Back to Journals » Journal of Asthma and Allergy » Volume 15
Targeting TSLP in Asthma
Authors Parnes JR, Molfino NA, Colice G, Martin U , Corren J, Menzies-Gow A
Received 23 November 2021
Accepted for publication 25 April 2022
Published 3 June 2022 Volume 2022:15 Pages 749—765
DOI https://doi.org/10.2147/JAA.S275039
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Luis Garcia-Marcos
Video abstract presented by Jane R Parnes.
Views: 1971
Jane R Parnes,1 Nestor A Molfino,1 Gene Colice,2 Ubaldo Martin,2 Jonathan Corren,3 Andrew Menzies-Gow4
1Amgen, Thousand Oaks, CA, USA; 2AstraZeneca, Gaithersburg, MD, USA; 3University of California, Los Angeles, CA, USA; 4Royal Brompton and Harefield Hospitals, London, UK
Correspondence: Jane R Parnes, Amgen, Thousand Oaks, CA, USA, Email [email protected]
Abstract: Thymic stromal lymphopoietin (TSLP) is an epithelial cell-derived cytokine implicated in the initiation and persistence of inflammatory pathways in asthma. Released in response to a range of epithelial insults (eg, allergens, viruses, bacteria, pollutants, and smoke), TSLP initiates multiple downstream innate and adaptive immune responses involved in asthma inflammation. Inhibition of TSLP is postulated to represent a novel approach to treating the diverse phenotypes and endotypes of asthma. Tezepelumab, the TSLP inhibitor farthest along in clinical development, is a human monoclonal antibody (IgG2λ) that binds specifically to TSLP, preventing interactions with its heterodimeric receptor. Results of recently published phase 2 and 3 studies, reviewed in this article, provide evidence of the safety and efficacy of tezepelumab that builds on initial findings. Tezepelumab is safe, well tolerated, and provides clinically meaningful improvements in asthma control, including reduced incidence of exacerbations and hospitalizations in patients with severe asthma. Clinical benefits were associated with reductions in levels of a broad spectrum of cytokines (eg, interleukin [IL]-5, IL-13) and baseline biomarkers (eg, blood eosinophils, immunoglobulin [Ig]E, fractional exhaled nitric oxide [FeNO]) and were observed across a range of severe asthma phenotypes (ie, eosinophilic and non-eosinophilic). These data strengthen the notion that anti-TSLP elicits broad inhibitory effects on pathways that are key to asthma inflammation rather than on narrower inhibition of individual downstream factors. This review presents the rationale for targeting TSLP to treat asthma, as well as the clinical effects of TSLP blockade on asthma outcomes, biomarkers of disease activity, airway inflammation, lung physiology, and patient symptoms.
Keywords: thymic stromal lymphopoietin, TSLP, asthma, exacerbation rates, anti-TSLP
Introduction
Asthma is a common chronic, noncommunicable, inflammatory airway disease characterized by respiratory symptoms, variable airflow limitation, and airway hyperresponsiveness (AHR).1,2 The multiple, somewhat overlapping patient phenotypes, including allergic asthma, non-allergic asthma, adult-onset asthma, asthma with persistent airflow limitation, and obesity-associated asthma,3 illustrate the heterogeneity of the disease.4 Asthma disease heterogeneity also exists at the endotype level.5 Allergic asthma and asthma with high eosinophil counts are associated with type 2 (T2) inflammation, mediated by the cytokines interleukin (IL)-4, IL-5, and IL-13, as well as by immunoglobulin (Ig)E. These endotypes can account for up to approximately 80% of all patients with asthma.6–8 The pathophysiology underlying the non-T2 asthma, or T2-low, endotype has not been fully elucidated, and may have biomarker elements that overlap with T2 asthma. Structural changes to the airways involving smooth muscle cell hypertrophy and neutrophil infiltration driven by IL-17 have been reported in the non-T2 endotype.2,9 Asthma phenotypes and endotypes in an individual patient can change over time, with nearly 1 in 2 patients with severe asthma assigned to a different biomarker cluster after 1 year of follow-up.5
Severe asthma is asthma that is uncontrolled despite high-dose inhaled corticosteroid (ICS)–long-acting beta2 agonist (LABA) therapy, or that requires high-dose ICS-LABA to remain controlled.10 Among individuals with asthma, between 5% and 10% have severe disease;11 of those, approximately 20% to 50% remain uncontrolled.12 Severe allergic or eosinophilic asthma can be treated with biologics if patients meet prescription requirements.13,14 In fact, the Global Initiative for Asthma (GINA) guidelines recommend biologics for patients with severe asthma driven by T2 inflammation.10,13 Available biologics inhibit specific downstream effectors of immune signaling pathways (eg, IL-5 and its receptor [IL-5R], IgE, and IL-4R) involved in asthma inflammation. Although blockade of IL-4R inhibits both IL-4 and IL-13 signaling, inhibition of IL-13 alone is not an approved therapy for asthma.15 Patients with severe asthma can have disease driven by multiple cytokines or exhibit variability in their cytokine profile, which may limit disease control by a single specific downstream anti-cytokine biologic.5,10,13,15 Furthermore, no biologics are currently approved for the treatment of non-T2 asthma.2 Severe asthma, regardless of phenotype or endotype, is associated with reduced quality of life (QoL), frequent use of oral corticosteroids (OCS), and increased healthcare resource utilization.16,17 Thus, an unmet need remains for new ways to address the complexity of severe asthma—across phenotypes and biomarkers—to help more patients achieve asthma control.
The epithelial cell-derived cytokine thymic stromal lymphopoietin (TSLP) has been implicated in the initiation and persistence of inflammatory pathways in asthma.9,18 TSLP is understood to promote T2 inflammatory responses, with recent evidence supporting the potential for TSLP to initiate non-T2 responses in asthma as well.9,19 Given its position at the top of the inflammatory cascade, TSLP can exert broad influence over airway inflammation through its impact on multiple cell types and pathways. Therefore, treatments targeting TSLP provide a novel approach to treat inflammation in asthma.9,18 We review the underlying rationale for treating severe asthma with anti-TSLP therapeutics and summarize clinical trial results, with a focus on tezepelumab—a human monoclonal antibody (IgG2λ) that binds and inhibits TSLP—as it is furthest along in clinical development.
Airway Epithelium and TSLP
An increased susceptibility of the airway epithelium to environmental or physical injury is key to triggering asthmatic airway inflammation.20,21 The ensuing inflammation involves release of a wide range of cytokines and chemokines that further damage respiratory epithelia, thickening the airway wall and limiting airflow. Although originally described in the supernatant of a thymic stromal cell line,22 TSLP is released predominantly by ciliated epithelial cells, mast cells, macrophages, and endothelial cells23,24 at the initial site of insult where local and systemic as well as adaptive and innate immune responses are shaped25 (see Figure 1). Cells that express the heterodimeric TSLP receptor include hematopoietic progenitor cells, eosinophils, basophils, mast cells, airway smooth muscle cells, type 2 innate lymphoid cells (ILC2s), lymphocytes, dendritic cells, and monocytes/macrophages.9 Airway structural cells also produce and are targets of TSLP, suggesting that TSLP may play a role in asthma-related, pathologic airway remodeling.26
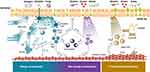 |
Figure 1 TSLP Acts Across the Spectrum of Asthma Inflammation. TSLP-driven mechanisms of disease in different asthma endotypes. Epithelial alarmins, including TSLP, are released in response to triggers at the epithelium. The alarmins activate multiple innate and adaptive immune responses that participate in overlapping and distinct pathways. TSLP may also mediate structural cell effects that contribute to airway hyperresponsiveness and remodeling. Figure adapted, with permission, from Gauvreau GM et al. Expert Opin Ther Targets. 2020;24(8):777–792.9,74–76 Abbreviations: IgE, immunoglobulin E; IL, interleukin; ILC2, type 2 innate lymphoid cell; T2, type 2; Th, T helper; TSLP, thymic stromal lymphopoietin. |
TSLP and the Asthma Inflammatory Cascade
Multiple lines of evidence suggest that TSLP coordinates effector functions of a diverse array of myeloid and lymphoid cell populations that participate in inflammatory responses in asthma.24 TSLP expression is increased in the airways of patients with asthma compared with healthy controls, and TSLP levels correlate with expression of T2-attracting chemokines, with disease severity, and with asthma exacerbation risk.27–30 Animal studies have demonstrated that TSLP is necessary and sufficient for the development of T2 cytokine–associated airway inflammation.31,32 Animal studies have demonstrated that TSLP is necessary and sufficient for the development of T2 cytokine-associated airway inflammation, mediated through distinct immune cell cascades in the context of innate and adaptive T2 inflammation.33 Certain TSLP gene variants have been identified, as outcomes of genome-wide association studies, in individuals with asthma.34,35 Evidence from clinical trials in patients with asthma treated with TSLP inhibitors has further corroborated the postulate that anti-TSLP treatment has broad anti-inflammatory properties.
