Back to Journals » Eye and Brain » Volume 8
Targeting VEGF in canine oxygen-induced retinopathy - a model for human retinopathy of prematurity
Received 6 October 2015
Accepted for publication 8 January 2016
Published 20 May 2016 Volume 2016:8 Pages 55—65
DOI https://doi.org/10.2147/EB.S94443
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Margaret Wong-Riley
D Scott McLeod, Gerard A Lutty
Department of Ophthalmology, Wilmer Ophthalmological Institute, Johns Hopkins Hospital, Baltimore, MD, USA
Abstract: Development of the dog superficial retinal vasculature is similar to the mechanism of human retinal vasculature development; they both develop by vasculogenesis, differentiation, and assembly of vascular precursors called angioblasts. Canine oxygen-induced retinopathy (OIR) was first developed by Arnall Patz in an effort to experimentally determine the effects of hyperoxia on the development of the retinal vasculature. The canine OIR model has many characteristics in common with human retinopathy of prematurity. Exposure of 1-day-old dogs to hyperoxia for 4 days causes a vaso-obliteration throughout the retina. Vasoproliferation, after the animals have returned to room air, is robust. The initial small preretinal neovascular formations anastomose to form large preretinal membranes that eventually cause tractional retinal folds. The end-stage pathology of the canine model is similar to stage IV human retinopathy of prematurity. Therefore, canine OIR is an excellent forum to evaluate the response to drugs targeting VEGF and its receptors. Evaluation of an antibody to VEGF-R2 and the VEGF-Trap demonstrated that doses should be titered down so that preretinal neovascularization is inhibited but retinal revascularization is able to proceed, vascularizing peripheral retina and preventing it from being a source of VEGF.
Keywords: angioblasts, blood vessels, endothelial cells, oxygen, retinopathy, retina, vascular endothelial cell growth factor
Introduction
Human retinopathy of prematurity (ROP) results when the developing retinal vasculature in the premature infant is exposed to higher-than normal oxygen levels (hyperoxia). Hyperoxia causes development of the retinal vasculature to cease and vaso-obliteration to occur. As the neural retina continues to develop and consumes greater levels of oxygen, the retina becomes hypoxic due to an attenuated retinal vasculature. The resultant hypoxia causes increased levels of hypoxia inducible factor (HIF) and subsequent increased expression of HIF-inducible angiogenic factors like VEGF cause the vasoproliferative stage in ROP. Oxygen-induced retinopathies (OIRs) are animal models for human ROP. None of the models exactly replicates human ROP, but each has some pathological characteristics that mimic the human disease. Canine OIR was first developed by Arnall Patz in an effort to experimentally determine the effects of hyperoxia on the developing retinal vasculature1,2 and modified by Flower et al.3 Dogs are similar to humans in regards to the mechanism of retinal vascular development, and also the end-stage pathology of the canine model is similar to stage IV human ROP having tractional retinal folds in retina.
Canine retinal vasculature forms by vasculogenesis
Unlike rodents, the superficial retinal vasculature of the dog develops by vasculogenesis and not angiogenesis. Vasculogenesis is the development of blood vessels by differentiation, aggregation, and assembly of vascular precursors or angioblasts (Figure 1). This process is occurring at birth in dogs and begins in humans at around 14 weeks gestation.4–6 The canine retina is only 60% vascularized at birth and angioblasts (positive for adenosine diphosphatase [ADPase] enzyme activity) are present throughout avascular peripheral retina at this time.4,7 The initial assembly of the angioblasts results in cords and then lumen develop within the cords. In dogs and humans, astrocyte (the inner retinal glia) differentiation trails vascular development and guidance is provided by Muller glia not astrocytes, unlike neonatal rodent retinas.5–7 The Muller cells, which span the retina from internal to external limiting membrane, form glycosaminoglycan-rich extracellular spaces in which vasculogenesis occurs4 while also providing adenosine via the enzyme 5′ nucleotidase to stimulate the vasculogenic process.7 Vascular endothelial cell markers (CD34 and von Willebrand’s factor) are not expressed in angioblasts and are expressed only in endothelial cells after lumen formation and presumably the presence of serum within the lumens.
