inflammation: key players beyond the Th2 cell pathway. Immunol Rev. 2017;278(1):145–161. | ||
Yagi R, Zhu J, Paul WE. An updated view on transcription factor GATA3-mediated regulation of Th1 and Th2 cell differentiation. Int Immunol. 2011;23(7):415–420. | ||
Bosnjak B, Stelzmueller B, Erb KJ, Epstein MM, Klaus J, Michelle M. Treatment of allergic asthma: modulation of Th2 cells and their responses. Respir Res. 2011;12:114. | ||
Afshar R, Medoff BD, Luster AD. Allergic asthma: a tale of many T cells. Clin Exp Allergy. 2008;38(12):1847–1857. | ||
Talaat RM, Mohamed SF, Bassyouni IH, Raouf AA. Th1/Th2/Th17/Treg cytokine imbalance in systemic lupus erythematosus (SLE) patients: Correlation with disease activity. Cytokine. 2015;72(2):146–153. | ||
Zhang H, Kong H, Zeng X, Guo L, Sun X, He S. Subsets of regulatory T cells and their roles in allergy. J Transl Med. 2014;12:125. | ||
Guzmán-Flores JM, Portales-Pérez DP. Mechanisms of suppression of regulatory T-cells (Treg). Gac Med Mex. 2013;149(6):630–638. | ||
Nakada EM, Shan J, Kinyanjui MW, Fixman ED. Adjuvant-dependent regulation of interleukin-17 expressing γδ T cells and inhibition of Th2 responses in allergic airways disease. Respir Res. 2014;15(1):90. | ||
Zhu J, Liu X, Wang W, Ouyang X, Zheng W, Wang Q. Altered expression of regulatory T and Th17 cells in murine bronchial asthma. Exp Ther Med. 2017;14(1):714–722. | ||
Jiang H, Wu X, Zhu H, Xie Y, Tang S. Jiang Y. FOXP3(+)Treg/Th17 cell imbalance in lung tissues of mice with asthma. Int J Clin Exp Med. 2015;8(3):4158–4163. | ||
Huang X, Chen Y, Zhang F, Yang Q, Zhang G. Peripheral Th17/Treg cell-mediated immunity imbalance in allergic rhinitis patients. Braz J Otorhinolaryngo. 2014;80(2):152–155. | ||
Shi YH, Shi GC, Wan HY, et al. Coexistence of Th1/Th2 and Th17/Treg imbalances in patients with allergic asthma. Chinese Medical Journal. 2011;124(13):1951–1956. | ||
Wei B, Zhag H, Li L, Li M, Shang Y. T Helper 17 Cells and Regulatory T-Cell Imbalance in Paediatric Patients with Asthma. J Int Med Res. 2011;39(4):1293–1305. | ||
Hartl D, Koller B, Mehlhorn AT, et al. Quantitative and functional impairment of pulmonary CD4+CD25hi regulatory T cells in pediatric asthma. J Allergy Clin Immunol. 2007;119(5):1258–1266. | ||
Strickland DH, Stumbles PA, Zosky GR, et al. Reversal of airway hyperresponsiveness by induction of airway mucosal CD4+ CD25+ regulatory T cells. J Exp Med. 2006;203(12):2649–2660. | ||
Park BS, Hong GU, Ro JY, Jy R. Foxp3+-Treg Cells Enhanced by Repeated Low-Dose Gamma-Irradiation Attenuate Ovalbumin-Induced Allergic Asthma in Mice. Radiat Res. 2013;179(5):570–583583. | ||
Wilson RH, Whitehead GS, Nakano H, Free ME, Kolls JK, Cook DN. Allergic Sensitization through the Airway Primes Th17-dependent Neutrophilia and Airway Hyperresponsiveness. Am J Respir Crit Care Med. 2009;180(8):720–730. | ||
Tao B, Ruan G, Wang D, Li Y, Wang Z. Imbalance of Peripheral Th17 and Regulatory T Cells in Children with Allergic Rhinitis and Bronchial Asthma. Iran J Allergy Asthma Immunol. 2015;14(3):273–279. | ||
Fuss IJ, Kanof ME, Smith PD, Zola H. Isolation of whole mononuclear cells from peripheral blood and cord blood. Curr Protoc Immunol. 2009:Chapter 7–Unit7.1. | ||
Zhang W, Zhang X, Sheng A, et al. γ-Secretase Inhibitor Alleviates Acute Airway Inflammation of Allergic Asthma in Mice by Downregulating Th17 Cell Differentiation. Mediators Inflamm. 2015;2015(1):258168, 7 pages. | ||
Zhao J, Lloyd CM, Noble A. Th17 responses in chronic allergic airway inflammation abrogate regulatory T-cell-mediated tolerance and contribute to airway remodeling. Mucosal Immunol. 2013;6(2):335–346. | ||
Zemedkun K, Woldemichael K, Tefera G. Assessing Control of Asthma in Jush, Jimma, South West Ethiopia. Ethiop J Health Sci. 2014;24(1):49–58. | ||
Manoharan A, Anderson WJ, Lipworth J, Lipworth BJ. Assessment of Spirometry and Impulse Oscillometry in Relation to Asthma Control. Lung. 2015;193(1):47–51. | ||
Chae EJ, Kim T-B, Cho YS, et al. Airway Measurement for Airway Remodeling Defined by Post-Bronchodilator FEV1/FVC in Asthma: Investigation Using Inspiration-Expiration Computed Tomography. Allergy, Asthma and Immunology Research. 2011;3(2):111–117. | ||
Zhao W, Yun X, Zhi W. Neutralization of interleukin-17 suppresses allergic rhinitis symptoms by downregulating Th2 and Th17 responses and upregulating the Treg response. Oncotarget. 2017;8(14):22361–22369. | ||
Wang L, Wan H, Tang W, et al. Critical roles of adenosine A2A receptor in regulating the balance of Treg/Th17 cells in allergic asthma. Clin Respir J. 2018;12(1):149–157. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.