Back to Journals » Research and Reports in Urology » Volume 7
Testosterone replacement alters the cell size in visceral fat but not in subcutaneous fat in hypogonadal aged male rats as a late-onset hypogonadism animal model
Authors Abdelhamed A , Hisasue S, Shirai M, Matsushita K, Wakumoto Y, Tsujimura A, Tsukamoto T, Horie S
Received 5 August 2014
Accepted for publication 19 September 2014
Published 5 March 2015 Volume 2015:7 Pages 35—40
DOI https://doi.org/10.2147/RRU.S72253
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jan Colli
Amr Abdelhamed,1,2 Shin-ichi Hisasue,1 Masato Shirai,3 Kazuhito Matsushita,1 Yoshiaki Wakumoto,1 Akira Tsujimura,1 Taiji Tsukamoto,4 Shigeo Horie1
1Department of Urology, Juntendo University, Graduate School of Medicine, Tokyo, Japan; 2Department of Dermatology, Venereology and Andrology, Sohag University, Graduate School of Medicine, Sohag, Egypt; 3Department of Urology, Juntendo University Urayasu Hospital, Urayasu, Japan; 4Department of Urology, School of Medicine, Sapporo Medical University, Sapporo, Japan
Background: Patients with late-onset hypogonadism (LOH) benefit from testosterone replacement by improvement in the parameters of the metabolic syndrome, but fat cell morphology in these patients is still unclear. This study aims to determine the effect of testosterone replacement on the morphology of fat cells in subcutaneous and visceral adipose tissue and on erectile function in hypogonadal aged male rats as a model of LOH.
Methods: Ten male Sprague-Dawley rats aged 20–22 months were randomly allocated to two groups, ie, aged male controls (control group, n=5) and aged males treated with testosterone replacement therapy (TRT group, n=5). Testosterone enanthate 25 mg was injected subcutaneously every 2 weeks for 6 weeks. At 6 weeks, the intracavernous pressure (ICP) and mean arterial blood pressure (MAP) ratio was assessed. Visceral and subcutaneous adipose tissue specimens were collected and analyzed using Image-J software.
Results: Body weight at 2, 4, and 6 weeks after TRT was 800.0±35.4 g, 767.5±46.3 g, and 780±40.4 g, respectively (not statistically significant). The ICP/MAP ratio was 0.341±0.015 in the TRT group and 0.274±0.049 in the control group (not statistically significant). The median subcutaneous fat cell size was 4.85×103 (range 0.85–12.53×103) µm2 in the control group and 4.93×103 (range 6.42–19.7×103) µm2 in the TRT group (not statistically significant). In contrast, median visceral fat cell size was significantly smaller in the TRT group (4.93×103 µm2 [range 0.51–14.88×103]) than in the control group (6.08×103 µm2 [0.77–19.97×103]; P<0.001, Mann-Whitney U test).
Conclusion: This is the first study clearly indicating that TRT can decrease visceral fat cell size, which is a key modulator in the metabolic syndrome. However, a short course of TRT could not improve the ICP response in hypogonadal aged male rats. Further investigation is necessary to clarify the exact rationale of TRT on the visceral fat cell.
