Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Terbinafine Induced Acute Generalized Exanthematous Pustulosis Treated with Adalimumab: Recalcitrant to Systemic Corticosteroid Therapy
Authors Deng L , He B, Ali K , Bu Z
Received 2 October 2022
Accepted for publication 16 December 2022
Published 4 January 2023 Volume 2023:16 Pages 9—15
DOI https://doi.org/10.2147/CCID.S391979
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Lin Deng,1 Beilei He,2 Kamran Ali,1,3 Zhangyu Bu1
1Department of Dermatology Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine, Hangzhou, People’s Republic of China; 2Department of the Fourth Clinical Medical College, Zhejiang Chinese Medical University, Hangzhou, People’s Republic of China; 3Department of Dermatology, International Education College of Zhejiang Chinese Medical University, Hangzhou, People’s Republic of China
Correspondence: Zhangyu Bu, Department of Dermatology, Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine, No. 261, Huansha Road, Hangzhou, People’s Republic of China, Tel +8657156006617, Email [email protected]
Abstract: Acute generalized exanthematous pustulosis (AGEP) is generally caused by drugs and is characterized by the rapid development of numerous non-follicular sterile pustules on an erythematous base, fever, and neutrophilia. We report an association between terbinafine, with AGEP, and adalimumab treatment. A 24-year-old teenage female patient with a history of onychomycosis was treated with terbinafine. On the second day of the first dose, multiple edematous and erythematous lesions appear with pinhead pustules. Neutrophilia was observed in the blood report. The clinical history, lesions, and laboratory evaluations were consistent with AGEP. We discontinued the terbinafine therapy, and the systemic corticosteroid was initiated; however, the patient’s condition worsened. Adalimumab subcutaneously was initiated, and the symptoms cleared up in weeks. The European Study of Severe Cutaneous Adverse Reactions (EuroSCAR) scoring system and Naranjo’s algorithm scale were used to check the possibility of a drug-induced adverse reaction. The Association of AGEP with the terbinafine drug is not rare. However, there are no reports or literature of drug-related rash or exanthematous eruptions unresponsive to corticosteroids and treated with adalimumab.
Keywords: acute generalized exanthematous pustulosis, AGEP, adalimumab, terbinafine, drug-induced adverse reaction, Naranjo’s algorithm scale, EuroSCAR
Introduction
An Acute generalized exanthematous pustulosis (AGEP) is a severe cutaneous reaction characterized by the acute onset of sterile non-follicular pinhead pustules on an erythematic base.1 Several drugs lead to AGEP, which causes more than 90% of cases, including antibiotics (Penicillin, vancomycin, and cephalosporins), antifungals (terbinafine, fluconazole), calcium channel blockers, and antimalarial agents.2 The AGEP is a self-limiting disease, and discontinuing the causative agent and topical and systemic corticosteroids will reduce hospitalization.3 Adalimumab is a human recombinant monoclonal antibody that targets soluble and cell-bound tumor necrosis factor-alpha (TNF-a inhibitor). It is approved for the treatment of inflammatory bowel diseases, rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, and psoriatic arthritis skin manifestations.4 However, the efficacy of Adalimumab in AGEP is not established. We report successful treatment of AGEP recalcitrant to systemic corticosteroid with adalimumab.
Case Presentation
A 24-year-old female with generalized erythema accompanied by fever for ten days, aggravated for four days, came to our hospital for treatment on April 19, 2022. Ten days ago, the patient developed erythema on the left shoulder and right forearm, with a few pustules accompanied by itching. The local community health hospital for treatment was misdiagnosed with “insect bite dermatitis.” “Ebastine and loratadine tablets for allergy”, Compound Glycyrrhizin Tablets for anti-inflammatory, and Topical Halometasone” were prescribed. The rash aggravated and generalized, and the skin lesions gradually developed to the trunk and extremities, showing largely edematous erythema with dense sterile pinhead to rice-sized pustules with a burning sensation. No improvement was seen.
By asking for a detailed medical history, the patient had “onychomycosis” and received oral antifungal therapy of “Terbinafine Tablets” two days before the lesions appeared. Other than this, the patient was healthy and had no history of any other systemic or topical medication. There was no family history of patients with similar diseases and psoriasis.
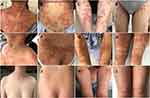
On physical examination, the patient’s temperature was 39°C, pulse 80 times/min, respiration rate 18 times/min, Blood pressure 103/61 mmHg (1 mmHg=0.133kPa), and all other system examinations were normal. On dermatological examination, prominent edematous erythema on the trunk and extremities with dense superficial partially fused sterile non-follicular pin-head size to rice-grain pustules and target lesions was seen the Nikolsky’s sign was negative (Figure 1A–D). There was no mucous membrane involvement.
