Back to Journals » Therapeutics and Clinical Risk Management » Volume 14
Tanezumab: a selective humanized mAb for chronic lower back pain
Authors Webb MP, Helander EM, Menard BL , Urman RD , Kaye AD
Received 27 September 2017
Accepted for publication 21 January 2018
Published 21 February 2018 Volume 2018:14 Pages 361—367
DOI https://doi.org/10.2147/TCRM.S144125
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Garry Walsh
Michael P Webb,1 Erik M Helander,2 Bethany L Menard,2 Richard D Urman,3 Alan D Kaye2
1Department of Anesthesiology, North Shore Hospital, Auckland, New Zealand; 2Department of Anesthesiology, LSU School of Medicine, New Orleans, LA, USA; 3Department of Anesthesiology, Perioperative and Pain Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA
Abstract: Chronic lower back pain is a significant disease that affects nearly 20% of the worldwide population. Along with hindering patients’ quality of life, chronic lower back pain is considered to be the second most common cause of disability among Americans. Treating chronic lower back pain is often a challenge for providers, especially in light of our current opioid epidemic. With this epidemic and an increased aging population, there is an imminent need for development of new pharmacologic therapeutic options, which are not only effective but also pose minimal adverse effects to the patient. With these considerations, a novel therapeutic agent called tanezumab has been developed and studied. Tanezumab is a humanized monoclonal immunoglobulin G2 antibody that works by inhibiting the binding of NGF to its receptors. NGF is involved in the function of sensory neurons and fibers involved in nociceptive transduction. It is commonly seen in excess in inflammatory joint conditions and in chronic pain patients. Nociceptors are dependent on NGF for growth and ongoing function. The inhibition of NGF binding to its receptors is a mechanism by which pain pathways can be interrupted. In this article, a number of recent randomized controlled trials are examined relating to the efficacy and safety of tanezumab in the treatment of chronic lower back pain. Although tanezumab was shown to be an effective pain modulator in major trials, several adverse effects were seen among different doses of the medication, one of which led to a clinical hold placed by the US Food and Drug Administration. In summary, tanezumab is a promising agent that warrants further investigation into its analgesic properties and safety profile.
Keywords: tanezumab, monoclonal antibody, chronic lower back pain, neurotrophin nerve growth factor (NGF), tropomyosin receptor A (TrkA), treatment
Introduction
Chronic lower back pain is common and is associated with a significant burden of illness. The Centers for Disease Control and Prevention lists back pain as the second most common cause of disability among American adults.1,2 A 2015 large, multinational epidemiological estimate of global burden of illness reported low back and neck pain as the leading cause of disability.3 In 2015, lower back pain was thought to affect 18.5% (16.4%–20.9%) of the worldwide population and was associated with significant disability.3 In countries sampled, representative of all continents, back pain was the most common cause of disability in all regions except those of Sub-Saharan Africa where human immunodeficiency virus (HIV) and hematological disorders were most disabling.3
Back pain was associated with 30 million person-years lost to disability in 2015 alone.3 Despite many advances, this global, disabling disease has remained the commonest cause of years lost to disability (YLD) since 1990. The population rates of lower back pain have remained relatively constant over interval assessments at 1990, 2005, and 2015. Owing to its management chiefly in primary care, and thus potentially outside of formalized registries, the incidence and thus estimates of disability may be falsely low.3
Musculoskeletal disease, including lower back pain, remains the leader in YLD across socioeconomic strata, and its occurrence was more prominent with increasing socioeconomic score.3 In most socioeconomic groups, the prevalence of the disease exceeds what is expected via epidemiological model. This rise is most noticeably evident in the highest of socioeconomic strata and particularly in women.