Keywords: testosterone replacement, visceral fat, erectile dysfunction, age
Introduction
Late-onset hypogonadism (LOH) is a clinical and biochemical syndrome in ageing men (who have had normal pubertal development and normal male secondary characteristics) associated with low testosterone, age-related comorbidities, and deterioration in general health status, including obesity.1 The prevalence of the metabolic syndrome (MetS) is increasing, especially in patients with low testosterone levels, who are at increased risk for later development of MetS.2 Patients with MetS commonly suffer from combinations of risk factors, like central abdominal obesity, high blood pressure, increased fasting glucose levels, hyperinsulinemia/insulin resistance (IR), and dyslipidemia in the form of elevated triglycerides and reduced high-density lipoprotein.3,4 Aging increases the prevalence of the MetS and is associated with hypogonadism5 and erectile dysfunction (ED).6–8 Hypogonadism, ED, visceral adiposity, IR, and MetS often coexist in the same population, increasing the risk of development of diabetes and cardiovascular disease, and affecting life expectancy.9
Although the etiology of MetS is multifactorial, visceral obesity is a key modulator in the development of this syndrome.10 Aging is an important factor in promoting visceral adiposity.11 Aging is associated with increased body fat mass, visceral adipose tissue (VAT), and ectopic fat deposition, which in turn is related to worse health conditions in the elderly12 with an enhanced cardiovascular disease risk profile.13,14 Although VAT and subcutaneous adipose tissue (SAT) are both associated with adverse cardiometabolic risk factors, VAT is more correlated with these risk factors.11
Accumulation of visceral fat may lead to development of IR, which is strongly associated with progression of cardiovascular disease.15 Visceral fat is an active endocrine organ which increases the production of inflammatory cytokines (eg, interleukin-6, tumor necrosis factor alpha), resulting in chronic inflammation and leading to the development of MetS parameters and ED.16 IR, which is strongly correlated with MetS, is a powerful predictor of ED.17,18 Moreover, improvement of IR ameliorates ED.19 Visceral fat is considered to be an independent predictor of dyslipidemia,20–22 IR,22,23 and all-cause mortality in men,24 and glucose tolerance and IR can be modified by changes in visceral fat.25 Therefore, in the future, we need to clarify the exact mechanism by which improvement in IR induces ED recovery in the LOH model.
Testosterone replacement therapy (TRT) improves MetS parameters in patients with LOH,26 reduces obesity parameters, and improves health-related quality of life in patients with ED and LOH.27 TRT was shown to reduce waist circumference and fat mass in hypogonadal men.28 Administration of testosterone is associated with positive effects on body composition via decreased fat mass.29–31 However, the mechanism of improvement in MetS parameters with TRT is still controversial, and the morphological changes occurring in the fat cell in this population is unclear. Therefore, in this study, we aimed to determine the effect of TRT on the morphology of fat cells in SAT and VAT, and on erectile function in hypogonadal aged male rats.
Materials and methods
Ten specific pathogen-free male Sprague-Dawley rats aged 20–22 months were randomly allocated into two groups, ie, aged male controls (control group, n=5) and aged males treated with testosterone (TRT group, n=5). All rats were subject to a baseline evaluation of weight and collection of blood samples for measurement of testosterone. The TRT group was injected with testosterone enanthate 25 mg subcutaneously every 2 weeks for 6 weeks (for a total of three injections). The testosterone level was confirmed at 1 week after the first injection of testosterone enanthate. Follow-up evaluations for all rats included body weight measurement and were done at 2, 4, and 6 weeks. The study was approved by the animal experiment committee of Sapporo Medical University, and its 1988 guidelines were followed as regards animal care, housing, and surgery.
Measurement of intracavernous pressure
After 6 weeks, the rats were anesthetized with pentobarbital sodium. The in vivo erectile response was evaluated by electrical field stimulation of the cavernous nerve. The response was expressed as the intracavernous pressure (ICP)/systemic mean arterial pressure (MAP) ratio.32 The rats were then sacrificed and samples of VAT and SAT were collected.
Histological analysis
VAT was collected from the omental depot. Samples were embedded in paraffin and transverse paraffin sections were cut to 3 μm. Each slide was stained with hematoxylin-eosin. From five randomly selected sections of each model, the average fat cell size was calculated using Image-J software (National Institutes of Health, Bethesda, MD, USA). All measurements were done by a single examiner (SH) blinded to other data.
Statistical analysis
The Student’s t-test was used to compare changes in body weight and ICP/MAP in both groups. The Mann-Whitney U test was used as a nonparametric test to compare changes in median subcutaneous and visceral fat cell size and testosterone levels in both groups. We used StatView version 5.0 for Windows software (SAS Institute, Cary, NC, USA) for the statistical analyses. A P-value of <0.05 was considered to be statistically significant.