Laboratory investigations were performed to rule out the infection and systemic complications. The blood routine results showed a high white blood cell count of 13.7×109/L (reference range, 4.0~10.0 ×109/L), leukocyte classification: Neutrophils (N) count of 0.8 (reference range, 0.40~0.75), Lymphocytes(L) 0.14 (reference range, 0.20~0.40), C-reactive protein (CRP) level of 1.4 mg/L (<10 mg/L). Urine routine: protein ±, the fecal routine was normal. The IgA, IgG, IgM, C3, C4, autoantibodies, and vasculitis-related antibodies were unremarkable. Bacterial cultures of pustular fluid were done twice, and the results were negative. Antinuclear antibodies were negative. Liver and renal functions were normal.
Abdominal and urinary system B-ultrasonography findings were normal. Bilateral cervical lymph nodes B-ultrasound showed multiple cervical lymph nodes on both sides. The chest CT showed a slightly thickened right oblique fissure.
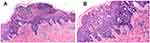
The skin biopsy was performed to rule out the differential diagnosis of Erythema multiforme (EM), generalized pustular psoriasis (GPP), and acute generalized exanthematous pustulosis (AGEP). The histopathological picture showed epidermal hyperkeratosis, parakeratosis, multiple abscess formation in the stratum corneum, spongiosis, and perivascular inflammatory cells containing lymphocytes, eosinophils, and neutrophils in the superficial dermis (Figure 2). The EuroSCAR study group scoring system for AGEP were 11 (Table 1), and the Naranjo Scale for the Adverse Drug Reaction (ADR) probability score was 10 (Table 2),5 giving a definite diagnosis of drug-induced AGEP. The above investigation results were consistent with AGEP. The diagnosis was made based on past medical history, disease course, clinical presentation, and blood and histopathological investigations.
 |
Table 1 The EuroSCAR Study Group Scoring System for AGEP |
 |
Table 2 Naranjo Adverse Drug Reaction (ADR) Probability Scale |
The patient was advised to discontinue the terbinafine, and prompt treatment was required for AGEP to prevent severe systemic complications. Methylprednisolone (40mg intravenous infusion, qd) and compound glycyrrhizin (80mg intravenous infusion, qd) for the inflammation, desloratadine citrate (8.8mg per oral, qd), cetirizine (10mg per oral, qn) and chlorpheniramine (10 mg intramuscularly, qn) were administrated. Omeprazole (40mg IV, qd) was given to protect the stomach complications and was supplemented with symptomatic treatment such as fluid rehydration and electrolyte supplementation. On the 2nd to 4th day after hospitalization, the new erythema and small pustules continued to appear on the trunk and lower extremities after cessation of causative drug and initiation of therapy. The patient was injected with diprosone intramuscular (betamethasone), considering the symptoms were not well controlled with methylprednisolone. On the 5th and 6th days of hospitalization, there were new lesions on the lower limbs, and the rash and pustules got severe on the neck. Considering the above regimen was ineffective, and lesions continued to spread, adalimumab 80mg (2 injections subcutaneously) was injected after discussing the benefits and risks and obtaining written informed consent. On the 3rd day of the adalimumab regimen, erythema and erythematic rash on the neck and trunk began to fade, some pustules dried up, and scaly skin gradually faded (Figure 1E–H). The rash on the upper and lower extremities gradually disappeared after eight days of adalimumab. However, there was a slow recovery of the lower extremities. The patient was discharged from the hospital; no recurrences were noted at the outpatient follow-up 14 days after discharge (Figure 1I–L).