Proof-of-Concept Studies
Proof-of-concept clinical studies were conducted with the anti-TSLP antibody tezepelumab36 and the inhaled anti-TSLP compound CSJ117,37 an antibody fragment against human TSLP. Tezepelumab and CSJ117 each inhibit binding of TSLP to its receptor thereby preventing downstream signaling. Tezepelumab was administered by intravenous infusion on days 1, 29, and 57 and allergen challenge was performed on days −14, 42, and 84.36 Both studies demonstrated that anti-TSLP treatments reduced allergen-induced bronchoconstriction and indices of airway inflammation in patients with mild, stable atopic asthma. Following an allergen inhalation challenge on day 84, tezepelumab demonstrated a significantly smaller maximum percentage decrease in forced expiratory volume in 1 second (FEV1) compared with placebo (–12% vs –22%, respectively; 95% CI: 1.59, 18.23; p=0.02) during the late asthmatic response 3 to 7 hours following allergen challenge.36 Tezepelumab treatment also significantly reduced the allergen-induced percentage reduction in FEV1 during the early asthmatic response (0 to 2 hours after allergen challenge) on days 42 and 84.36 The CSJ117 proof-of-concept study yielded results comparable to those obtained with tezepelumab for the late, but not the early, asthmatic response on day 84.38 An exploratory endpoint in the tezepelumab proof-of-concept study was the provocative concentration of methacholine required to reduce the FEV1 by 20% (methacholine PC20).36 Tezepelumab therapy was associated with an increase in methacholine PC20 on day 83 compared with the placebo group (nominal p = 0.04).36 In addition, treatment with tezepelumab decreased levels of blood and sputum eosinophils before and after the allergen challenge, and reduced the fraction of exhaled nitric oxide (FeNO) during the study. CSJ117 also reduced sputum eosinophilia and pre challenge FeNO levels.37,38 These findings suggest that anti-TSLP treatment inhibited multiple inflammatory pathways.
Categories of Clinical Trial Evidence for Tezepelumab Efficacy Across Different Dimensions of Asthma
Informed by proof-of-concept study results, the phase 2b PATHWAY and phase 3 NAVIGATOR trials were conducted to investigate the safety and efficacy of tezepelumab in reducing exacerbations and improving lung function in patients with severe, uncontrolled asthma.39–41 Additional clinical trials have investigated the effects of tezepelumab on airway inflammation, airway remodeling, efficacy in OCS-dependent asthma, and patient reported outcomes (see Table 1).
 |
Table 1 Tezepelumab Clinical Trials in Asthma |
Asthma Exacerbation Rates
The phase 2b PATHWAY trial investigated the effects of tezepelumab in patients with uncontrolled moderate to severe asthma39 (see Table 1). The primary endpoint was the annualized rate of asthma exacerbations (AAER) at week 52.39 Asthma exacerbations were defined as a worsening of asthma symptoms that led to any of the following: the use, or increased dose, of systemic glucocorticoids (oral or injectable); an emergency department (ED) visit due to asthma that resulted in systemic glucocorticoid treatment; or an inpatient hospitalization due to asthma. In the trial, 550 patients were randomized to receive one of three subcutaneous doses of tezepelumab or placebo.39 Tezepelumab reduced the AAER by 62% (95% CI: 42, 75), 71% (95% CI: 54, 82), and 66% (95% CI: 47, 79), in the 70-mg-every-4 weeks (Q4W), 210-mg-Q4W, 280-mg-Q2W tezepelumab groups, respectively, relative to placebo, (p < 0.001 for each dose vs placebo).39 This AAER reduction level exceeded the 20% to 40% recommended reduction range considered clinically relevant for a given asthma treatment regimen and/or intervention in randomized controlled trials.42
NAVIGATOR was the 52-week, phase 3, multicenter, double-blind confirmatory trial of tezepelumab for the treatment of asthma that included 1059 adults and adolescents with severe, uncontrolled asthma. Patients were randomized to receive tezepelumab (n=528; 210 mg Q4W) or placebo (n=531)40,41 (see Table 1). The primary endpoint was the AAER at week 52. In the overall population, tezepelumab treatment resulted in a 56% reduction (rate ratio [RR], 0.44: 95% CI: 0.37, 0.53; p < 0.001) in the AAER relative to placebo at week 52, which met the study primary endpoint criterion and confirmed the PATHWAY study findings.40,41
AAER in Asthma Phenotypes/Endotypes
In both studies—PATHWAY and NAVIGATOR—AAERs were analyzed across different asthma phenotypes and endotypes.39,41 Subgroups of patients defined by baseline blood and airway eosinophil counts, FeNO levels, and IgE levels were analyzed. For each of these inflammatory biomarker subgroups, in both trials, patients treated with tezepelumab exhibited a lower AAER compared with patients in comparable subgroups receiving placebo39,41 (see Figure 2). One key aspect of the NAVIGATOR study design was the enrollment of equal proportions of patients with baseline blood eosinophil counts ≥300 cells/µL and <300 cells/µL. In this study, for patients with baseline blood eosinophil counts ≥300 cells/µL, <300 cells/µL, <150 cells/µL, and ≥150 cells/µL, tezepelumab reduced the AAER vs placebo by 70% (RR, 0.30; 95% CI: 0.22, 0.40), 41% (RR, 0.59; 95% CI: 0.46, 0.75; p < 0.001), 39% (RR, 0.61:95% CI: 0.42, 0.88), and 61% (RR, 0.39; 95% CI: 0.32, 0.49), respectively41(see Figure 2).
 |
Figure 2 NAVIGATOR: Annualized Rate of Asthma Exacerbations at Week 52, According to Baseline Biomarker Status. Note: Reproduced from The New England Journal of Medicine , Menzies-Gow A, Corren J, Bourdin A, et al, Tezepelumab in Adults and Adolescents with Severe, Uncontrolled Asthma, 384., 1800-1809. Copyright © 2021 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.41Abbreviations: AAER, annualized asthma exacerbation rate; CI, confidence interval; Eos, eosinophils; FEIA, fluorescence enzyme immunoassay; FeNO, fractional exhaled nitric oxide; IgE, immunoglobulin E; ppb, parts per billion; Q4W, every 4 weeks. |
Analyses of PATHWAY and NAVIGATOR data have examined the effect of tezepelumab on the AAER in patients with severe, uncontrolled asthma with or without allergy to a perennial aeroallergen.41,43 In both studies, allergic asthma was defined as a baseline positive signal from an IgE fluorescence enzyme immunoassay to ≥1 region-specific allergens. In PATHWAY, treatment with tezepelumab 210 mg Q4W resulted in AAER reductions of 78% and 67%, vs placebo, in patients with allergic (n = 71) and non-allergic asthma (n = 57), respectively.43 In NAVIGATOR, tezepelumab reduced the AAER over 52 weeks by 58% and 51%, vs placebo, in patients positive (n=339) vs negative (n=184), respectively, for perennial allergen.41 In PATHWAY, tezepelumab 210 mg Q4W treatment was found to reduce the AAER, relative to placebo, by 82% and 77% among patients eligible for omalizumab treatment according to US or EU prescribing information, respectively. While in the omalizumab-ineligible US or EU populations, the same tezepelumab dose reduced the AAER by 63% and 69%, respectively.43 Similarly, in NAVIGATOR, tezepelumab reduced the AAER over 52 weeks by 59% and 67% among patients eligible for omalizumab according to US and EU prescribing information, respectively, whereas in omalizumab-ineligible patients, tezepelumab reduced the AAER by 54% and 48%, respectively.44,45 These findings concur with the observations that tezepelumab therapy reduces AAER regardless of patient baseline IgE level. Together, these results demonstrate that tezepelumab improves asthma outcomes in a broad range of patients with severe asthma, regardless of baseline blood eosinophil count, FeNO level, allergic status, or omalizumab eligibility (see Figure 2).