Canine model of oxygen-induced retinopathy
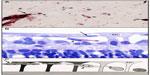
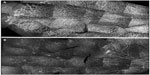
Newborn dogs are placed into 100% oxygen for 4 days to induce retinopathy. This exposure to hyperoxia arrests the development of the superficial retinal vasculature and causes the vaso-obliterative phase of OIR (Figure 2). This results in 60% loss in vascular area and the vaso-obliteration is the greatest in the capillaries, whereas large blood vessels appear viable as determined by ADPase activity (Figure 3).8 The vaso-obliteration occurs throughout the retina but islands of surviving endothelial cells can be found throughout the once-vascularized areas (Figure 3). The loss of vasculature in the presence of a differentiating neuroblastic layer (neuronal progenitors) after removal from hyperoxia results in extreme hypoxia, the initiating event in the vaso-proliferative stage in canine OIR.
  | Figure 2 Vaso-obliteration in the dog model of OIR. |
  | Figure 3 Hyperoxia affects all regions uniformly in the dog OIR model. |
Revascularization of retina occurs in a burst of disorganized angiogenesis that results in a very dense capillary network in the inner retina (Figure 4).9 The extracellular spaces created by Muller cell processes are filled with proliferating astrocytes, and multiple layers of capillaries form the inner retina. At the periphery of the surviving retinal vasculature, glial proliferation occurs resulting in gliosis; in humans, gliosis causes the formation of a ridge of tissue between vascularized and nonvascularized retina, but no anatomical ridge occurs in dogs.10 By postnatal day 15 (P15), tufts of neovascularization can be seen in vitreous with fundus and red free photography (Figure 5).9 Vitreous, the gel in posterior chamber of the eye, normally has had regression of the fetal vasculature occur by this time and is becoming avascular. Fluorescein angiography demonstrates the leaky nature of these formations and the extreme tortuosity of arteries (Figure 5).11 In cross sections, the dense cellularity of these preretinal formations is apparent. Often adjacent tufts of neovascularization will anastomose with each other forming a vascular complex (Figure 6). Eventually the complexes will anastomose forming a dense mat-like structure of new blood vessels, which expands to form an extensive preretinal vascular membrane (Figure 6).12 The membranes, when visualized with fundus photography and fluorescein angiography at P22, appear at times to cover an entire vascular arcade (Figure 7).11 The preretinal membranes persist at least until P45 (Figure 8) when they appear as tented membranes putting tractional force on the retinas. The result is tractional retinal folds in retina at P45 (Figure 8) similar to those formed in stage IV human ROP.
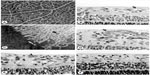  | Figure 4 Vasoproliferative phase of OIR in dog. |
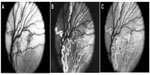  | Figure 5 Intravitreal neovascularization in the dog OIR model. |
  | Figure 6 Possible sequence leading to the evolution of inter-anastomosing neovascular networks in the vitreous from a 22-day-old oxygen-treated animal. |
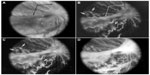  | Figure 7 Clinical appearance of vascularized membranes in oxygen-treated animals. |
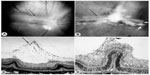  | Figure 8 Tented vascularized membrane and tractional retinal folds in a 45-day-old oxygen-treated animal. |
Anti-VEGF therapy evaluation in the canine OIR model
In all of the OIR models, VEGF plays a key role in vasculogenic and angiogenic processes in retina.12–14 VEGF expression is upregulated in retina during development because there is no retinal vasculature and neurons have become metabolically active, consuming the oxygen that is available. In the dog retina, VEGF receptor-2 (VEGFR2 or kinase domain receptor [KDR]) is present on angioblasts and endothelial cells during development.14 However, this receptor is greatly upregulated in vasoproliferative stage of OIR in forming intra-retinal as well as extra-retinal neovascularization (Figure 9).14 The effects of neutralization of VEGFR2 in the canine OIR model was evaluated by implanting ELVAX slow-release polymer pellets (Elvax 40; Aldrich Chemical, Milwaukee, WI) with 300 μg of anti-VEGFR2 (ImClone Systems, New York City, NY, USA) or control non-immune immunoglobulin in the vitreous at P6, 1 day after the removal of the animals from hyperoxia.14 The animals were sacrificed at P21 and vitreous as well as retinas were incubated for ADPase activity. Anti-VEGFR2 inhibited the formation of preretinal neovascularization, which remains attached to the vitreous body in dog (Figure 10).14 However, anti-VEGFR2 also inhibited the revascularization of retina compared to control IgG, resulting in a reduced retinal vasculature present only in central retina (Figure 10). Analysis of the area of retinal vasculature demonstrated a significant reduction in retinal vasculature in anti-VEGFR2-implanted eyes (Figure 11). Similarly, the reduction in the area of neovascularization in vitreous was significant in anti-VEGFR2 eyes compared to IgG control eyes; therefore, anti-VEGFR2 at the doses used inhibits the formation of preretinal neovascularization as well as revascularization of inner retina.