Given an increase in aging population, the rate of back pain and corresponding disability is likely to rise. It has been observed that slight increases have occurred in back pain-related YLD in older patients from 1990 to 2015, suggesting that people are living longer but are increasingly disabled at the same time.3 This is a threat to healthy aging, of which mobility is a basic component. These data have implications for global health in the broadest sense. Interestingly, lower back pain typically becomes apparent as a disability in adolescents and young adults (age 15–39).3 Peak prevalence of lower back pain is thought to occur between the ages of 35 and 55.4
In addition to the disability and quality of life burden of lower back pain, an economical burden must also be considered. Individuals, their families, employers, and governments all face costs associated with back pain. Various estimates have been calculated from around the world, but all suggest a significant figure annually accrued by this condition. A study from the United Kingdom estimated 100 million work days lost per year.5 The US estimates from 2007 suggest that 100–200 billion dollars and 149 million workdays are lost per year due to lower back pain.6 The bulk of cost comes from loss of productivity or absence from work. It is worth noting that some analyses have demonstrated that a small fraction of back pain sufferers (5%) account for ~75% of the costs associated with lower back pain.7
Lower back pain is a common, disabling global affliction that has remained problematic for many decades despite advances in pharmacological and physical treatment and their availability. The effective treatment of lower back pain has global implications in economical terms of productive working years as well as quality of life.
Pharmacology
General
Tanezumab is a humanized monoclonal immunoglobulin G2 antibody against the neurotrophin NGF. NGF regulates the growth and function of sensory neurons, including small diameter afferent fibers involved in nociceptive transduction. This drug has an analgesic effect related to its inhibition of peripheral nociception. Tanezumab has shown promise and efficacy in the management of pain via the drug’s ability to inhibit the interaction between NGF and TrkA and p75.8
Mechanism of action
Tanezumab’s primary effect is to inhibit the interaction between NGF and its high affinity receptor TrKA and low-affinity receptor p75. The TrKA is a tyrosine kinase receptor, while p75 is a specific receptor for the NGF ligand.9 Peripheral nociceptive fibers and nociceptors, rich in p75 and TrKA receptors, are dependent upon NGF for both genesis and ongoing function.9 Nociceptor development is dependent upon NGF. Pain processing is heavily reliant on the presence of NGF, particularly in “C” fibers. These are small unmyelinated fibers that synthesize and secrete the neurotransmitter substance P and calcitonin gene-related peptide (CGRP). These “C” fibers have been implicated in both acute and chronic pain.9
Tanezumab binds to human NGF with high affinity, having a dissociation constant of less than 2 Pm.9 The IC50 of tanezumab is 20 Pm, which is roughly equivalent to the EC50 of NGF for TrKA and t75.9
While tanezumab has been shown to bind tightly to NGF, it is highly selective with a relative 1000-fold decrease in affinity for other substances in the NGF family, specifically BDNF, NT-3 or NT-4/5, NTF, GDNF, and VEGF.9
An excess of NGF is associated with inflammatory joint conditions and is critical in the etiology of chronic pain. Chronic stimulation of peripheral nociceptors and firing of C type fibers is thought to be part of the underpinning of central sensitization, hyperalgesia, and allodynic aspects of chronic pain.10
Pharmacokinetics
Related to the mechanism of action of monoclonal antibodies, the pharmacokinetics of these drugs are complex and exhibit both linear and nonlinear kinetics.11 Pharmacokinetic assessment of tanezumab was initially performed as a dose-finding experiment early in drug development with these data suggesting a 2-compartment model with linear elimination kinetics.12 The pharmacokinetics have been further analyzed from assays taken in Phase III trials.12 In a 2-compartment model investigation, a mixed linear/nonlinear elimination model was developed.12 This model was based on a “typical” study patient in Phase III trials – an 85 kg female patient with normal renal function (creatinine clearance 93.5 mL/min). Initial volume of distribution (Vd) was estimated at 2.71 L (approximating plasma volume) with Vd at steady state of 4.69 L.12
The elimination of tanezumab is via endocytosis and catabolism in the reticuloendothelial system. Scavenging of the antibody from this system prolongs elimination and thus can alter elimination kinetics. As would be expected, clearance models for tanezumab differ for linear vs nonlinear models (Clearance 0.135 L/day vs 0.207 L/day); but this effect is small and is only relevant for small doses of tanezumab (<2.5 mg).12 The low total clearance is manifest as residual plasma tanezumab up to and including 8 weeks after dosing.