AAER in Other Patient Subgroups
Patients included in the PATHWAY study had poorly controlled asthma despite prior treatment with LABA combined with a medium- or high-dose of ICS fluticasone (or equivalent).39 Among patients in the high-dose inhaled glucocorticoid baseline stratum, tezepelumab reduced the AAER by 70% (95% CI: 41, 84), 77% (95% CI: 52, 89), and 76% (95% CI: 50, 88) in the 70-mg-Q4W, 210-mg-Q4W, and 280-mg-Q2W groups, respectively, relative to placebo (nominal p < 0.001 vs placebo for each dose).39 AAER reductions were lower, relative to placebo, among patients in the baseline medium-dose inhaled glucocorticoid group by 48% (RR, 0.52; 95% CI:−15, 76), 60% (RR, 0.40; 95% CI: 5, 83), and 48% (RR, 0.52; 95% CI: −14, 76), respectively.39
A post hoc subgroup analysis from the PATHWAY study showed that tezepelumab 210 mg Q4W reduced the AAER by 75% (RR, 0.25; 95% CI:15, 93) and 73% (RR, 0.27; 95% CI:47, 86) in patients with (n=23) and without (n=112) nasal polyposis (NP), respectively, relative to placebo.46 Of 1059 patients in NAVIGATOR, 83 had NP in the past 2 years and 976 did not. Tezepelumab reduced AAER by 86% (95% CI: 70, 93) and 52% (95% CI: 42, 61) vs placebo in NP+ and NP– patients, respectively.47 Patients with asthma experience higher rates of asthma exacerbations in the autumn and winter, compared with the spring and summer, likely due to heightened aeroallergen exposure and respiratory virus prevalence.48 Another post hoc analysis of data from the PATHWAY trial demonstrated that tezepelumab consistently reduced exacerbation rates across all seasons, compared with placebo, regardless of asthma endotype.49
As part of the investigation of the effects of tezepelumab vs placebo on AAER, the NAVIGATOR trial included adolescent patients with uncontrolled, severe asthma (n=41 for each study arm).41 A 30% (RR, 0.70; 95% CI: 0.34, 1.46) reduction, relative to placebo, in AAER was observed among adolescent patients treated with tezepelumab over 52 weeks.41
There were differences between the NAVIGATOR and PATHWAY study patient populations. The NAVIGATOR study included an adolescent cohort. Further, more patients were enrolled in NAVIGATOR than in PATHWAY.39,41 Higher percentages of patients in NAVIGATOR vs PATHWAY were on high-dose ICS (~75% vs ~50%, respectively); experienced >2 exacerbations in the previous year (~40% vs ~20%, respectively); and had perennial aeroallergen positivity (~69% vs ~46%, respectively).39,41 Additionally, patients in NAVIGATOR had higher baseline levels of FeNO (mean, 43.8 ppb vs 33.5 ppb in PATHWAY) and serum IgE (median, 195.6 IU/mL vs 130.0 IU/mL in PATHWAY).39,41 Despite these differences, however, clinically significant AAER reductions with tezepelumab, relative to placebo, were observed in these two studies.39,41 Moreover, in the SOURCE trial—a second, smaller, 48-week phase 3 trial of tezepelumab in adults with oral corticosteroid-dependent asthma (see Table 1)—tezepelumab reduced the AAER by 31% (95% CI: –9, 56), relative to placebo, over 48 weeks.50,51
Hospitalizations
The impact of tezepelumab treatment on exacerbations requiring hospitalization and ED visits was investigated in both the NAVIGATOR and the PATHWAY trials.39,41 In the NAVIGATOR trial hospitalization and ED visits were prespecified secondary analyses.41 Tezepelumab reduced patient hospitalizations by 85% (RR, 0.15; 95% CI: 0.07, 0.33), relative to placebo.41 A supportive analysis of NAVIGATOR found that a lower proportion of patients in the tezepelumab group, compared with the placebo group, required asthma-related hospitalizations (3% vs 7%, respectively); ED visits (4% vs 9%, respectively); and unscheduled visits to a specialist (35% vs 44%, respectively), as well as other health-care related services.52
A post hoc analysis of PATHWAY results demonstrated that tezepelumab reduced hospitalizations by 74% (RR, 0.26; 95% CI: 0.08, 0.92), 84% (RR, 0.16; 95% CI: 0.04, 0.70), and 74% (RR, 0.26; 95% CI: 0.07, 0.98) across the 70-mg-Q4W, 210-mg-Q4W, and 280-mg-Q2W dose groups, respectively, relative to placebo, over 52 weeks.53 Tezepelumab also reduced all-cause ED visits by 56% (RR, 0.44; 95% CI: 0.14, 1.41), 84% (RR, 0.16; 95% CI: 0.04, 0.69), and 37% (RR, 0.63; 95% CI: 0.22, 1.81), relative to placebo, across the 70-mg-Q4W, 210-mg-Q4W, and 280-mg-Q2W dose groups, respectively.
Combined data from the independent PATHWAY and NAVIGATOR trials provide consistent evidence that inhibition of TSLP with tezepelumab reduced the AAER, compared with placebo, in patients with poorly controlled asthma, regardless of baseline inflammatory biomarker level, allergic status, ICS dose, presence or absence of NP, adolescent or adult status, as well as across different seasons of the year. These subgroup analyses provide strong support for the hypothesis that TSLP plays an upstream role in asthma exacerbations and that TSLP inhibition exhibits efficacy across broad patient populations with asthma (see Figure 2).
Lung Function
A secondary endpoint in the PATHWAY trial explored the effects of tezepelumab on lung function by measuring changes from baseline in the prebronchodilator FEV1 (pre-BD) at week 52.39 The least square (LS) mean change from baseline at week 52 in the pre-BD FEV1 was greater by 120 mL (95% CI: 20, 220), 130 mL (95% CI: 30, 230), and 150 mL (95% CI: 50, 250) in the tezepelumab 70-mg-Q4W, 210-mg-Q4W, and 280-mg-Q2W groups, respectively, relative to placebo.39 The minimal clinically important increase indicating improved lung function is 100 to 200 mL.54 This finding was observed at the earliest time point examined (week 4) and was sustained for the duration of the trial. Tezepelumab delivered clinically important improvement in lung function in this uncontrolled asthma population.39
Improvement in lung function with tezepelumab was also observed in NAVIGATOR.41 Tezepelumab rapidly improved lung function, compared with placebo, at the earliest time point assessed (week 2) and sustained the improvement through week 52. Pre-BD FEV1 increases from baseline at week 52 were 230 mL and 90 mL in the tezepelumab vs placebo groups, respectively (LS mean difference: 130 mL; 95% CI: 80, 180;p < 0.001)41 (see Figure 3).
 |
Figure 3 NAVIGATOR: Change from Baseline to Week 52 in Prebronchodilator FEV1. Abbreviation: FEV1, forced expiratory volume in 1 second. I bars indicate 95% confidence intervals. Note: Adapted from The New England Journal of Medicine, Menzies-Gow A, Corren J, Bourdin A, et al, Tezepelumab in Adults and Adolescents with Severe, Uncontrolled Asthma, 384., 1800-1809. Copyright © 2021 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.41 |
Lung function at week 48 was also assessed as a secondary endpoint in the SOURCE trial.50,51 In OCS-dependent patients, tezepelumab increased pre-BD FEV1 by 210 mL compared with a reduction of 40 mL in the placebo group (LS mean difference: 260 mL; 95% CI:130, 390).51 As observed in the PATHWAY trial, increased FEV1 was observed at the earliest assessment—week 4—and was sustained through week 48, regardless of reductions of daily maintenance OCS dose.39 Overall, across three trials—that is, PATHWAY, NAVIGATOR, and SOURCE—tezepelumab treatment was associated with clinically significant improvements in lung function in patients with severe asthma.39,41,51
Asthma Control and Related Quality of Life
Regulatory bodies recognize the importance of patient reports of their own health status to inform treatment decisions for both physicians and patients as well as for health authorities and payers to assess treatment benefit.55 Patient-reported outcomes (PROs) are increasingly being included in asthma clinical trials as tools to assess patient-centered outcomes.