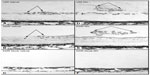  | Figure 9 KDR (VEGFR2) localizationin the canine OIR model. |
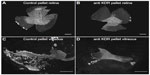  | Figure 10 Effect of anti-KDR on dog OIR. |
  | Figure 11 Effect of anti-KDR on intravitreal and retinal vascular area. |
The effect of VEGF neutralization in canine OIR model was evaluated using the VEGF-Trap (Aflibercept, Regeneron, Tarrytown, NY, USA).The VEGF-Trap is a recombinant fusion protein invented by Regeneron that has the binding sites for VEGF-R1 and -R2 fused with the fragment, crystallizable (Fc) portion of human IgG1. A single 5, 25, or 250 μg dose of VEGF-Trap in 50 μL was injected into the vitreous at P7. Human Fc at the same doses was injected into control eyes. The effects of these reagents were evaluated at P21 by incubating both the vitreous and the retina for ADPase activity. All doses of VEGF-Trap resulted in the complete prevention of preretinal neovascularization (Figure 12).16 The VEGF-Trap also resulted in smaller areas of retinal vasculature at 25 and 250 μg doses (Figure 13); however, the 5 μg trap eyes had normal areas of retinal vasculature (Figure 13). The area of retinal vasculature in 5 μg dose eyes was comparable to control Fc injected eyes, so inner retina could revascularize and reduce hypoxia and VEGF production by retina.
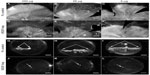  | Figure 12 Retinal vasculature and intravitreal neovascularization after treatment with VEGF-Trap. |
  | Figure 13 Retinal and intravitreal vasculature areas after treatment with VEGF-Trap |
Conclusion
The canine OIR model has many features in common with human ROP. There is profound global vaso-obliteration resulting in the loss of predominantly capillaries and a 50% reduction in vascular density. The neovascularization is robust in retina and in vitreous. The angiogenesis is so exuberant that large membranes can completely cover the retinal vascular arcades. The model is excellent for evaluating antiangiogenic therapies, including targeting VEGF or its receptor, provided that the reagents recognize dog proteins. The newborn dog eye is comparable in size and its vitreous body is comparable in volume to the premature human eye, so preclinical dosing studies are appropriate in canine OIR. Evaluation of reagents targeting VEGFR2 or VEGF in canine model of OIR demonstrates a very important point: preretinal neovascularization must be inhibited without inhibiting revascularization of retina. Therefore, dosing of the reagents is critical and adult human doses are not appropriate for a premature human eye.17 Avery predicted that 6.5 μg would seem an appropriate dose for a human premature infant eye, which is almost comparable to our 5 ug dose in the canine OIR model, because the premature human eye is slightly larger than neonatal dog model. The goal is to determine a titer that inhibits preretinal neovascularization but permits revascularization of inner retina. The Japanese gardener’s philosophy is excellent guidance in dosing: less is more.
