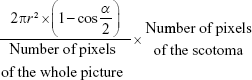Back to Journals » Clinical Ophthalmology » Volume 11
Testing the clinical value of multifocal electroretinography and microperimetry and the effects of intravitreal therapy with ranibizumab on macular function in the course of wet age-related macular degeneration: a 1-year prospective study
Authors Reinsberg M, Hilgers RD, Lüedeke I, Nassar K, Grisanti S , Grisanti S, Lüke J, Lüke M
Received 29 September 2016
Accepted for publication 13 December 2016
Published 6 April 2017 Volume 2017:11 Pages 621—629
DOI https://doi.org/10.2147/OPTH.S123513
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Mihaela Reinsberg,1 Ralf-Dieter Hilgers,2 Inger Lüdeke,1 Khaled Nassar,1 Swaantje Grisanti,1 Salvatore Grisanti,1 Julia Lüke,1,* Matthias Lüke1,*
1University Eye Hospital, University of Lübeck, Lübeck, 2Department of Medical Statistics, RWTH Aachen University, Aachen, Germany
*These authors contributed equally to this work
Purpose: To investigate the clinical value of multifocal electroretinography (mfERG) and microperimetry and the effects of intravitreal therapy with ranibizumab (Lucentis®) on macular function in the course of neovascular age-related macular degeneration (nAMD).
Materials and methods: We conducted a prospective single-arm interventional cohort study with 20 nAMD patients older than 50 years. Examinations were scheduled monthly for 1 year during intravitreal therapy with ranibizumab. The examinations included mfERG, microperimetry, spectral domain optical coherence tomography, and best-corrected visual acuity using ETDRS score.
Results: During the 12-month observation period, a significant positive linear correlation between the logarithm of minimum angle of resolution (logMAR) and scotoma area (r=0.28, 95% confidence interval [CI] 0.21–0.35), between logMAR and fovea thickness in optical coherence tomography (r=0.11, 95% CI 0.04–0.2), and a significant negative correlation between logMAR and mfERG (-0.37, 95% CI -0.43 to -0.31) were observed. A significant ranibizumab effect on logMAR was found (P=0.0065). From a total of 25 relapses, 14 were able to be predicted correctly by mfERG P1 decrease in the preceding month. However, there was no statistically significant relation between prediction and observed relapses (Fisher’s exact test, P=0.6726).
Conclusion: Our results indicate a possible role of mfERG and microperimetry in the monitoring of macular function and prediction of recurrence during intravitreal pharmacotherapy in wet AMD.
Keywords: macular function testing, microperimetry, multifocal electroretinogram, therapy monitoring
Introduction
Age-related macular degeneration (AMD) is a disease of the elderly (>50 years of age) and is the leading cause of blindness in developed countries around the world.1 Because of increasing life expectancy and the resulting increased elderly population, AMD has become a major public health issue in recent decades. AMD is classified into one of two general subgroups: the nonexudative form of the disease and the exudative form of the disease.2 Although less prevalent than the nonexudative form the exudative or neovascular form of AMD (nAMD) causes more sudden, often substantial, loss of central vision than the nonexudative form, and is responsible for most cases of severe loss of visual acuity (VA) in this disease.
The treatment of nAMD has undergone major changes during the past decade. Ranibizumab (Lucentis®; Novartis International AG, Basel, Switzerland) is a recombinant humanized IgG1κ-isotype monoclonal antibody fragment (Fab) that selectively binds all isoforms of VEGF. Ranibizumab (intravitreal injections of 0.5 mg) is approved for nAMD.3,4 Ninety percent of subjects treated with ranibizumab can maintain their vision status, and 30% experience significant improvement with monthly administration of the drug.3,4
However, the frequent injections are a substantial burden to patients and health care systems. Therefore, different treatment regimens have been investigated to maintain efficacy with a reduced number of injections or visits.5–7 Studies published to date have used ophthalmoscopy, VA, and optical coherence tomography (OCT) criteria to guide retreatment but have failed to show improved effectiveness with regimen changes.8
Objectives of the study
Little information (except for VA) exists regarding the development of central retinal function during the course of nAMD under ranibizumab therapy. Discontinuing intravitreal therapy during remission of the disease could result in a relapse, but chronic usage of ranibizumab every month could also result in retinal toxicity or tachyphylaxis, leading to further visual deterioration. Therefore, sophisticated and frequent monitoring of subjects is required to find the right moment to restart therapy before visual loss occurs due to a relapse of nAMD.