Results
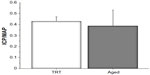
The median testosterone level before the study was 0.60 (range 0.56–1.52) ng/mL and at 1 week after the first TRT was 3.00 (range 1.70–3.90) ng/mL. Baseline body weight in the control group was 937.5±45.5 g and in the TRT group was 862.5±31.5 g (difference not statistically significant). The change in body weight at 2, 4, and 6 weeks after TRT tended to decrease with time, but the change was not statistically significant (Figure 1). ICP/MAP was 0.427±0.042 in the TRT group and 0.384±0.146 in the control group (difference not statistically significant, Figure 2).
  | Figure 1 The body weight change at 2, 4, and 6 weeks after TRT tended to decrease with time, although the change was not statistically significant. |
Representative microscopic findings in subcutaneous and visceral fat cells are shown in Figure 3A and B. The median subcutaneous fat cell size was 4.85×103 (range 0.85–12.53×103) μm2 in the control group and 4.93×103 (range 6.42–19.7×103) μm2 in the TRT group (difference not statistically significant, Figure 4A and B, respectively). In contrast, the median visceral fat cell size was significantly smaller in the TRT group (4.93×103 μm2 [range 0.51–14.88×103]) than in the control group (6.08×103 μm2 [range 0.77–19.97×103]; P<0.0001, Mann-Whitney U test).
  | Figure 3 Microscopic findings for (A) subcutaneous and (B) visceral fat cells (×200). |
Discussion
In the current study, we set out to address the important question about the underlying mechanisms of TRT in improving MetS parameters with a focus on adipose tissue changes which could modulate both cardiovascular disease and sexual health using an aged hypogonadal rat model. The reduction in visceral fat cell size using TRT was reported in a rabbit model of MetS33 and in a rat model of inherent obese type 2 diabetes.34 LOH is induced by reduction in testosterone with aging in the male.35 Physical alteration, including obesity, is one of the features of LOH; however, the mechanism via which TRT ameliorates obesity in the aged population is still unclear. Therefore, we conducted this study to determine how testosterone reduces the adipose tissue volume in aged hypogonadal rats.
Fat cells maintain triglyceride and free fatty acid levels, in addition to determination of IR, which is influenced more by visceral fat cells than by subcutaneous fat cells.36 VAT fat cells are more metabolically active, more sensitive to lipolysis, have more lipoprotein lipase activity,37 and have a stronger correlation with IR than SAT fat cells.38 Hypertrophic fat cells in VAT secrete adipocytokines, leading to development of a chronic low-grade inflammation, which is associated with IR.39 The outcome of our study is supported by VAT reduction with TRT in some clinical studies. TRT prevents a gain in VAT and loss of skeletal muscle in nonobese aging men.40 Also, TRT in eugonadal middle-aged abdominally obese men results in decreased VAT based on computed tomography, but no change in SAT.41,42
The androgen receptor (AR) density is higher in VAT adipocytes than in SAT adipocytes.43,44 Therefore, androgens levels are more concentrated in VAT.45 AR affects the energy balance in male metabolism by its negative influence on adiposity.46 However, results are conflicting with regard to the effect of androgen on IR. Some male AR knockout mice studies showed almost intact insulin sensitivity, due to enhanced adiponectin secretion which is insulin-sensitizing;46,47 and others showed increased IR in AR knockout mice.48 The current study shows an insignificant weight reduction in aged male rats after TRT. This is consistent with a report by Vanderschueren et al,49 who showed that body weight and fat mass were not significantly different after TRT in aged orchidectomized rats. This could be explained by the fact that TRT achieved significant positive changes in body composition via increased muscle mass and reduction in fat mass.50,51 Overall, reduction of visceral fat cell size is induced by a direct pathway via AR and by an indirect pathway via accelerated metabolism through increased muscle mass. This reduction in visceral fat cell size may explain the sustained and significant weight reduction that has been reported with long-term use of TRT in clinical studies.27,52
This study also failed to demonstrate improvement in erectile function after TRT. In contract with our results, the erectile response could be improved in aged rats with a testosterone implant.53 In clinical studies, TRT in men with LOH resulted in improved sexual function.54 This inadequate improvement might be explained by the slowly progressive nature of the influence of androgens on erection.55 In the clinical context, up to 6 months of TRT may be required before significant improvement in erectile function is observed.1 In our study, the subjects were old and close to the end of their life span, so we could not confirm the long-term effects of TRT on erectile function. In the future, we need to observe erectile function after long-term TRT.