Asthma control was assessed using the PRO Asthma Control Questionnaire-6 (ACQ-6) in several tezepelumab clinical trials.39,41,51,56 (see Table 2). In each of these studies, treatment with tezepelumab was associated with greater improvements in mean ACQ-6 scores from baseline than placebo, with statistically significant results observed in NAVIGATOR. However, mean change score differences from placebo did not meet the minimally clinically important difference (MCID) of 0.5 score points.57 In a PATHWAY supportive analysis, tezepelumab increased the proportion of ACQ-6 responders (defined as a reduction of ≥0.5 from baseline in ACQ-6 total score) in all tezepelumab treatment groups vs placebo.58
 |
Table 2 Tezepelumab Effect on Patient-Reported Outcomes of Asthma Control and Patient Quality of Life |
Asthma-related QoL was evaluated in tezepelumab clinical trials using the Asthma Quality of Life Questionnaire standardized for patients 12 years or older (AQLQ[S]+12) scale.39,41,51 In the NAVIGATOR study, tezepelumab treatment was associated with improved AQLQ(S)+12 scores from baseline that reached statistical significance compared with placebo; however, the difference in increase from baseline did not meet the criterion for MCID (see Table 2).
Treatment with tezepelumab was also associated with improved scores from baseline vs placebo in the St George’s Respiratory Questionnaire (SGRQ), the Asthma Symptom Diary (ASD), and the 5-dimension 5-level EuroQol questionnaire (see Table 2). Importantly, in the NAVIGATOR study, an MCID in SGRQ score improvement from baseline vs placebo was met in the tezepelumab group.57,59 The ASD score improvement from baseline obtained with tezepelumab vs placebo in NAVIGATOR was nominally statistically significant.41 These results indicate that across multiple PRO instruments used in various clinical trials, tezepelumab improved asthma control and symptoms compared with placebo, although not always reaching statistical significance despite achieving an MCID, which may be related to placebo response rates.
One of several factors that contribute to observed high placebo PRO response rates in clinical trials of severe asthma is adherence to background corticosteroid medications during the studies.60 Lack of monitoring of adherence to background medications has been shown to compromise the ability of trials to demonstrate statistically significant differences between placebo and investigational drugs.61
Efficacy in Oral Corticosteroid-Dependent Asthma
Patients with asthma who receive long-term treatment with OCS experience acute and chronic comorbidities, including reduced bone density, cardiovascular events, elevated blood sugar, and infections, in addition to an increased risk for mortality.62 An analysis of 100 patients in the NAVIGATOR trial who were receiving maintenance OCS at baseline and throughout the 52-week study demonstrated that tezepelumab-treated patients had a numerically lower AAER compared with placebo-treated patients (2.12 vs 2.94; 28% reduction relative to placebo; 95% CI: −26, 59) at week 52.47
Clinical trial results have indicated that tezepelumab promotes improved disease control and reductions in inflammatory biomarkers, thus raising the question of whether tezepelumab-treated patients might require the use of less OCS treatment. The SOURCE trial evaluated this question in 150 adults with OCS-dependent asthma who were randomized to tezepelumab 210 Q4W or to placebo50,51 (see Table 1). The primary study endpoint was categorized percentage reduction from baseline in daily OCS doses through week 48 while not losing asthma control.51 Included in the SOURCE study were adult patients with asthma who had been receiving the following treatments prior to the first study visit: medium-dose or high-dose ICS for 12 months, LABA and high-dose ICS for ≥3 months, OCS for treatment of asthma for ≥6 months and a stable dose of OCS for ≥1 month.50
Although the SOURCE study did not meet its primary endpoint, tezepelumab was shown to numerically raise the odds for OCS reduction, compared with placebo, at week 48 (odds ratio [OR], 1.28, 95% CI: 0.69, 2.35; p = 0.43).51 The median percentage reduction from baseline in daily maintenance OCS dose was 100% (95% CI: 70.0, 100) in the tezepelumab group and 75% (95% CI: 66.7, 100) in the placebo group at week 48. In a subgroup analysis tezepelumab increased the cumulative odds of achieving a category of greater percentage reduction in daily OCS use (OR, 2.58; 95% CI: 1.16, 5.75 and OR, 3.49; 95% CI: 1.16, 10.49) in patients with ≥150 cells/µL or ≥300 cells/µL, respectively, compared with placebo.51 In patient groups with low levels of eosinophils—that is, <150 cells/µL and <300 cells/µL—ORs favored placebo (OR, 0.40; 95% CI: 0.14, 1.13 and OR, 0.70; 95% CI: 0.33, 1.47, respectively).51 The placebo group in the SOURCE trial had higher reductions in OCS than those observed in other steroid-sparing clinical trials in asthma, which might be related to the longer OCS reduction phase in SOURCE and the fact that OCS reductions were allowed in patients who experienced asthma exacerbations. Post hoc analyses of SOURCE study data were performed to assess the impact of study design features on the placebo response for the primary endpoint.51
Mechanistic Studies Supporting Efficacy in Asthma
The phase 2 UPSTREAM trial was conducted to investigate AHR and airway inflammation in patients with symptomatic asthma who were receiving maintenance therapy with ICS and were treated with tezepelumab or placebo63 (see Table 1). The primary objective was to evaluate the effect of tezepelumab on AHR to mannitol. Secondary outcomes included changes in airway tissue levels of eosinophils, total mast cells, mast cells positive for tryptase only, mast cells positive for tryptase and chymase, and neutrophils from baseline to week-12 in airway mucosal biopsies. Patients eligible to participate had uncontrolled asthma and AHR to inhaled mannitol at baseline (provoking dose [PD] of mannitol causing a 15% reduction in FEV1 [PD15] of ≤315 mg) despite any stable doses of ICS.63
In UPSTREAM, tezepelumab improved AHR to mannitol with a mean change in PD15 of 1.9 (95% CI: 1.2, 2.5) vs 1.0 (95% CI: 0.3, 1.6) with placebo (p = 0.06).63 The number of patients with a negative mannitol test (PD15 >635 mg) at visit 6 was higher in the tezepelumab group compared with the placebo group (9 of 20 patients vs 3 of 19 patients), respectively; (p = 0.04).63 This result suggests that tezepelumab improves a core physiological abnormality in asthma—that is, the hyperresponsiveness of patients with asthma to mannitol challenge. Supporting this interpretation are secondary outcome findings that tezepelumab reduced airway tissue eosinophil cell levels by 74%, whereas placebo increased airway tissue eosinophil cell levels by 28% (p = 0.004 vs placebo).63
The CASCADE study investigated tezepelumab effects on airway inflammation using primarily bronchoscopy-based assessments56,64 (see Table 1). The primary endpoint was change from baseline to week 28 in airway submucosal inflammatory cells (ie, eosinophils, neutrophils, T cells and mast cells) from bronchoscopic biopsies.64 CASCADE randomized 116 adult patients who had been receiving medium-dose or high-dose ICS for 6 to ≥12 months at screening to tezepelumab 210 mg Q4W or placebo Q4W. Patients were required to have received medium-dose or high-dose ICS plus ≥1 additional asthma controller medication, with or without maintenance OCS, for ≥3 months at screening.64 The study was designed to ensure inclusion of approximately 30% of participants with blood eosinophil counts of <150 cells/μL, 30% with blood eosinophil counts of 150 to <300 cells/μL, and 40% with blood eosinophil counts of ≥300 cells/μL at screening.64
Results from analyses of bronchoscopic biopsies showed that tezepelumab reduced patient bronchial submucosal eosinophils, vs placebo, with a geometric LS mean ratio of eosinophil counts of 0.15 (95% CI: 0.05, 0.41; nominal p < 0.0010 vs placebo),56 consistent with findings from UPSTREAM.63 Airway submucosal eosinophils were reduced across all baseline inflammatory biomarker subgroups, including Th2 cytokines.56 Reductions in other investigated inflammatory cell types did not differ between tezepelumab- and placebo-treated groups. No significant differences were observed between tezepelumab and placebo with respect to changes in patient respiratory airway reticular basement membrane thickness and epithelial integrity.56
An exploratory endpoint in the CASCADE study was AHR to mannitol.56,64 In the trial tezepelumab reduced AHR to mannitol by a mean PD15 difference vs placebo of 138.8 mg (95% CI: 14.2, 263.3; nominal p = 0.030).56 This result is consistent with the decline in mannitol AHR in conjunction with eosinophil blood and airway reductions observed with tezepelumab treatment in the UPSTREAM trial.63 Results from the proof-of-concept methacholine challenge showed that tezepelumab-treated patients had reduced AHR to methacholine, also suggesting that attenuated AHR and eosinophil depletion are a consequence of TSLP inhibition.36 Variants of the TSLP gene have been discovered that diminish TSLP expression and are associated with protection from asthma and AHR in patients.33,65 Together these findings support the hypothesis that TSLP activity drives asthma susceptibility. Neither the UPSTREAM nor the CASCADE study completely elucidated the mechanisms by which tezepelumab reduces airway inflammation and further studies will be required. For example, additional exploration into effects of tezepelumab on mast cells is ongoing.