Multifocal electroretinography (mfERG) and microperimetry are sensitive techniques to assess macular function. mfERG is a technique developed by Sutter and Tran,9 which allows detailed study of localized disorders affecting the outer retinal layers.9,10 mfERG gives an objective evaluation and mapping of retinal function from several retinal areas and is thought to reflect the cone-mediated responses of the photoreceptor and bipolar cells under normal test conditions.11 Eyes with nAMD show reduced mfERG responses, and they remain largely unchanged during photodynamic therapy.12–16
Perimetry examines the sensitivity of different spatial locations of the retina and is dependent on retinal morphology.17 Microperimetry assesses the sensitivity of the central retina and allows precise topographic correlations of macular anatomy and light sensitivity. These noninvasive measurements also allow detailed analysis of macular function in nAMD.18
The objective of the present study was to investigate central retinal function in subjects with subfoveal choroidal neovascularization (CNV) due to nAMD during intravitreal treatment with ranibizumab and to evaluate the value of mfERG as a sensitive monitoring tool and early predictor of nAMD recurrence. Main outcome measures of the study were changes in macular function observed by mfERG and microperimetry as measured during 12 months.
Materials and methods
This prospective single-center, single-arm longitudinal interventional cohort study was conducted at the University Eye Hospital, University of Lübeck, Germany. The study was approved by the Ethics Committee of the University of Lübeck (10-184, 2010) and conducted in accordance with the Declaration of Helsinki. The study was registered on ClinicalTrials.gov (NCT01269151), and had the EudraCT number 2010-021777-37.
Participants and interventions
All subjects screened for inclusion in the study had had diagnosed nAMD in their clinical history. Subjects meeting the inclusion criteria were included in the study after they had signed the written informed consent. Inclusion criteria were a diagnosis of nAMD, over 50 years of age, written informed consent, available for 12 months of follow-up, and best-corrected VA (BCVA) of 0.1 or better. Exclusion criteria were concomitant systemic or ocular conditions such as severe cardiovascular, renal, or hepatic disease, stroke, ocular inflammatory and infectious disease, angle-block glaucoma, and macular and retinal dystrophies.
After a prescreening evaluation, the subjects underwent a full ophthalmic examination, including BCVA measurement, dilated fundus examination, intraocular pressure measurement, OCT, color fundus photos, and fluorescein angiography (FlA) (Table 1). BCVA was measured in a full-refraction protocol with standardized illumination and recorded as letters read at 4 m and 1 m on ETDRS charts. If a subject read fewer than 20 letters at 4 m, then the BCVA test was performed at 1 m. Final BCVA (logarithm of minimum angle of resolution [logMAR]) letter score was calculated by the number of letters read at 4 m plus 30 (or letters read at 1 m). After an explanation of the procedures and assessment, subjects who consented to participate in the study were monitored monthly.
Subjects included in the study received three intravitreal injections of 0.5 mg ranibizumab (one every 4 weeks) if the disease relapsed. The treatment was adapted to the PrONTO study and did not differ from standard clinical practice in cases with nAMD.19 Subjects underwent assessments before the injections and were monitored monthly. After three injections, monthly therapy with ranibizumab was continued if no further improvement or increased visual function could be achieved. All assessments were repeated monthly as performed at baseline. FlA was performed only if a recurrence was suspected. Reinjection was considered based on changes in BCVA, OCT, and FlA findings. The decision to retreat was made by an independent third person and based on any of the criteria of recurrence: visual loss (five letters), subretinal fluid observed using OCT or cystoid macular edema, new retinal bleeding, and active leakage observed using FlA.
Amplitudes of mfERG 1 month before relapse of the disease (month – 1) were compared to mfERG amplitude 2 months before the relapse (month – 2). If amplitude at month – 1 was smaller than amplitude at month – 2, the relapse was classified as electrophysiologically detectable and could have been recognized as such.
Study assessments at each visit are summarized in Table 1. OCT scans were all obtained with the same spectral domain OCT (SD-OCT) machine (Spectralis, software version 5.3.3.0, Eye Explorer software 1.8.6.0; Heidelberg Engineering GmbH, Heidelberg, Germany). Central foveal thickness (CFT) was calculated as the mean thickness of the central subfield in the map generated from the raster scans by the provided software. Any segmentation errors at the internal limiting membrane and/or at the apical retinal pigment epithelium within the central foveal region (center 1 mm) were identified and corrected manually before analysis. FlA was performed with a scanning laser ophthalmoscope (Retina Angiograph; Heidelberg Engineering) or via SD-OCT.