This study has some limitations that need to be acknowledged. First, the number of animals included was small. We used very old rats close to the end of their life span, so the number of animals available for inclusion was limited. Second, we used a hypogonadal aged male rat model, which is usually associated with a high prevalence of one or more of the MetS parameters; however, we did not use MetS rat model and confirm the other cytokines other than testosterone after TRT. Third, it is unclear how much visceral fat cell reduction is needed to induce favorable metabolic changes. In the future study, it will be necessary to clarify the exact mechanism and long-term effects of TRT on visceral fat cell size, erectile function, and other cytokines in a larger number of animals. This should be investigated in a rat model of MetS in addition to aged rats.
Conclusion
This is the first study clearly indicating that TRT can decrease the size of the visceral fat cell, which is a key modulator of MetS, but could not improve the ICP response in hypogonadal aged male rats. Further investigation is needed to clarify the exact rationale of TRT on the visceral fat cell.
Disclosure
The authors report no conflicts of interest in this work. This study was presented in abstract form at the American Urological Association annual meeting, Washington, DC, USA, held on April 16–20, 2011.
References
Lunenfeld B, Mskhalaya G, Kalinchenko S, Tishova Y. Recommendations on the diagnosis, treatment and monitoring of late-onset hypogonadism in men – a suggested update. Aging Male. 2013;16(4):143–150. | |
Kapoor D, Jones TH. Androgen deficiency as a predictor of metabolic syndrome in aging men: an opportunity for intervention? Drugs Aging. 2008;25(5):357–369. | |
National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. | |
Einhorn D, Reaven GM, Cobin RH, et al. American College of Endocrinology position statement on the insulin resistance syndrome. Endocr Pract. 2003;9(3):237–252. | |
Rodriguez A, Muller DC, Metter EJ, et al. Aging, androgens, and the metabolic syndrome in a longitudinal study of aging. J Clin Endocrinol Metab. 2007;92(9):3568–3572. | |
Filippi S, Vignozzi L, Morelli A, et al. Testosterone partially ameliorates metabolic profile and erectile responsiveness to PDE5 inhibitors in an animal model of male metabolic syndrome. J Sex Med. 2009;6(12):3274–3288. | |
Demir T, Demir O, Kefi A, Comlekci A, Yesil S, Esen A. Prevalence of erectile dysfunction in patients with metabolic syndrome. Int J Urol. 2006;13(4):385–388. | |
Kupelian V, Shabsigh R, Araujo AB, O’Donnell AB, McKinlay JB. Erectile dysfunction as a predictor of the metabolic syndrome in aging men: results from the Massachusetts Male Aging Study. J Urol. 2006;176(1):222–226. | |
Corona G, Mannucci E, Forti G, Maggi M. Hypogonadism, ED, metabolic syndrome and obesity: a pathological link supporting cardiovascular diseases. Int J Androl. 2009;32(6):587–598. | |
Riedner CE, Rhoden EL, Ribeiro EP, Fuchs SC. Central obesity is an independent predictor of erectile dysfunction in older men. J Urol. 2006;176(4 Pt 1):1519–1523. | |
Liu J, Fox CS, Hickson DA, et al. Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: the Jackson Heart Study. J Clin Endocrinol Metab. 2010;95(12):5419–5426. | |
Zamboni M, Rossi AP, Fantin F, et al. Adipose tissue, diet and aging. Mech Ageing Dev. 2014;136–137:129–137. | |
Sironi AM, Petz R, De Marchi D, et al. Impact of increased visceral and cardiac fat on cardiometabolic risk and disease. Diabet Med. 2012;29(5):622–627. | |
Klein S, Allison DB, Heymsfield SB, et al. Waist circumference and cardiometabolic risk: a consensus statement from Shaping America’s Health: Association for Weight Management and Obesity Prevention; NAASO, The Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Am J Clin Nutr. 2007;85(5):1197–1202. | |