Acknowledgments
This study was supported by NIH grants RO1-EY09357 (GAL) and EY001765 (Wilmer Ophthalmological Institute CORE grant) and unrestricted funds from Research to Prevent Blindness (unrestricted funds to Wilmer). The Johns Hopkins School of Medicine Institutional Animal Care and Use Committee provided Ethics approval and also approved the use of dogs for this study in accordance with the guidelines of the Association for Research in Vision and Ophthalmology on use of laboratory animals.
Disclosure
The authors report no other conflict of interest in this work.
References
Patz A, Eastham A, Higgenbotham D, Kleh T. Oxygen studies in retrolental fibroplasia: II. the production of the microscopic changes of retrolental fibroplasia in experimental animals. Am J Ophthalmol. 1953;36:1511–1522. | |
Kimura T, Chen CH, Patz A. Light and electron microscopic studies of intravitreal proliferative tissues in human and puppy eyes. Nippon Ganka Gakkai Zasshi. 1979;83:255–265. | |
Flower RW, Blake DA, Wajer SD, Egner PG, McLeod DS, Pitts SM. Retrolental fibroplasia: evidence for a role of the prostaglandin cascade in the pathogenesis of oxygen-induced retinopathy in the newborn beagle. Ped Res. 1981;15:1293–1302. 1. Hasegawa T, McLeod DS, Prow T, Merges C, Grebe R, Lutty GA. Vascular precursors in developing human retina. Invest Ophthalmol Vis Sci. 2008;49:2178–2192. | |
McLeod DS, Lutty GA, Wajer SD, Flower RW. Visualization of a developing vasculature. Microvasc Res. 1987;33:257–269. | |
McLeod DS, Hasegawa T, Prow T, Merges C, Lutty G. The initial fetal human retinal vasculature develops by vasculogenesis. Dev Dyn. 2006;235:3336–3347. | |
Chan-Ling T, McLeod DS, Hughes S, et al. Astrocyte-endothelial cell relationships during human retinal vascular development. Invest Ophthalmol Vis Sci. 2004;45:2020–2032. | |
McLeod DS, Merges C, Selden SC, Lutty GA. Histochemical localization of 5′ nucleotidase and adenosine in the normal and oxygen exposed neonatal beagle retina. Invest Ophthalmol Vis Sci. 1992;33:1085. | |
McLeod DS, Brownstein R, Lutty GA. Vaso-obliteration in the canine model of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 1996;37:300–311. | |
McLeod DS, Crone SN, Lutty GA. Vasoproliferation in the neonatal dog model of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 1996;37:1322–1333. | |
Lutty GA, McLeod DS. Increased retinal glial expression of GFAP and 5′ nucleotidase in response to hyperoxia. Invest Opthalmol Vis Sci. 1987;28:204. | |
McLeod DS, D’Anna SA, Lutty GA. Clinical and histopathologic features of canine oxygen-induced proliferative retinopathy. Invest Ophthalmol Vis Sci. 1998;39:1918–1932. | |
Baba T, McLeod DM, Edwards MM, et al. VEGF165b in the developing vasculatures of the fetal human eye. Dev Dyn. 2012;241:595–607. | |
Lutty GA, McLeod DS. Retinal vascular development and oxygen-induced retinopathy: a role for adenosine. Prog Ret Eye Res. 2003;22:95–111. | |
McLeod DS, Taomoto M, Cao J, Zhu Z, Witte L, Lutty GA. Localization of VEGF receptor-2 (KDR/Flk-1) and effects of blocking it in oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2002;43:474–482. | |
Murata T, Nakagawa K, Khalil A, Ishibashi T, Inomata H, Sueishi K. The temporal and spatial vascular endothelial growth factor expression in retinal vasculogenesis of rat neonates. Lab Invest. 1996; 74:68–77. | |
Lutty GA, McLeod DS, Bhutto I, Wiegand SJ. Effect of VEGF trap on normal retinal vascular development and oxygen-induced retinopathy in the dog. Invest Ophthalmol Vis Sci. 2011;52:4039–4047. | |
Avery RL. Bevacizumab (Avastin) for retinopathy of prematurity: wrong dose, wrong drug, or both? J AAPOS. 2012;16:2–4. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.