Multifocal ERG
mfERG was recorded with a cathode-ray tube monitor (RetiScan 3.22.0.1; Roland Instruments, Wiesbaden, Germany) projecting stimuli from 61 segments across 28° of the field and with a red-fixation cross extending to all four corners of the screen to facilitate central fixation. Furthermore, subjects were coached for steady fixation and observed throughout the test. The stimuli from each of the 61 segments were controlled by a binary sequence (m-sequence 511 samples with eight cycles). The mean screen luminance during testing was 120 cd/m2. mfERG scans were recorded monocularly from each eye with Dawson–Trick–Litzkow thread electrodes referenced to each lateral canthus and a ground electrode placed at the earlobe. The responses were registered at 10–100 Hz and filtered by a smooth filter at 50 Hz. The 61 segments were then averaged into five concentric rings and described by P1-amplitude density (nV/deg2) and latency (ms). P1 amplitude was measured from N1 trough to P1 peak, whereas P1 latency was the time from the onset of the light stimulus until the P1 peak.
Microperimetry
Macular sensitivity was evaluated by scanning laser ophthalmoscopy (SLO; Rodenstock GmbH, Düsseldorf, Germany), software version 3.0x. SLO microperimetry enables an exact, point-to-point correspondence between fundus image and perimetric results. A helium–neon laser beam (633 nm) and an infrared diode laser (780 nm) are simultaneously projected onto the retina and detected through a selection of confocal apertures. The infrared laser, which is invisible to the subject, is used for retinal imaging, allowing a satisfactory exploration when lens or vitreous opacities are present. Confocal SLO with graphic capabilities allows the investigator to determine the retinal location of visual stimuli on the retinal image in real time. The stimuli are observed by the subject while images of the stimuli are seen on the subject’s retina by the observer. The central fixation stimulus is a cross directly projected onto the foveal area, visualized on the monitor display. SLO provides a 33°×21° image of the fundus, with a minimum resolution of a 4-minute arc (20 μm) for measurement and positioning of targets. Microperimetry can be performed with different-sized point stimuli, ranging from Goldmann I to IV.20–22 The intensity of the stimulus is inversely related to the tested retinal sensitivity, expressed as alphabetic letters from A (corresponding to 0 dB) to G (corresponding to 32 dB). Missed stimuli, represented by the corresponding letter, were also stored and visualized with a small dark square. Manual static perimetry was performed with a concentric grid centered on the fovea and the image then fixed on the monitor of the SLO. The first stimulus was presented at 15 dB. Thereafter, light intensity was reduced by 2 dB after a correct answer and increased by 1 dB if the stimulus was not perceived. The operator selected a retinal landmark that could be easily identified, such as a vessel bifurcation. Immediately after the detection of each stimulus, the operator positioned a cursor over the retinal landmark, which enabled the computer to calculate the real point of fixation, as well as the actual location of the stimulus.
Macular sensitivity, expressed in decibels of the fixation point, was tested and analyzed. SLO examination enabled assessment of the relationship between the retinal fixation locus and CNV. The extent of the absolute scotoma was determined using a 0 dB stimulus in all subjects. Once the absolute scotoma was completely defined, the digital fundus image was frozen and saved on the hard disk.
The digital fundus image was exported as a TIFF file and edited with ImageJ (version 1.45s). Points that were seen by the subject were depicted in green and the others in red. The scotoma area was plotted manually between the seen and not-seen points. The scotoma area measured in pixels of the image was transformed to square millimeters using the individual length of the eye (r) and a scanned angle (α) of 40° according to the following formula:
|
Outcomes
The main outcome measures were changes in central retinal function monitored by mfERG and microperimetry, as measured at 12 months compared with baseline. Secondary outcomes were changes in BCVA and OCT (CFT) compared to baseline. We compared and statistically correlated the mfERG P1-response amplitudes (nV/deg2) and the area of the scotoma measured using microperimetry (mm2) with BCVA and fovea thickness (μm), measured using SD-OCT, and analyzed the development of these four criteria during the 12-month follow-up.
Statistical methods
Results are described as mean and standard deviation (SD). Bootstrap estimates (medians) and 95% confidence intervals (CIs; quintile estimates) for intersubject correlations based on the Hamlett multilevel model or Roy multilevel model are presented.23,24 The bootstrap settings were seed 1,567, sample size 10,000, and no stratification. Where the Hamlett multilevel model could not be fitted to the data, weighted Pearson correlation coefficients with 95% CIs (via Fisher z-transformation) for intersubject correlations were calculated. LogMAR BCVA, OCT, mf ERG P1, and scotoma area were correlated pairwise.