Nonlinear kinetics is postulated to be related to the interaction of the monoclonal antibody with its target and the formation of multimers and the internalization of tanezumab-NGF receptor complexes. Free drug clearance is estimated at 100 L/day. This estimate comes from other monoclonal antibody research data and the high capacity function of the reticuloendothelial system.12 Elimination half-life of tanezumab is 21 days, which is typical of other monoclonal antibodies.13
Females exhibited slower elimination of the drug, and this is possibly related to hormone- and sex-based differences in receptor expression and bulk.12 Renal dysfunction appears to have a significant effect on total body clearance of tanezumab.12 This is unexpected, given the relative permeability of the glomerulus to monoclonal antibodies and it is suspected to be related to transcytosis.14 Body weight has the largest impact on total body clearance with a 10% increase in weight, resulting in a 10% increase in clearance; however this variation has not been explained. Drug dosing has a minimal effect on clearance and exhibits no effect at all on weight-based or fixed dose serum tanezumab levels at their maximum levels following initial infusion. This finding is consistent with infusion of other drugs of this class.15
Efficacy and safety
Tanezumab has been primarily studied for its analgesic efficacy in the treatment of three major conditions: cystitis, osteoarthritis (OA), and chronic lower back pain. There is a small volume of work revolving around its use as a cancer pain agent, but there are few Phase III trials with data for this indication. The bulk of the efficacy data for tanezumab is from its use as an analgesic in OA of the hip and knee. Initially, the drug was favorably received for this indication; however, safety data reviewed in 2010 suggested that its use was associated with osteonecrosis (ON) of affected joints.16 The US Food and Drug Administration (FDA) issued a research hold on the drug at this time for all indications other than that for cancer pain. A formal review in 2012 analyzed the reported 87 cases of ON, with 81 cases reported from Phase III OA trails and 6 from Phase II chronic lower back pain trials. It was determined that there were in fact only two cases of ON and both were from OA trials. These events were deemed to be primarily due to worsening OA, with rapid progression of osteoarthritis (RPOA) as a less common cause. The pathological changes were thought to occur due to the analgesia from tanezumab, prostaglandin paucity-mediated bone destruction from co-prescribed non-steroidal anti-inflammatory drugs, and poor underlying bone architecture rather than a primary drug-related effect on bone matrix.16,17
With regard to clinical efficacy and safety of tanezumab in lower back pain, three randomized controlled trials and one meta-analysis including tanezumab trials are available for analysis. In 2011 Katz et al analyzed efficacy and safety of tanezumab.18 This was a proof-of-concept, three-parallel arm, prospective, placebo/active-controlled, double-blind, randomized controlled trial of 217 patients with chronic lower back pain. The study’s primary outcome was average lower back pain (aLBP) intensity score, and secondary outcomes included proportional aLBP decrement, global assessments of disability, and concomitant analgesic use. Patients enrolled were non-morbidly obese adults with non-radicular, non-traumatic Quebec Task Force category 1 or 2 lower back pain requiring the chronic use of simple and mild opiate analgesics. Patients underwent washout and baseline assessments and were then randomized to receive either a) tanezumab infusion 200 mcg/kg + tablet placebo, b) naproxen 1 g/day + infusion placebo, or c) infusion and placebo tablet. Follow-up consisted of safety and efficacy measures for 16 weeks, with efficacy at 6 weeks being the primary end point.18 The study had high attrition from dropout/loss to follow-up, and only 67%, 73%, and 61% of patients in the respective tanezumab, naproxen, and placebo arms completed the study.18 The baseline demographics among the groups were nonheterogenous. All patients included in the analysis demonstrated the efficacy of treatment irrespective of cohort. At 6 weeks, there was a statistically significant improvement in aLBP in the tanezumab group vs both other cohorts. This held true for secondary endpoints as well.18
Safety data were collected, and adverse effect rates were similar among the groups. Nine patients discontinued the trial related to adverse effects. This was disproportionately weighted to the placebo and tanezumab group (4.9% and 4.5% of patients respectively). Adverse effects were uniformly nonserious, with 5% or more of patients who received tanezumab reporting arthralgia, headache, hyperesthesia, and myalgia. Feelings of abnormal peripheral sensation were reported in the tanezumab, naproxen, and placebo groups at 12.5%, 3.4%, and 2.4%, respectively. None of these complaints resulted in discontinuation of the study drug, all patients had normal neurological examinations, and all hyperesthesia resolved within the 16-week study window. It should also be noted that in this study there were no severe adverse reactions reported in the tanezumab group. The study team concluded that tanezumab was an efficacious analgesic over 16 weeks in the treatment of lower back pain and had a similar rate of adverse effects to that of naproxen and placebo.18 This study was of a small sample size and employed an intention to treat strategy. Statistical analysis was unusual in that an alpha of 0.1 was accepted as statistically significant. The study also endured a high rate of attrition, though it was for all groups. The study had a short duration and a single infusion of tanezumab, so safety data may be incomplete.