Kobayashi H, Nakamura T, Miyaoka K, et al. Visceral fat accumulation contributes to insulin resistance, small-sized low-density lipoprotein, and progression of coronary artery disease in middle-aged non-obese Japanese men. Jpn Circ J. 2001;65(3):193–199. | |
Traish AM, Feeley RJ, Guay A. Mechanisms of obesity and related pathologies: androgen deficiency and endothelial dysfunction may be the link between obesity and erectile dysfunction. FEBS J. 2009;276(20):5755–5767. | |
Russo GI, Cimino S, Fragala E, et al. Insulin resistance is an independent predictor of severe lower urinary tract symptoms and of erectile dysfunction: results from a cross-sectional study. J Sex Med. 2014;11(8):2074–2082. | |
Chen S, Wu R, Huang Y, et al. Insulin resistance is an independent determinate of ED in young adult men. PLoS One. 2013; 8(12):e83951. | |
Rey-Valzacchi GJ, Costanzo PR, Finger LA, et al. Addition of metformin to sildenafil treatment for erectile dysfunction in eugonadal nondiabetic men with insulin resistance. A prospective, randomized, double-blind pilot study. J Androl. 2012;33(4):608–614. | |
Pouliot MC, Despres JP, Nadeau A, et al. Visceral obesity in men. Associations with glucose tolerance, plasma insulin, and lipoprotein levels. Diabetes. 1992;41(7):826–834. | |
Nguyen-Duy TB, Nichaman MZ, Church TS, Blair SN, Ross R. Visceral fat and liver fat are independent predictors of metabolic risk factors in men. Am J Physiol Endocrinol Metab. 2003;284(6):E1065–E1071. | |
Banerji MA, Buckley MC, Chaiken RL, Gordon D, Lebovitz HE, Kral JG. Liver fat, serum triglycerides and visceral adipose tissue in insulin-sensitive and insulin-resistant black men with NIDDM. Int J Obes Relat Metab Disord. 1995;19(12):846–850. | |
Despres JP, Lemieux S, Lamarche B, et al. The insulin resistance-dyslipidemic syndrome: contribution of visceral obesity and therapeutic implications. Int J Obes Relat Metab Disord. 1995;19 Suppl 1:S76–S86. | |
Kuk JL, Katzmarzyk PT, Nichaman MZ, Church TS, Blair SN, Ross R. Visceral fat is an independent predictor of all-cause mortality in men. Obesity. 2006;14(2):336–341. | |
Ross R, Dagnone D, Jones PJ, et al. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann Intern Med. 2000;133(2):92–103. | |
Stanworth RD, Jones TH. Testosterone in obesity, metabolic syndrome and type 2 diabetes. Front Horm Res. 2009;37:74–90. | |
Yassin DJ, Doros G, Hammerer PG, Yassin AA. Long-term testosterone treatment in elderly men with hypogonadism and erectile dysfunction reduces obesity parameters and improves metabolic syndrome and health-related quality of life. J Sex Med. 2014;11(6):1567–1576. | |
Aversa A, Bruzziches R, Francomano D, Spera G, Lenzi A. Efficacy and safety of two different testosterone undecanoate formulations in hypogonadal men with metabolic syndrome. J Endocrinol Invest. 2010; 33(11):776–783. | |
Kenny AM, Prestwood KM, Gruman CA, Marcello KM, Raisz LG. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels. J Gerontol A Biol Sci Med Sci. 2001;56(5):M266–M272. | |
Srinivas-Shankar U, Roberts SA, Connolly MJ, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010;95(2):639–650. | |
Katznelson L, Finkelstein JS, Schoenfeld DA, Rosenthal DI, Anderson EJ, Klibanski A. Increase in bone density and lean body mass during testosterone administration in men with acquired hypogonadism. J Clin Endocrinol Metab. 1996;81(12):4358–4365. | |
Mehta N, Sikka S, Rajasekaran M. Rat as an animal model for male erectile function evaluation in sexual medicine research. J Sex Med. 2008;5(6):1278–1283. | |
Maneschi E, Morelli A, Filippi S, et al. Testosterone treatment improves metabolic syndrome-induced adipose tissue derangements. J Endocrinol. 2012;215(3):347–362. | |
Fujioka K, Kajita K, Wu Z, et al. Dehydroepiandrosterone reduces preadipocyte proliferation via androgen receptor. Am J Physiol Endocrinol Metab. 2012;302(6):E694–E704. | |
Wu FC, Tajar A, Beynon JM, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363(2):123–135. | |