Response following ranibizumab application was analyzed by fitting a two-level mixed-effect model to the data.25,26 The subjects served as random effect, and ranibizumab application (yes/no) and measurement before treatment were the fixed covariables. Effects were assessed as significant if the P-value was below 0.05 (5% level). Statistical Analysis System software (9.3 [TS1M2]) on Windows X64_7PRO was used for the statistical calculations.
Results
Participant flow and recruitment
A total of 20 subjects were enrolled in this study between December 2010 and November 2011 at the University Eye Hospital of Lübeck. The enrollment of the subjects is shown in Figure 1. Of the 27 subjects who were assessed for eligibility, seven declined to participate, although they met the inclusion criteria, and were thus excluded from the study. All 20 subjects included in the study received the allocated intervention. Two of them were lost to follow-up: one due to hospitalization, and a second withdrew voluntarily. In one case, the intervention was discontinued, due to a transient ischemic attack. The data of all 20 subjects were analyzed.
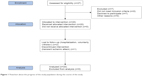  | Figure 1 Flowchart about the progress of the study population during the course of the study. |
Baseline data
All 20 subjects were Caucasian, and 15 of the subjects were female. The mean age was 76 (SD 6.8) years, with the youngest subject being 60 years old and the oldest subject 89 years old. There were 13 left eyes and seven right eyes enrolled in the study. Nine of the 20 eyes were pseudophakic, ten had a cataract, and one subject had a clear lens. Mean BCVA at screening was 0.4 logMAR (SD 0.2, range 0–0.7). Mean intraocular pressure at screening was 15 (SD 2.5, range 10–19) mmHg. Mean mfERG-amplitudes at screening were P1 52.6 (SD 44.8) nV/deg2, P2 31.2 (SD 15.1) nV/deg2, P3 24.9 (SD 8.8) nV/deg2, P4 19 (SD 6.4) nV/deg2, and P5 15.3 (SD 6.1) nV/deg2. Mean scotoma area at screening was 0.6 (SD 0.8) mm2, and mean fovea thickness on OCT was 284 (SD 100.1) μm (Table 2).
Hypertension was a concomitant medical condition in 13 of the 20 subjects. None of the 20 subjects suffered from arrhythmia. Other diseases were present in 17 subjects. Nine of the 20 subjects had received systemic anticoagulation or antiaggregation therapy when they were incorporated in the study. Seventeen subjects had already been treated previously with ranibizumab injections, and three had received local antiglaucoma therapy (Table 2).
Outcomes
At the screening visit, we observed a mean logMAR of 0.38 (SD 0.22). At the 12-month follow-up, mean logMAR was 0.41 (SD 0.29), resulting in a decrease in VA (increase of LogMAR of 0.04 (SD 0.27)). Mean fovea thickness in OCT was 284 (SD 101) μm at screening and had decreased by 22 (SD 166) μm to 267 (SD 106) μm at the 12-month follow-up. Mean P1 response amplitude in mfERG had increased by 1.6 (SD 26.9) nV/deg2 from 52.6 (SD 44.9) nV/deg2 at screening to 54.3 (SD 40.3) nV/deg2 at the 12-month follow-up. We observed a mean scotoma area of 0.55 (SD 0.84) mm2 at screening and a mean scotoma area of 0.89 (SD 1.37) mm2 at the 12-month follow-up, resulting in an increase of 0.33 (SD 1.44) mm2 (Table 3). We found a significant positive linear correlation between logMAR and scotoma area of 0.28 (95% CI 0.21–0.35), as well as with OCT of 0.11 (95% CI 0.04–0.2). We also found a negative correlation between logMAR and ERG P1 of −0.37 (95% CI −0.43 to −0.31).
We investigated the treatment effect of ranibizumab on logMAR, OCT, mfERG P1, and scotoma area by fitting a two-level mixed-effect model. A significant treatment effect on logMAR was observed with ranibizumab (F=7.55, ndf=1, ddf=204; P=0.0065), with adjusted least-square means of 0.31 (SD 0.02) and 0.36 (SD 0.02) without ranibizumab. In addition, there was a significant treatment effect seen with ranibizumab (F=34.09, ndf=1, ddf=135; P<0.0001) on OCT, with adjusted least-square means of 229.45 (SD 5.81) with ranibizumab and 254.11 (SD 6) without. We selected an unstructured covariance of the time effect and deleted the observations of subjects 1, 2, 7, 11, 10, 13, and 17 from the analysis set, because of their high influence on the parameter estimates. We observed a nonsignificant treatment effect under Lucentis (F=34.09, ndf=1, ddf=135; P=0.8554) on mfERG P1. We selected an unstructured covariance of the time effect and deleted the observations of subjects 1, 3, 9, and 12 from the analysis set, because of their high influence on the parameter estimates. We also observed a nonsignificant treatment effect under Lucentis (F=2.05, ndf=1, ddf=133; P=0.1541) on scotoma area. We selected an unstructured covariance of the time effect and deleted the observations of subjects 6, 7, 8, 13, 14, 15, and 18 from the analysis set, because of their high influence on the parameter estimates.