None of the studies to date focused on the mechanism(s) by which TSLP inhibition might influence airway remodeling. Nevertheless, data from both the PATHWAY and NAVIGATOR studies lend insight into potential effects of tezepelumab on airway remodeling. A post hoc analysis elucidated the mechanistic underpinnings of tezepelumab activity in patients from the PATHWAY study by examining serum proteins at baseline and post-treatment.68 At baseline, matrix metalloproteinase-10 (MMP-10) and periostin levels correlated significantly (p < 0.01) with patients’ blood eosinophil counts and FeNO levels.68 Tezepelumab decreased levels from baseline of matrix remodeling proteins (MMP-10 and periostin), eosinophil-related proteins (interleukin 5 receptor subunit alpha [IL5RA] and pregnancy-associated plasma protein A or pappalysin-1 [PAPP‑A]), IgE, and TARC/CCL17 proteins by >10% vs placebo (nominal p < 0.05) in serum samples at week 52.68 Effects of tezepelumab on MMP-10 and MMP-3 levels were also evaluated as exploratory endpoints in the NAVIGATOR trial. Early (week 2) and sustained reductions from baseline in MMP-10 and MMP-3 levels, vs placebo, were observed. At week 52 MMP-10 and MMP-3 levels were reduced from baseline by 18.58% (95% CI: 14.38–22.58) and by 11.44% (95% CI: 5.96–16.61) vs placebo, respectively.69
As mentioned earlier, a consideration when comparing outcomes across studies in this review is that endpoints and patient populations differed between trials. For example, the CASCADE study was small and patients had relatively stable disease, which is in contrast to trials focused primarily on clinical outcomes.56 In CASCADE, no differences were observed between groups in change from baseline to the end of treatment in FEV1 and there were only minor improvements with tezepelumab in ACQ-6 score and exacerbations.56
Biomarkers
Baseline blood eosinophil count, FeNO level, and serum specific IgE status are inflammatory biomarkers used by clinicians to inform treatment decisions.6 Biomarker assessment can be useful for defining asthma molecular phenotypes, as well as for predicting disease course or response to therapy. In the tezepelumab development program subgroup analyses of patients in clinical trials defined by baseline inflammatory biomarker status demonstrated that tezepelumab treatment responses were comparable across subgroups. In addition, biomarker evaluation before and after treatment documented that tezepelumab reduced inflammatory biomarkers of T2 asthma.
In the PATHWAY study, substantial and persistent decreases in blood eosinophil counts and FeNO levels were observed in each tezepelumab dose group compared with placebo, beginning at week 4 after treatment initiation.39 Progressive decreases were also observed in total serum IgE levels across all tezepelumab groups.39 These inflammatory biomarkers were also reduced in patients treated with tezepelumab in the NAVIGATOR trial.41 Mean changes from baseline in FeNO level were −17.3 vs −3.5 ppb in the tezepelumab vs placebo groups, respectively at week 52. Tezepelumab reduced the mean blood eosinophil count from baseline by 170 cells/µL vs 40 cells/µL with placebo. Additionally, tezepelumab reduced mean serum total IgE levels from baseline by 164.4 IU/mL vs 43.64 IU/mL with placebo.41
A post hoc analysis of inflammatory biomarkers in patients from the PATHWAY study provided insight into relationships between inflammatory biomarkers and asthma exacerbation rates.66 In this analysis, baseline blood eosinophil counts and FeNO levels correlated with baseline serum levels of IL-5, IL-13, and periostin. Levels of FeNO, IL-5, and to a lesser extent blood eosinophil counts, each correlated with higher exacerbation rates in patients treated with placebo, suggesting that these biomarkers are also indicators of severe disease. Tezepelumab reduced exacerbation rates regardless of baseline T2 inflammatory marker levels, including blood eosinophil count, FeNO level, and serum levels of IgE, IL-5, IL-13, periostin, thymus and activation-regulated chemokine (TARC), and TSLP. Moreover, by week 52 tezepelumab reduced expression of IL-5 from baseline by 60%, compared with an increase of 3% with placebo; furthermore, the agent reduced IL-13 by 52%, vs 2% with placebo. In patients with severe, uncontrolled asthma, treatment with tezepelumab was associated with levels of IL-5 and IL-13 that approached levels observed in the healthy cohort.67 After 52 weeks of treatment, greater changes from baseline in blood eosinophil counts (–50.0% vs –3.0%), FeNO levels (–25% vs 0), serum total IgE (–20.1% vs 1.4% increase) and TARC levels (–19.0% vs –6.7%) were observed in the tezepelumab and placebo groups, respectively.66
One of the secondary endpoints in the SOURCE trial was analysis of inflammatory biomarkers at 48 weeks.50,51 Tezepelumab treatment led to greater sustained reductions from baseline, compared with placebo, in blood eosinophil counts, FeNO levels, and IgE levels. Inflammatory biomarkers were also found to be reduced with tezepelumab in the CASCADE trial.56 Treatment with tezepelumab was associated with greater reductions from baseline in blood eosinophils, FeNO levels, IL-5, IL-13, and plasma eosinophil-derived neurotoxin, vs placebo, at week 12, end of treatment, and at follow-up.56 Greater reductions with tezepelumab vs placebo in patient submucosal eosinophils in CASCADE are consistent with observed reductions in blood eosinophils in the NAVIGATOR study and in a PATHWAY post hoc analysis that reported reductions in markers of blood eosinophils.68
The dual findings that tezepelumab demonstrated efficacy across asthma endotypes defined by baseline inflammatory biomarkers and that tezepelumab reduced levels of these biomarkers during treatment are consistent with the postulated broad, upstream mechanism of action of anti-TSLP therapy. These attenuated biomarker findings across clinical trials confirm that TSLP plays an upstream role in the hierarchy of inflammatory pathways, such that its inhibition reduces inflammatory biomarkers across diverse pathways including those affecting epithelial structure (eg, periostin). These findings also provide molecular biomarker evidence that may establish the foundation for the broad clinical benefit of tezepelumab observed in clinical trials.