Dhaliwal SS, Welborn TA. Central obesity and multivariable cardiovascular risk as assessed by the Framingham prediction scores. Am J Cardiol. 2009;103(10):1403–1407. | |
Despres JP, Couillard C, Gagnon J, et al. Race, visceral adipose tissue, plasma lipids, and lipoprotein lipase activity in men and women: the Health, Risk Factors, Exercise Training, and Genetics (HERITAGE) family study. Arterioscler Thromb Vasc Biol. 2000; 20(8):1932–1938. | |
Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11(1):11–18. | |
Capurso C, Capurso A. From excess adiposity to insulin resistance: the role of free fatty acids. Vascul Pharmacol. 2012;57(2–4):91–97. | |
Allan CA, Strauss BJ, Burger HG, Forbes EA, McLachlan RI. Testosterone therapy prevents gain in visceral adipose tissue and loss of skeletal muscle in nonobese aging men. J Clin Endocrinol Metab. 2008;93(1):139–146. | |
Marin P, Holmang S, Gustafsson C, et al. Androgen treatment of abdominally obese men. Obes Res. 1993;1(4):245–251. | |
Marin P, Holmang S, Jonsson L, et al. The effects of testosterone treatment on body composition and metabolism in middle-aged obese men. Int J Obes Relat Metab Disord. 1992;16(12):991–997. | |
Freedland ES. Role of a critical visceral adipose tissue threshold (CVATT) in metabolic syndrome: implications for controlling dietary carbohydrates: a review. Nutr Metab (Lond). 2004;1(1):12. | |
Bjorntorp P. Neuroendocrine factors in obesity. J Endocrinol. 1997;155(2):193–195. | |
Belanger C, Hould FS, Lebel S, Biron S, Brochu G, Tchernof A. Omental and subcutaneous adipose tissue steroid levels in obese men. Steroids. 2006;71(8):674–682. | |
Yanase T, Fan W, Kyoya K, et al. Androgens and metabolic syndrome: lessons from androgen receptor knock out (ARKO) mice. J Steroid Biochem Mol Biol. 2008;109(3–5):254–257. | |
Fan W, Yanase T, Nomura M, et al. Androgen receptor null male mice develop late-onset obesity caused by decreased energy expenditure and lipolytic activity but show normal insulin sensitivity with high adiponectin secretion. Diabetes. 2005;54(4):1000–1008. | |
Lin HY, Xu Q, Yeh S, Wang RS, Sparks JD, Chang C. Insulin and leptin resistance with hyperleptinemia in mice lacking androgen receptor. Diabetes. 2005;54(6):1717–1725. | |
Vanderschueren D, Vandenput L, Boonen S, Van Herck E, Swinnen JV, Bouillon R. An aged rat model of partial androgen deficiency: prevention of both loss of bone and lean body mass by low-dose androgen replacement. Endocrinology. 2000;141(5):1642–1647. | |
Di Sante S, Conners WP, Morgentaler A. Influence of baseline serum testosterone on changes in body composition in response to testosterone therapy. J Sex Med. 2012;9(2):585–593. | |
Steidle C, Schwartz S, Jacoby K, Sebree T, Smith T, Bachand R. AA2500 testosterone gel normalizes androgen levels in aging males with improvements in body composition and sexual function. J Clin Endocrinol Metab. 2003;88(6):2673–2681. | |
Saad F, Haider A, Doros G, Traish A. Long-term treatment of hypogonadal men with testosterone produces substantial and sustained weight loss. Obesity. 2013;21(10):1975–1981. | |
Garban H, Marquez D, Cai L, Rajfer J, Gonzalez-Cadavid NF. Restoration of normal adult penile erectile response in aged rats by long-term treatment with androgens. Biol Reprod. 1995;53(6):1365–1372. | |
Permpongkosol S, Tantirangsee N, Ratana-olarn K. Treatment of 161 men with symptomatic late onset hypogonadism with long-acting parenteral testosterone undecanoate: effects on body composition, lipids, and psychosexual complaints. J Sex Med. 2010;7(11):3765–3774. | |
Hackett G, Cole N, Bhartia M, Kennedy D, Raju J, Wilkinson P. Testosterone replacement therapy with long-acting testosterone undecanoate improves sexual function and quality-of-life parameters vs placebo in a population of men with type 2 diabetes. J Sex Med. 2013;10(6):1612–1627. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.