We further considered the number of ERG P1 predicted relapses, based on the criteria that define a relapse using VA, SD-OCT, FlA, and funduscopy. Of 186 observations, a total of 25 relapses were observed, of which 14 were able to be predicted correctly by ERG P1 decrease in the preceding month. There was no statistically significant relation between predicted and observed relapses (Fisher’s exact test, P=0.6726). Overall, total injections during the 12-month period numbered 156.
Adverse events
A total of 12 adverse events and five severe adverse events were reported during this trial. Three of them were hospitalization; one was a transient ischemic attack and one subject died. No serious adverse reactions or suspected unexpected serious adverse reactions were identified or reported during this study, and no new relevant safety findings were identified. The safety data were in accordance with the previous cumulative experience for the investigational medicinal product ranibizumab.
Discussion
In nAMD patients, a functional documentation of central and peripheral retinal areas is required to estimate a patient’s reading and mobility performance. Large clinical trials investigating anti-VEGF therapy have focused on VA as a functional outcome measure, though VA reflects only the function of about the central 1° of the retina. In contrast, mfERG measures neuroretinal function up to 25° in diameter and reflects potentially hypoxic effects on retinal function.27 Hypoxia is likely to occur in cases of choroidal atrophy, which have been described in relation to deprivation of VEGF from the choroid during anti-VEGF therapy.28,29
This prospective longitudinal interventional cohort study showed that mfERG improves in subjects with nAMD during intravitreal ranibizumab treatment. However, the effects on P1 amplitude were not statistically significant, in contradiction to another study that showed a significant improvement during the initiation phase of ranibizumab treatment, with a significant increase in the density of the central ring of mfERG after the first injection that remained stable during the treatment (three injections, one per month). However, in this study, mfERG was recorded using a 19-segment stimulus over the central 42° diameter field; this is a deviation from International Society for Clinical Electrophysiology of Vision (ISCEV) guidelines, which recommend a stimulus containing 61 or 103 segments.30 The technique applied in this cohort of elderly patients allowed the rapid recording of high-quality traces from subjects with reduced ability for stable fixation.
We performed this study according to ISCEV guidelines, using 61 segments.31 The missing significant improvement in P1 could be explained by unstable fixation during the measurements, which is a problem in subjects with nAMD. Another explanation is that most of our subjects were not native subjects with freshly diagnosed nAMD. The limited improvement in P1 could also have been a sign of limited recuperation capability of the macula during nAMD.
The mechanisms of visual loss in nAMD are unclear, and the dysfunction of many different retinal cells is probably involved. The standard mfERG is largely shaped by On- and Off-bipolar cell activity, with smaller contributions from the photoreceptors and inner retinal (eg, amacrine and ganglion) cells.11 The mean increase of the mfERG signal that we recorded in subjects after intravitreal injection of ranibizumab suggests that this treatment induces the expected recovery in the function of one or more cell populations contributing to the genesis of this signal.
In this study, we demonstrated for the first time that most relapses in nAMD could be detected by mfERG P1 before they could be detected using VA testing, SD-OCT, FlA, or funduscopy. However, mfERG failed to predict all relapses based on the criteria for a relapse. Further studies are warranted to identify a subgroup that can benefit from a more intense functional evaluation, including mfERG, to determine the perfect injection rhythm and prevent functional loss in a long course of therapy.