Discussion
The cutaneous side effects of a variety of systemically administered medications are common. The clinical features and mechanisms of these reactions vary, but pustular drug eruptions are uncommon. AGEP is a severe cutaneous rash generally caused by medications or infections. Recognizing AGEP to avoid confusion with systemic infection and improper therapy is essential. The development of multiple disseminated sterile pustules, along with fever, massive neutrophilia, and occasionally eosinophilia, are clinical features of AGEP.6
In this report, the patients had terbinafine for fungal infection, which caused erythematic pustular lesions with abnormal white blood cells, neutrophils, lymphocytes, and C-reactive proteins. The histopathological image showed epidermal hyperkeratosis, parakeratosis, multiple abscess formation in the stratum corneum, spongiosis, lymphocytes, eosinophils, and neutrophil infiltration around the blood vessels in the superficial dermis. AGEP is distinguished from GPP by its abrupt onset, association with recent drug eruptions, spontaneous recovery after discontinuation of the causative agent, absence of recurrence, and absence of personal or family history of psoriasis. Although GPP may show tortuous dilated vessels and psoriasiform hyperplasia, neither of which were observed in our patient’s histopathological image.7,8 The absence of epidermal sloughing and negative Nikolsky sign differentiates it from Toxic epidermal necrolysis(TEN).9 The EuroSCAR study group scoring system for AGEP10 and the Naranjo Scale for the Adverse Drug Reaction(ADR)11 probability score resulted in the definite diagnosis of drug-related AGEP. The diagnosis of AGEP were made based on clinical presentation, blood, histopathological investigation, and scoring system.
Several drugs related to AGEP include pristinamycin, aminopenicillins, quinolones, cephalosporins, vancomycin, diltiazem, hydroxychloroquine, sulfonamides, carbamazepine, ketoconazole, terbinafine, and fluconazole. However, corticosteroids, macrolides, and oxicam are less strongly associated with AGEP.2,5,12,13
The AGEP is a self-limiting disease and discontinuing the causative agent and topical and systemic corticosteroids will reduce hospitalization.3,6,14 The systemic corticosteroids were ineffective in our patient, and the lesions continued to spread. Adalimumab was given after discussing the potential risks and benefits with the patient, and informed consent was taken. Significant improvement was observed; the erythematic rash faded, pustules dried up, and desquamation gradually faded in days.
Adalimumab inhibits the production of TNF alpha by binding to its receptors (soluble and membrane-bound). It inhibits TNF alpha’s interaction with the p55 (TNFR1) and p75 (TNFR2) TNF receptors on cell surfaces, thus interrupting cytokine-driven inflammation. Since it is identical in structure and function to the naturally occurring human IgG1, it is highly selective for TNF alpha and has a low immunogenic potential.4
A study reported that new biological drugs, including anti-IgE (Omalizumab) and Tumor Necrosis Factor-α inhibitors, might be helpful in cases of immediate-type hypersensitivity and cell-mediated hypersensitivity to medications.15
IL-17 has also been implicated in the pathogenesis of AGEP. Patients with AGEP have elevated levels of IL-17, IL-17A, and IL-22 in their peripheral blood. Furthermore, it has been reported that inflammation enhanced by TNF-α plays a crucial role in the pathophysiology of AGEP. According to a study by Gulsum et al, at a molecular level, in the treatment of AGEP with etanercept (TNF-α). TNF-α stimulates inflammation and triggers p53-related apoptosis in tissue cells to prevent genome damage and malign growth. According to these results, although inflammation enhanced by TNF-α appears to be the primary mechanism of AGEP, p53-related apoptosis is also believed to contribute to this disease.16 However, the molecular mechanism of Adalimumab in AGEP is not clear.
Prednisolone therapy failed to benefit our patient, primarily suppressing inflammation, whereas rapid improvement of skin lesions after adalimumab treatment revealed that adalimumab accelerated the healing period in AGEP. TNF-alpha inhibitors have been used in the treatment of drug-induced rashes, including Stevens-Johnson syndrome and toxic epidermal necrolysis. They have been treated with TNF-alpha inhibitors.17 To the best of our knowledge, there are no reports on the treatment of terbinafine-induced AGEP with Adalimumab. However, large-scale studies are required before “anti-TNF- treatment” can be confirmed as a new “gold standard” in treating AGEP.
Key Summary Points
A Case Report of Terbinafine Induced Acute Generalized Exanthematous Pustulosis Treated with Adalimumab: Recalcitrant to Systemic Corticosteroid Therapy.
AGEP related to terbinafine is not rare. To the best of our knowledge, there are no case studies on terbinafine-induced AGEP, which is recalcitrant to systemic corticosteroid and treated with adalimumab (TNF-α) inhibitor.
The drug-induced reaction was confirmed by medical history, histopathological image, EuroSCAR, and Naranjo’s algorithm scale.
Further studies are required to investigate the mechanism of adalimumab in the treatment of AGEP to use it in standard clinical practice.
Ethics Statement
The authors certify that all necessary patient consent forms have been obtained. Written informed consent for publication of their details was obtained from the patients. The ethical committee approval was not needed to publish this case report.
Acknowledgment
The patient in this manuscript has given written informed consent to publish the case details and any accompanying images to be published.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
Funding
This research was conducted without any commercial or financial funding.