Safety in PATHWAY and NAVIGATOR Trials
In all clinical trials to date tezepelumab was well tolerated. The safety profiles of the PATHWAY39 and NAVIGATOR41 studies were comparable (see Table 3). In PATHWAY, three serious adverse events (SAEs) were deemed by the investigator to be related to the trial agent. Two SAEs (pneumonia and stroke) occurred in the same patient in the low-dose (70 mg Q4W) tezepelumab group and one (Guillain–Barré syndrome) occurred in the medium-dose group (210 mg Q4W).39 No cases of Guillain–Barré syndrome were reported in the NAVIGATOR trial.41 The proportion of patients experiencing severe infections during the NAVIGATOR trial was 8.7% in both treatment arms.41 Other SAEs were also balanced between the tezepelumab and placebo cohorts, except for two deaths reported during the NAVIGATOR trial—both in the placebo group. Anti-drug antibodies were detected in ≤10% of patients in both trials;39,41 two cases—one in each treatment group—were neutralizing in the NAVIGATOR trial,41 whereas none had neutralizing activity in the PATHWAY study.39
 |
Table 3 Summary of Adverse Events |
The DESTINATION trial is an ongoing phase 3 extension study intended to further evaluate the safety and tolerability of tezepelumab in adults and adolescents with severe, uncontrolled asthma.70 Individuals previously randomized to tezepelumab in NAVIGATOR or SOURCE were eligible to enroll and remain on tezepelumab dosing in DESTINATION. Participants randomized to the placebo arm in the predecessor studies who enrolled in DESTINATION were re-randomized in a 1:1 ratio to either tezepelumab or placebo. The primary objective of the study is to evaluate the long-term safety and tolerability of tezepelumab over 104 weeks (inclusive of the treatment period of either predecessor study).70
Conclusions
TSLP is an epithelial cell-derived cytokine that serves as an upstream regulator of airways inflammation, by direct and indirect actions on a host of immune and structural cells. Tezepelumab inhibition of TSLP function has demonstrated efficacy in patients with severe asthma—a population in which an unmet need remains despite existing therapeutics. Phase 2 and phase 3 registrational studies demonstrated that tezepelumab delivers clinically significant, rapid, and sustained relief from asthma exacerbations regardless of asthma phenotype, including patients with eosinophil low asthma, for whom there are no specific treatments (see Figure 4). These efficacy results, regardless of baseline biomarker status, confirm a role for TSLP across broad inflammatory processes. Anti-TSLP treatment also improved the lung function of patients with asthma across all trials and decreased AHR to mannitol in clinical trials.56,63 Tezepelumab efficacy data in patients with eosinophil counts of <150 is unique and is supported by the US label.71 These benefits were associated with improved asthma control and asthma QoL. Furthermore, tezepelumab reduced the number of airway eosinophils, regardless of patient baseline biomarker status, supporting a cellular mechanism based on inhibition of signals upstream of eosinophil activation, for the observed clinical response. Future longer-term studies may provide evidence of extended benefits of tezepelumab on airway structure and function. Tezepelumab is a well-tolerated, novel treatment option for patients with severe asthma, regardless of phenotype or endotype, that delivers clinically meaningful improvements in asthma outcomes.
 |
Figure 4 Summary Figure: Tezepelumab Demonstrated Efficacy in a Range of Outcomes Across a Broad Population of Patients.39,41,53 aPATHWAY included three tezepelumab doses; data from the 210-mg dose only are presented; bNominal p value; cScore difference meets criteria for minimal clinically important difference. nsp ≥ 0.05; *p < 0.05; **p < 0.01; ***p < 0.001 compared with placebo group. Abbreviations: AAER, annualized asthma exacerbation rate; ACQ-6, Asthma Control Questionnaire-6; AQLQ(S)+12, Asthma Quality of Life Questionnaire standardized for patients 12 years or older; BD, bronchodilator; eos, eosinophils; FEV1, forced expiratory volume in 1 second; NA, not applicable; Q4W, every 4 weeks. |
Data Sharing Statement
Qualified researchers may request data from Amgen clinical studies. Complete details are available at the following: https://wwwext.amgen.com/science/clinical-trials/clinical-data-transparency-practices/.
Funding
Funding for tezepelumab clinical studies was provided by Amgen Inc. and AstraZeneca. Medical writing support was provided by Kate Smigiel, PhD, of Amgen Inc. and Kathryn Miles, PhD, of BioScience Communications, whose work was funded by Amgen Inc. and AstraZeneca.
Disclosure
Jane R Parnes reports a patent Treatment of asthma with anti-TSLP antibody: US-10828365-B2 issued to Assigned to Amgen and AstraZeneca. Jane R. Parnes and Nestor A. Molfino are employees and stockholders of Amgen Inc. Gene Colice and Ubaldo Martin are employees and stockholders of AstraZeneca. Jonathan Corren has received grant support, consulting fees, fees for a speakers bureau, and advisory board fees from AstraZeneca and Regeneron; grant support, advisory board fees, and fees for a speakers bureau from Genentech; and grant support from Sanofi, Teva Pharmaceutical Industries, and OptiNose. Andrew Menzies-Gow has received grants, advisory board fees, lecture fees, and consulting fees from AstraZeneca; advisory board fees from GlaxoSmithKline; advisory board fees and lecture fees from Novartis; advisory board fees, lecture fees, and travel expenses from Teva; advisory board fees, lecture fees, and consulting fees from Sanofi. The authors report no other conflicts of interest in this work.
References
1. Papi A, Brightling C, Pedersen SE, Reddel HK. Asthma. Lancet. 2018;391(10122):783–800. doi:10.1016/S0140-6736(17)33311-1
2. Dorey-Stein ZL, Shenoy KV. Tezepelumab as an emerging therapeutic option for the treatment of severe asthma: evidence to date. Drug Des Devel Ther. 2021;15:331–338. doi:10.2147/DDDT.S250825
3. Holgate ST, Wenzel S, Postma DS, Weiss ST, Renz H, Sly PD. Asthma. Nat Rev Dis Primers. 2015;1(1):15025. doi:10.1038/nrdp.2015.25
4. Kaur R, Chupp G. Phenotypes and endotypes of adult asthma: moving toward precision medicine. J Allergy Clin Immunol. 2019;144(1):1–12. doi:10.1016/j.jaci.2019.05.031
5. Kupczyk M, Dahlén B, Sterk PJ, et al. BIOAIR Investigators. Stability of phenotypes defined by physiological variables and biomarkers in adults with asthma. Allergy. 2014;69(9):1198–1204. doi:10.1111/all.12445
6. de Llano LP, Rivas DD, Cid NB, Robles IM. Phenotype-guided asthma therapy: an alternative approach to guidelines. J Asthma Allergy. 2021;14:207–217. doi:10.2147/JAA.S266999
7. Sze E, Bhalla A, Nair P. Mechanisms and therapeutic strategies for non-T2 asthma. Allergy. 2020;75(3):311–325. doi:10.1111/all.13985
8. Wang E, Wechsler ME, Tran TN, et al. Characterization of severe asthma worldwide: data from the International Severe Asthma Registry. Chest. 2020;157(4):790–804. doi:10.1016/j.chest.2019.10.053
9. Gauvreau GM, Sehmi R, Ambrose CS, Griffiths JM. Thymic stromal lymphopoietin: its role and potential as a therapeutic target in asthma. Expert Opin Ther Targets. 2020;24(8):777–792. doi:10.1080/14728222.2020.1783242
10. Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention; 2021. Available from. https://ginasthma.org/wp-content/uploads/2021/05/GINA-Main-Report-2021-V2-WMS.pdf.