Our further mfERG results were similar to results of previous studies that have investigated mfERG in eyes receiving intravitreal bevacizumab. A recently reported similarly sized study on subjects receiving a pro re nata (PRN) bevacizumab treatment regimen showed improvements in mfERG at 1 week, persisting over 6 months of follow-up; the earliest improvement occurred in central rings, with improvement extending to peripheral rings in later examinations during the follow-up period. Significant improvements at 2 weeks and 1 month after single doses of bevacizumab have been reported previously in studies with fewer patients.32–34 One of these studies noted a loss of effect at 3 months after a single injection.34 Pedersen et al investigated safety using a full-field ERG and detected a reduction in response to bevacizumab treatment at month 3, and speculated that this may have indicated some effect on choroidal function, possibly related to drug penetration of the retina.32 In a previous study, Moschos et al investigated the effects of the intravitreal use of bevacizumab on the retinal function of subjects with nAMD by means of mfERG during a follow-up of 3 months.34 They found that neuroretinal function after bevacizumab did not change in most of the subjects compared to baseline values. The preliminary results of Feigl et al35 in an observational case series of three patients with nAMD receiving ranibizumab confirmed the findings of Moschos et al. The authors found no significant change in the central and peripheral neuroretinal function of subjects with nAMD before and after treatment with ranibizumab. Although the number of subjects included in our study was relatively small, the results suggest that ranibizumab has no adverse effect on cellular function as measured by mfERG, at least in the short term, with similar findings to the ones for bevacizumab.33,34
Finally, we found a relationship between VA and the retinal response density of the foveal area (ring 1). However, no significant correlation was observed between the mfERG and any of the retinal thickness measured with OCT. Comparably, Yip et al reported no significant correlation between the mfERG of various rings and the OCT parameters in eyes with central serous chorioretinopathy.36 These findings demonstrate that functional assessment using mfERG better reflects the level of VA, and not anatomical evaluation by OCT. Therefore, macular thickness does not necessarily reflect the course of VA, as supported by other studies.37
However, we found a significant positive linear correlation between logMAR and scotoma area, as well as fovea thickness. We also found a negative correlation between logMAR and mfERG. Retinal sensitivity, as measured by microperimetry, may be more sensitive to changes in macular function, because it assesses a larger retinal area than the conventional distance BCVA.38 The ability to perform activities of daily living is strongly dependent on preservation of the central visual field.39 The size of the central scotoma has already been established as an important determinant of reading capability, reading speed, and the ability to benefit from handheld magnifying lenses.40
Examination of the effect of anti-VEGF treatment on retinal sensitivity of the central visual field has been examined in previous studies with similar findings. In a retrospective 24-month follow-up study, Parravano et al used ranibizumab to treat 18 subjects with nAMD and noted that although VA and retinal thickness changes were maximal at 4 weeks after intravitreal ranibizumab, retinal sensitivity continued to show an improvement and only two of the 18 subjects showed a reduction in threshold sensitivity despite treatment with ranibizumab.41 Prager et al treated 14 subjects (21 eyes) with systemic bevacizumab therapy, administered as three initial infusions (one infusion every 2 weeks), followed by retreatment in the event of CNV recurrence. They noted that systemic bevacizumab induced a significant increase in mean retinal sensitivity and a reduction in absolute scotoma size.42 Ozdemir et al found that intravitreal bevacizumab therapy in previously untreated subjects induced a significant increase in mean retinal sensitivity and a reduction in mean scotoma size.43
Our study documented and correlated additionally functional and morphological changes in the maintenance phase of intravitreal ranibizumab therapy and found that all subjects maintained VA over the study period. No signs of retinal toxicity were observed, such as induction of choroidal or retinal pigment-epithelium atrophy during nAMD under long-term ranibizumab therapy, which were observed elsewhere recently.44
Hatef et al showed that in subjects with a retinal thickness of 280 mm or less at the fovea, retinal thinning was associated with decreased retinal sensitivity. However, above 280 mm, retinal edema was associated with decreased sensitivity.45 These data support the view that there is an optimal range of retinal thickness that correlates with optimal retinal sensitivity.
There are several psychophysical tests that can be used to quantify macular function, including VA, contrast sensitivity, photo stress testing, mfERG, and visual field sensitivity. Of these, VA remains the most important parameter to quantify the functional impact of AMD and make treatment decisions in individual subjects in daily practice, although further functional tests such as microperimetry and mfERG may add valuable additional information. Our study has provided novel information on the relationship between macular sensitivity, retinal thickness, and VA in the maintenance phase of ranibizumab therapy for nAMD. Subjects may have stable VA and retinal thickness but can still have deteriorating retinal sensitivity, suggesting that this is a late-stage manifestation of the disease.
Conclusion
Our results show that in subjects with nAMD, intravitreal ranibizumab therapy is associated with an improvement in mfERG, as well as a reduction in OCT thickness (CFT) and an improvement in VA. mfERG appears to be a useful tool in evaluating the changes in macular function during anti-VEGF therapy. Larger studies are warranted to determine whether the increased mfERG response is maintained, the influence of a PRN treatment regimen, and the general prognostic value of mfERG in predicting changes in VA.
Limitations
This study was performed according to the ISCEV guidelines and used 61 hexagons in mfERG. The missing significant improvement in P1 could be explained by an unstable fixation during the measurements, which is a problem in subjects with nAMD. Additionally, some subjects who had very unstable, unreproducible responses were excluded from the final analysis.