Disclosure
All authors declare that the research was conducted without any commercial or financial relationships that could be construed as potential conflicts of interest.
References
1. Creadore A, Desai S, Alloo A, et al. Clinical characteristics, disease course, and outcomes of patients with acute generalized exanthematous pustulosis in the US. JAMA Dermatol. 2022;158(2):176–183. doi:10.1001/jamadermatol.2021.5390
2. Oh DAQ, Yeo YW, Choo KJL, Pang SM, Oh CC, Lee HY. Acute generalized exanthematous pustulosis: epidemiology, clinical course, and treatment outcomes of patients treated in an Asian academic medical center. JAAD Int. 2021;3:1–6. doi:10.1016/j.jdin.2020.12.004
3. Safa I, Ines L, Noureddine L, et al. Acute localized exanthematous pustulosis: clinical features, pathophysiology, and therapy. Dermatol Ther. 2021;34(5):e15087. doi:10.1111/dth.15087
4. Ellis CR, Azmat CE. Adalimumab. In: StatPearls. Treasure Island (FL): StatPearls Publishing LLC; 2022.
5. Wu L, Ali K, Qiu Y, Li M, Da J. Dupilumab-induced acute generalized exanthematous pustulosis in a 17-year-old female Chinese patient with atopic dermatitis. Acta Derm Venereol. 2022;102:adv00743. doi:10.2340/actadv.v102.1079
6. Hadavand MA, Kaffenberger B, Cartron AM, Trinidad JCL. Clinical presentation and management of atypical and recalcitrant acute generalized exanthematous pustulosis. J Am Acad Dermatol. 2020;87(3):632–639. doi:10.1016/j.jaad.2020.09.024
7. Isom J, Braswell DS, Siroy A, Auerbach J, Motaparthi K. Clinical and histopathologic features differentiating acute generalized exanthematous pustulosis and pustular psoriasis: a retrospective series. J Am Acad Dermatol. 2020;83(1):265–267. doi:10.1016/j.jaad.2020.03.015
8. Sussman M, Napodano A, Huang S, Are A, Hsu S, Motaparthi K. Pustular psoriasis and acute generalized exanthematous pustulosis. Medicina. 2021;57(10):1004. doi:10.3390/medicina57101004
9. Copaescu AM, Bouffard D, Masse MS. Acute generalized exanthematous pustulosis simulating toxic epidermal necrolysis: case presentation and literature review. Allergy Asthma Clin Immunol. 2020;16:9. doi:10.1186/s13223-020-0407-5
10. Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)-results of a multinational case-control study (EuroSCAR). Br J Dermatol. 2007;157(5):989–996. doi:10.1111/j.1365-2133.2007.08156.x
11. Rawat BPS, Jagannatha A, Liu F, Inferring YH. ADR causality by predicting the Naranjo score from clinical notes. AMIA Annu Symp Proc. 2020;2020:1041–1049.
12. Lee J, Endicott A, Shinkai K. Acute generalized exanthematous pustulosis. JAMA Dermatol. 2021;157(5):589. doi:10.1001/jamadermatol.2020.5187
13. Liu J, Ali K, Lou H, Wang L, Wu L. First-trimester impetigo herpetiformis leads to stillbirth: a case report. Dermatol Ther. 2022;12(5):1271–1279. doi:10.1007/s13555-022-00735-9
14. Islamoğlu ZGK, Karabağli P. A case of recalcitrant acute generalized exanthematous pustulosis with Sjogren’s syndrome: successfully treated with low-dose cyclosporine. Clin Case Rep. 2019;7(9):1721–1724. doi:10.1002/ccr3.2352
15. Calogiuri G, Brockow K, Nettis E, Macchia L, Foti C, Vacca A. Promising use of the new biologics in the management of drug-induced hypersensitivity reactions: preliminary approaches. Endocr Metab Immune Disord Drug Targets. 2020;20(9):1456–1469. doi:10.2174/1871530320666200515113736
16. Gencoglan G, Tosun M, Aktepe F. The molecular mechanism of etanercept, an anti-tumour necrosis factor-alpha receptor-fusion protein, in the treatment of acute generalized exanthematous pustulosis. J Dermatolog Treat. 2009;20(4):241–245. doi:10.1080/09546630802683843
17. Zhang S, Tang S, Li S, Pan Y, Ding Y. Biologic TNF-alpha inhibitors in the treatment of Stevens-Johnson syndrome and toxic epidermal necrolysis: a systemic review. J Dermatolog Treat. 2020;31(1):66–73. doi:10.1080/09546634.2019.1577548
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.