11. Dharmage SC, Perret JL, Custovic A. Epidemiology of asthma in children and adults. Front Pediatr. 2019;7:246. doi:10.3389/fped.2019.00246
12. Chen S, Golam S, Myers J, Bly C, Smolen H, Xu X. Systematic literature review of the clinical, humanistic, and economic burden associated with asthma uncontrolled by GINA Steps 4 or 5 treatment. Curr Med Res Opin. 2018;34(12):2075–2088. doi:10.1080/03007995.2018.1505352
13. Menzies-Gow A, Wechsler ME, Brightling CE. Unmet need in severe, uncontrolled asthma: can anti-TSLP therapy with tezepelumab provide a valuable new treatment option? Respir Res. 2020;21(1):268. doi:10.1186/s12931-020-01505-x
14. Gallelli L, Busceti MT, Vatrella A, Maselli R, Pelaia G. Update on anticytokine treatment for asthma. Biomed Res Int. 2013;2013:104315. doi:10.1155/2013/104315
15. Rogliani P, Calzetta L, Matera MG, et al. Severe asthma and biological therapy: when, which, and for whom. Pulm Ther. 2020;6(1):47–66. doi:10.1007/s41030-019-00109-1
16. Caminati M, Senna G. Uncontrolled severe asthma: starting from the unmet needs. Curr Med Res Opin. 2019;35(2):175–177. doi:10.1080/03007995.2018.1528218
17. Tan L, Reibman J, Ambrose C, et al. Clinical and economic burden of patients with uncontrolled severe asthma with low blood eosinophil levels in the United States. J Allergy Clin Immunol. 2021;147(2 suppl):AB47. doi:10.1016/j.jaci.2020.12.199
18. Comeau MR, Ziegler SF. The influence of TSLP on the allergic response. Mucosal Immunol. 2010;3(2):138–147. doi:10.1038/mi.2009.134
19. Kitajima M, Lee H-C, Nakayama T, Ziegler SF. TSLP enhances the function of helper type 2 cells. Eur J Immunol. 2011;41(7):1862–1871. doi:10.1002/eji.201041195
20. Holgate ST. The airway epithelium is central to the pathogenesis of asthma. Allergol Int. 2008;57(1):1–10. doi:10.2332/allergolint.R-07-154
21. Bartemes KR, Kita H. Dynamic role of epithelium-derived cytokines in asthma. Clin Immunol. 2012;143(3):222–235. doi:10.1016/j.clim.2012.03.001
22. Friend SL, Hosier S, Nelson A, Foxworthe D, Williams DE, Farr A. A thymic stromal cell line supports in vitro development of surface IgM+ B cells and produces a novel growth factor affecting B and T lineage cells. Exp Hematol. 1994;22(3):321–328.
23. Patel NN, Kohanski MA, Maina IW, Workman AD, Herbert DR, Cohen NA. Sentinels at the wall: epithelial-derived cytokines serve as triggers of upper airway type 2 inflammation. Int Forum Allergy Rhinol. 2019;9(1):93–99. doi:10.1002/alr.22206
24. Ziegler SF, Artis D. Sensing the outside world: TSLP regulates barrier immunity. Nat Immunol. 2010;11(4):289–293. doi:10.1038/ni.1852
25. Headley MB, Zhou B, Shih WX, Aye T, Comeau MR, Ziegler SF. TSLP conditions the lung immune environment for the generation of pathogenic innate and antigen-specific adaptive immune responses. J Immunol. 2009;182(3):1641–1647. doi:10.4049/jimmunol.182.3.1641
26. Redhu NS, Gounni AS. Function and mechanisms of TSLP/TSLPR complex in asthma and COPD. Clin Exp Allergy. 2012;42(7):994–1005. doi:10.1111/j.1365-2222.2011.03919.x
27. Ying S, O’Connor B, Ratoff J, et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol. 2005;174(12):8183–8190. doi:10.4049/jimmunol.174.12.8183
28. Ying S, O’Connor B, Ratoff J, et al. Expression and cellular provenance of thymic stromal lymphopoietin and chemokines in patients with severe asthma and chronic obstructive pulmonary disease. J Immunol. 2008;181(4):2790–2798. doi:10.4049/jimmunol.181.4.2790
29. Li Y, Wang W, Lv Z, et al. Elevated expression of IL-33 and TSLP in the airways of human asthmatics in vivo: a potential biomarker of severe refractory disease. J Immunol. 2018;200(7):2253–2262. doi:10.4049/jimmunol.1701455
30. Ko H-K, Cheng S-L, Lin C-H, et al. Blood tryptase and thymic stromal lymphopoietin levels predict the risk of exacerbation in severe asthma. Sci Rep. 2021;11(1):8425. doi:10.1038/s41598-021-86179-1
31. Zhou B, Comeau MR, De Smedt T, et al. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6(10):1047–1053. doi:10.1038/ni1247
32. Ziegler SF, Roan F, Bell BD, Stoklasek TA, Kitajima M, Han H. The biology of thymic stromal lymphopoietin (TSLP). Adv Pharmacol. 2013;66:129–155. doi: 10.1016/B978-0-12-404717-4.00004-4
33. Kabata H, Flamar A-L, Mahlakõiv T, et al. Targeted deletion of the TSLP receptor reveals cellular mechanisms that promote type 2 airway inflammation. Mucosal Immunol. 2020;13(4):626–636. doi:10.1038/s41385-020-0266-x
34. He J-Q, Hallstrand TS, Knight D, et al. A thymic stromal lymphopoietin gene variant is associated with asthma and airway hyperresponsiveness. J Allergy Clin Immunol. 2009;124(2):222–229. doi:10.1016/j.jaci.2009.04.018
35. Sun Y, Wei X, Deng J, et al. Association of IL1RL1 rs3771180 and TSLP rs1837253 variants with asthma in the Guangxi Zhuang population in China. J Clin Lab Anal. 2019;33(6):e22905. doi:10.1002/jcla.22905
36. Gauvreau GM, O’Byrne PM, Boulet LP, et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med. 2014;370(22):2102–2110. doi:10.1056/NEJMoa1402895
37. Gauvreau G, Hohlfeld J, Boulet L-P, et al. Efficacy of CSJ117 on allergen-induced asthmatic responses in mild atopic asthma patients. Eur Respir J. 2020;56:3690. doi:10.1183/13993003.congress-2020.3690
38. Novartis Pharmaceuticals. Novartis clinical trial results website. Protocol Number CCSJ117X2201. Available from: https://www.novctrd.com/ctrdweb/trialresult/trialresults/pdf?trialResultId=17681.
39. Corren J, Parnes JR, Wang L, et al. Tezepelumab in adults with uncontrolled asthma. N Engl J Med. 2017;377(10):936–946. doi:10.1056/NEJMoa1704064
40. Menzies-Gow A, Colice G, Griffiths JM, et al. NAVIGATOR: a phase 3 multicentre, randomized, double-blind, placebo-controlled, parallel-group trial to evaluate the efficacy and safety of tezepelumab in adults and adolescents with severe, uncontrolled asthma. Respir Res. 2020;21(1):266. doi:10.1186/s12931-020-01526-6
41. Menzies-Gow A, Corren J, Bourdin A, et al. Tezepelumab in adults and adolescents with severe, uncontrolled asthma. N Engl J Med. 2021;384(19):1800–1809. doi:10.1056/NEJMoa2034975
42. Bonini M, Di Paolo M, Bagnasco D, et al. Minimal clinically important difference for asthma endpoints: an expert consensus report. Eur Respir Rev. 2020;29(156):190137. doi:10.1183/16000617.0137-2019
43. Corren J, Ambrose CS, Sałapa K, et al. Efficacy of tezepelumab in patients with severe, uncontrolled asthma and perennial allergy. J Allergy Clin Immunol Pract. 2021;9(12):4334–4342.e6. doi:10.1016/j.jaip.2021.07.045
44. Israel E, Chupp G, Colice G, et al. Tezepelumab efficacy according to US omalizumab eligibility: results from the NAVIGATOR phase 3 study.
45. Corren J, Menzies-Gow A, Ambrose CS, et al. The effect of tezepelumab in patients with allergic and non-allergic asthma: results from the NAVIGATOR phase 3 study.
46. Emson C, Corren J, Sałapa K, Hellqvist Å, Parnes JR, Colice G. Efficacy of tezepelumab in patients with severe, uncontrolled asthma with and without nasal polyposis: a post hoc analysis of the phase 2b PATHWAY study. J Asthma Allergy. 2021;14:91–99. doi:10.2147/JAA.S288260
47. Menzies-Gow A, Brightling C, Ambrose C, et al. Effect of tezepelumab in oral corticosteroid-dependent patients with severe asthma: results from the phase 3 NAVIGATOR study. Am J Respir Crit Care Med. 2021;203:A1442.