Acknowledgments
This study was supported by a grant of Novartis GMBH, Nuremberg, Germany. The authors would like to thank Dr Claudia Frumento for editorial support.
Disclosure
The authors report no conflicts of interest in this work. Swaantje and Salvatore Grisanti are husband and wife, as are Matthias Lüke and Julia Lüke.
References
Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1992;99:933–943. | ||
Bressler N, Bressler S, Fine S. Age-related macular degeneration. Surv Ophthalmol. 1988;32:375–413. | ||
Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. | ||
Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–1444. | ||
Regillo CD, Brown DM, Abraham P, et al. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER study year 1. Am J Ophthalmol. 2008;145:239–248. | ||
Lalwani GA, Rosenfeld PJ, Fung AE, et al. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO Study.Am J Ophthalmol. 2009;148(1):43–58.e1. | ||
Kumar A, Sahni JN, Stangos AN, Campa C, Harding SP. Effectiveness of ranibizumab for neovascular age-related macular degeneration using clinician-determined retreatment strategy. Br J Ophthalmol. 2011;95:530–533. | ||
Mitchell P, Korobelnik JF, Lanzetta P, et al. Ranibizumab (Lucentis) in neovascular age-related macular degeneration: evidence from clinical trials. Br J Ophthalmol. 2010;94:2–13. | ||
Sutter EE, Tran D. The field topography of ERG components in man – I: the photopic luminance response. Vision Res. 1992;32:433–446. | ||
Hood DC. Assessing retinal function with the multifocal technique. Prog Retin Eye Res. 2000;19:607–646. | ||
Hood DC, Frishman LJ, Saszik S, Viswanathan S. Retinal origins of the primate multifocal ERG: implications for the human response. Invest Ophthalmol Vis Sci. 2002;43:1673–1685. | ||
Jurklies B, Weismann M, Hüsing J, Sutter EE, Bornfeld N. Monitoring retinal function in neovascular maculopathy using multifocal electroretinography: early and long-term correlation with clinical findings. Graefes Arch Clin Exp Ophthalmol. 2002;240:244–264. | ||
Heinemann-Vernaleken B, Palmowski A, Allgayer R, Ruprecht KW. Comparison of different high resolution multifocal electroretinogram recordings in subjects with age-related maculopathy. Graefes Arch Clin Exp Ophthalmol. 2001;239:556–561. | ||
Huang S, Wu D, Jiang F, et al. The multifocal electroretinogram in age-related maculopathies. Doc Ophthalmol. 2001;101:115–124. | ||
Ruther K, Breidenbach K, Schwartz R, Hassenstein A, Richard G. Prüfung der zentralen Netzhautfunktion mit dem multifokalen Elektroretinogramm vor und nach photodynamischer Therapie [Testing central retinal function with multifocal electroretinography before and after photodynamic therapy]. Ophthalmologe. 2003;100:459–464. German. | ||
Mackay AM, Brown MC, Hagan RP, Fisher AC, Grierson I, Harding SP. Deficits in the electroretinogram in neovascular age-related macular degeneration and changes during photodynamic therapy. Doc Ophthalmol. 2007;115:69–76. | ||
Lang G. Ophthalmology. 2nd ed. New York: Thieme; 2007. | ||
Querques G, Forte R, Longo C, et al. La microperimetrie dans la degenerescence maculaire liee a l’age [Microperimetry in age-related macular degeneration]. J Fr Ophtalmol. 2008;31:515–521. French. | ||
Fung AE, Lalwani GA, Rosenfeld PJ, et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007;143:566–583. | ||
Varano M, Scassa C. Scanning laser ophthalmoscope microperimetry. Semin Ophthalmol. 1998;13:203–209. | ||
Rohrschneider K, Fendrich T, Becker M, et al. Static fundus perimetry using the scanning laser ophthalmoscope with an automated threshold strategy. Graefes Arch Clin Exp Ophthalmol. 1995;233:743–749. | ||
Rohrschneider K, Becker M, Schumacher N, Krastel H, Kruse FE, Völcker HE. Normal values for fundus perimetry with the scanning laser ophthalmoscope. Am J Ophthalmol. 1998;126:52–58. | ||
Hamlett A, Ryan L, Wolfinger R. On the use of Proc Mixed to estimate correlation in the presence of repeated measures. 2004. Available from: http://www2.sas.com/proceedings/sugi29/198-29.pdf. Accessed January 19, 2017. | ||
Roy A. Estimating correlation coefficient between two variables with repeated observations using mixed effects model. Biom J. 2006;48:286–301. | ||
Singer J. Using SAS Proc Mixed to fit multilevel models, hierarchical models, and individual growth models. J Educ Behav Stat. 1998;23:323–355. | ||
Van den Noortgate W, Onghena P. Combining single case experimental data using hierarchical linear models. Sch Psychol Q. 2003;18:325–346. | ||
Feigl B, Brown B, Lovie-Kitchin J, Swann P. Functional loss in early age-related maculopathy: the ischaemia postreceptoral hypothesis. Eye (Lond). 2007;21:689–696. | ||
McLeod D, Taomoto M, Otsuji T, Green W, Sunness J, Lutty G. Quantifying changes in RPE and choroidal vasculature in eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2002;43:1986–1993. | ||
Marneros A, Fan J, Yokoyama Y, et al. Vascular endothelial growth factor expression in the retinal pigment epithelium is essential for choriocapillaris development and visual function. Am J Pathol. 2005;167:1451–1459. | ||
Campa C, Hagan R, Sahni JN, et al. Early multifocal electroretinogram findings during intravitreal ranibizumab treatment for neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52:3446–3451. | ||
Hood DC, Bach M, Brigell M, et al. ISCEV guidelines for clinical multifocal electroretinography (2007 edition). Doc Ophthalmol. 2008;116:1–11. | ||
Pedersen KB, Møller F, Sjølie AK, Andréasson S. Electrophysiological assessment of retinal function during 6 months of bevacizumab treatment in neovascular age-related macular degeneration. Retina. 2010;30:1025–1033. | ||
Karanjia R, Eng KT, Gale J, Sharma S, ten Hove MW. Electrophysiological effects of intravitreal Avastin (bevacizumab) in the treatment of exudative age-related macular degeneration. Br J Ophthalmol. 2008;92:1248–1252. | ||
Moschos M, Brouzas D, Apostolopoulos M, Koutsandrea C, Loukianou E, Moschos M. Intravitreal use of bevacizumab (Avastin) for choroidal neovascularization due to ARMD: a preliminary multifocal-ERG and OCT study – multifocal-ERG after use of bevacizumab in ARMD. Doc Ophthalmol. 2007;114:37–44. | ||
Feigl B, Greaves A, Brown B. Functional outcomes after multiple treatments with ranibizumab in neovascular age-related macular degeneration beyond visual acuity. Clin Ophthalmol. 2007;1:167–175. | ||
Yip YW, Ngai JW, Fok AC, et al. Correlation between functional and anatomical assessment by multifocal electroretinography and optical coherence tomography in central serous chorioretinopathy. Doc Ophthalmol. 2010;120:193–200. | ||
Lipski A, Bornfeld N, Jurklies B. Multifocal electroretinography in subjects with exudative AMD and intravitreal treatment with pegaptanib sodium. Retina. 2007;27:864–872. | ||
Okada K, Kubota-Taniai M, Kitahashi M, Baba T, Mitamura Y, Yamamoto S. Changes in visual function and thickness of macula after photodynamic therapy for age-related macular degeneration. Clin Ophthalmol. 2009;3:483–488. | ||
McClure ME, Hart PM, Jackson AJ, Stevenson MR, Chakravarthy U. Macular degeneration: do conventional measurements of impaired visual function equate with visual disability? Br J Opthalmol. 2000;84:244–250. | ||
Ergun E, Maár N, Radner W, Barbazetto I, Schmidt-Erfurth U, Stur M. Scotoma size and reading speed in subjects with subfoveal occult choroidal neovascularization in age-related macular degeneration. Ophthalmology. 2003;110:65–69. | ||
Parravano M, Oddone F, Tedeschi M, et al. Retinal functional changes measured by microperimetry in neovascular age-related macular degeneration treated with ranibizumab: 24-month results. Retina. 2010;30:1017–1024. | ||
Prager F, Michels S, Simader C, Geitzenauer W, Schmidt-Erfurth U. Changes in retinal sensitivity in subjects with neovascular age-related macular degeneration after systemic bevacizumab (Avastin) therapy. Retina. 2008;28:682–688. | ||
Ozdemir H, Karacorlu M, Senturk F, Karacorlu SA, Uysal O. Microperimetric changes after intravitreal bevacizumab injection for exudative age-related macular degeneration. Acta Ophthalmol. 2012;90:71–75. | ||
Grunwald JE, Daniel E, Huang J, et al. Risk of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2014;121:150–161. | ||
Hatef E, Colantuoni E, Wang J, et al. The relationship between macular sensitivity and retinal thickness in eyes with diabetic macular edema. Am J Ophthalmol. 2011;152:400–405. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.