48. Gerhardsson de Verdier M, Gustafson P, McCrae C, Edsbäcker S, Johnston N. Seasonal and geographic variations in the incidence of asthma exacerbations in the United States. J Asthma. 2017;54(8):818–824. doi:10.1080/02770903.2016.1277538
49. Corren J, Karpefors M, Hellqvist Å, Parnes JR, Colice G. Tezepelumab reduces exacerbations across all seasons in patients with severe, uncontrolled asthma: a post hoc analysis of the PATHWAY phase 2b study. J Asthma Allergy. 2021;14:1–11. doi:10.2147/JAA.S286036
50. Wechsler ME, Colice G, Griffiths JM, et al. SOURCE: a phase 3, multicentre, randomized, double-blind, placebo-controlled, parallel group trial to evaluate the efficacy and safety of tezepelumab in reducing oral corticosteroid use in adults with oral corticosteroid dependent asthma. Respir Res. 2020;21(1):264. doi:10.1186/s12931-020-01503-z
51. Wechsler ME, Menzies-Gow A, Brightling CE, et al. SOURCE study group. Evaluation of the oral corticosteroid-sparing effect of tezepelumab in adults with oral corticosteroid-dependent asthma (SOURCE): a randomised, placebo-controlled, phase 3 study. Lancet Respir Med. 2022;S2213–S2600(21):00537-3. doi:10.1016/S2213-2600(21)00537-3
52. Bourdin A, Menzies-Gow A, Chupp G, et al. Reductions in asthma exacerbation-related hospitalizations and emergency department visits in patients with severe, uncontrolled asthma treated with tezepelumab: results from the phase 3 NAVIGATOR study. Am J Respir Crit Care Med. 2021;203:A1203.
53. Corren J, Chen S, Callan L, Gil EG. The effect of tezepelumab on hospitalizations and emergency department visits in patients with severe asthma. Ann Allergy Asthma Immunol. 2020;125(2):211–214. doi:10.1016/j.anai.2020.05.020
54. Tepper RS, Wise RS, Covar R, et al. Asthma outcomes: pulmonary physiology. J Allergy Clin Immunol. 2012;129(3 suppl):S65–S87. doi:10.1016/j.jaci.2011.12.986
55. Braido F, Baiardini I, Canonica GW. Patient-reported outcomes in asthma clinical trials. Curr Opin Pulm Med. 2018;24(1):70–77. doi:10.1097/MCP.0000000000000440
56. Diver S, Khalfaoui L, Emson C, et al. CASCADE study investigators. Effect of tezepelumab on airway inflammatory cells, remodelling, and hyperresponsiveness in patients with moderate-to-severe uncontrolled asthma (CASCADE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med. 2021;9(11):1299–1312. doi:10.1016/S2213-2600(21)00226-5
57. Shen Q, von Maltzahn R, Nelsen L, Revicki D. Psychometric properties of the asthma symptom index in patients with severe asthma. J Allergy Clin Immunol Pract. 2021;9(1):400–409.e1. doi:10.1016/j.jaip.2020.08.019
58. Corren J, Garcia Gil E, Griffiths JM, et al. Tezepelumab improves patient-reported outcomes in patients with severe, uncontrolled asthma in PATHWAY. Ann Allergy Asthma Immunol. 2021;126(2):187–193. doi:10.1016/j.anai.2020.10.008
59. Bourbeau J, Maltais F, Rouleau M, Guímont C. French-Canadian version of the Chronic Respiratory and St George’s respiratory questionnaires: an assessment of their psychometric properties in patients with chronic obstructive pulmonary disease. Can Respir J. 2004;11(7):480–486. doi:10.1155/2004/702421
60. Luc F, Prieur E, Whitmore GA, Gibson PG, Vandemheen KL, Aaron SD. Placebo effects in clinical trials evaluating patients with uncontrolled persistent asthma. Ann Am Thorac Soc. 2019;16(9):1124–1130. doi:10.1513/AnnalsATS.201901-071OC
61. Mokoka MC, McDonnell MJ, MacHale E, et al. Inadequate assessment of adherence to maintenance medication leads to loss of power and increased costs in trials of severe asthma therapy: results from a systematic literature review and modelling study. Eur Respir J. 2019;53(5):1802161. doi:10.1183/13993003.02161-2018
62. Volmer T, Effenberger T, Trautner C, Buhl R. Consequences of long-term oral corticosteroid therapy and its side-effects in severe asthma in adults: a focused review of the impact data in the literature. Eur Respir J. 2018;52(4):1800703. doi:10.1183/13993003.00703-2018
63. Sverrild A, Hansen S, Hvidtfeldt M, et al. The effect of tezepelumab on airway hyperresponsiveness to mannitol in asthma (UPSTREAM). Eur Respir J. 2021. doi:10.1183/13993003.01296-2021
64. Emson C, Diver S, Chachi L, et al. CASCADE: a phase 2, randomized, double-blind, placebo-controlled, parallel-group trial to evaluate the effect of tezepelumab on airway inflammation in patients with uncontrolled asthma. Respir Res. 2020;21(1):265. doi:10.1186/s12931-020-01513-x
65. Moorehead A, Hanna R, Heroux D, et al. A thymic stromal lymphopoietin polymorphism may provide protection from asthma by altering gene expression. Clin Exp Allergy. 2020;50(4):471–478. doi:10.1111/cea.13568
66. Corren J, Pham T-H, Gil EG, et al. Baseline type 2 biomarker levels and response to tezepelumab in severe asthma. Allergy. 2021. doi:10.1111/all.15197
67. Pham T-H, Chen C, Colice G, Parnes JR, Griffiths JM, Cook B. Tezepelumab normalizes serum interleukin-5 and −13 levels in patients with severe, uncontrolled asthma. Ann Allergy Asthma Immunol. 2021;127(6):689–691. doi:10.1016/j.anai.2021.08.008
68. Pham T-H, Cook B, Parnes JR, Colice G, Griffiths JM Tezepelumab reduces biomarkers of airway remodeling, MMP-10 and MMP-3: exploratory results from the phase 3 NAVIGATOR study.
69. Sridhar S, Zhao W, Pham T-H, et al. Tezepelumab decreases matrix remodelling and inflammatory pathways in patients with asthma. Eur Respir J. 2019;54(suppl 63):RCT3785. doi:10.1183/13993003.congress-2019.RCT3785
70. Menzies-Gow A, Ponnarambil S, Downie J, Bowen K, Hellqvist Å, Colice G. DESTINATION: a phase 3, multicentre, randomized, double-blind, placebo-controlled, parallel-group trial to evaluate the long-term safety and tolerability of tezepelumab in adults and adolescents with severe, uncontrolled asthma. Respir Res. 2020;21(1):279. doi:10.1186/s12931-020-01541-7
71. TEZSPIRE™ Prescribing information. Amgen Inc. and AstraZeneca AB; 2021. Available from: https://den8dhaj6zs0e.cloudfront.net/50fd68b9-106b-4550-b5d0-12b045f8b184/e306dc06-d580-4457-b15f-9f28545ad63a/e306dc06-d580-4457-b15f-9f28545ad63a_viewable_rendition__v.pdf.
72. Alpizar S, Megally A, Chen C, Raj A, Downie J, Colice G. Functionality and performance of an accessorized pre-filled syringe and an autoinjector for at-home administration of tezepelumab in patients with severe, uncontrolled asthma. J Asthma Allergy. 2021;14:381–392. doi:10.2147/JAA.S305114
73. Khusial RJ, Honkoop PJ, van der Meer V, Snoeck-Stroband JB, Sont JK. Validation of online asthma control questionnaire and asthma quality of life questionnaire. ERJ Open Res. 2020;6(1):00289–2019. doi:10.1183/23120541.00289-2019
74. Brusselle GG, Maes T, Bracke KR. Eosinophils in the spotlight: eosinophilic airway inflammation in nonallergic asthma. Nat Med. 2013;19(8):977–979. doi:10.1038/nm.3300
75. Brusselle G, Bracke K. Targeting immune pathways for therapy in asthma and chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11(Suppl 5):S322–S328. doi:10.1513/AnnalsATS.201403-118AW
76. Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015;16(1):45–56. doi:10.1038/ni.3049
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