In 2013 Kivitz et al published a similar, but larger study regarding the efficacy and safety of tanezumab. This time the authors compared differing doses of tanezumab to naproxen and placebo in concurrent arms.19 Study inclusion and exclusion criteria were nearly identical to those of Katz et al. Again, a washout period occurred, and patients had baseline measures taken and were subsequently randomized to receive either placebo, tanezumab 5 mg, 10 mg, or 20 mg, or naproxen in a double-blind, double-dummy fashion. Infusions took place at weeks 1 and 8, and naproxen/placebo was given daily. This study enlisted 1,347 subjects, and patients were randomized into placebo, tanezumab 5 mg, 10 mg, or 20 mg, or naproxen groups. Efficacy was measured via aLBP daily (Table 1) from baseline to week 16, and much like that of Katz et al, secondary measures of incremental changes of lower back pain, and global assessments of function (Table 2) and satisfaction, and rescue analgesia were employed. Safety was measured by assessment of any serious or nonserious adverse effects adjudicated by external clinicians. Statistical significance was set at the more traditional alpha of 0.05, and intention to treat was employed.
  | Table 1 Comparison of average lower back pain intensity scores |
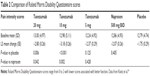  | Table 2 Comparison of Roland Morris Disability Questionnaire scores |
Baseline demographics and medical comorbidities were equivalent among groups. In this study, 20 mg and 10 mg tanezumab were statistically significantly more efficacious than placebo and naproxen for the primary endpoint along with two additional pain-related secondary endpoints. It is worth noting that 20 mg tanezumab had little additional analgesic effect over the 10 mg dose. The 5 mg dose of tanezumab had no statistically significant effect vs naproxen or placebo. Pain-related efficacy and global assessments of function were improved with the higher doses of study drug right out to the 16-week mark. Adverse effects were most common in the tanezumab groups. In the 20 mg dose group, 64.4% of patients experienced an adverse event, which is compared with 52% in the placebo group, 60.8% with tanezumab 5 mg, 58% with the 10 mg dose, and 48.1% in the naproxen group. Neurological sequelae, including paresthesia and headache, as well as arthralgia were the most common complaints and appeared to be dose dependent. Gastrointestinal adverse effects were reported in the tanezumab group at 12.1%, 8.8%, and 9.1% for the 5 mg, 10 mg, and 20 mg doses, respectively, and 10% in those treated with placebo. The highest rate of reported gastrointestinal adverse effects was in the naproxen group at 14.6%. Higher dose tanezumab was associated with hyperesthesia and dysesthesia at a rate of 12.9%, which far exceeded smaller doses, naproxen, or placebo. These events subsided by the end of the trial follow-up. Serious adverse effects were rare, but occurred more commonly in those receiving tanezumab; of note, none of the effects included ON, and there were no reported deaths in any groups.19
The study committee deemed these findings to demonstrate the efficacy and safety of tanezumab at the 10 mg and 20 mg doses compared to naproxen or placebo. Clinically meaningful pain relief was estimated to be a reduction in pain scores of ~30%, and this was achieved in the 10 and 20 mg tanezumab dose groups. They found that the rates of adverse effects and patient attrition, which were again high, were not dissimilar to other trials of therapy for chronic lower back pain.19
This larger trial demonstrated statistically and clinically significant analgesic effects of tanezumab and elucidated some potential therapeutic dosing regimens. The percentage of patients that responded to treatment were consistently higher in the tanezumab 10 mg and 20 mg arms compared to both naproxen and placebo arms. The overall safety profile of tanezumab was similar to other trials and similar to both placebo and naproxen treatment groups.19
A follow-up safety analysis subset of the Kivitz et al trial was performed by Gimbel et al in 2014.20 In this trial, those who completed the initial trial were then enrolled in a further noncontrolled, dose-blinded study in which 10 mg or 20 mg tanezumab intravenous (three doses) and subcutaneous (four doses) were given at 8-week intervals. This “add-on” study sought to determine the long-term efficacy of the drug. The primary endpoint of efficacy was measured by the change in reporting of Brief Pain Inventory Short Form. Secondary measures were global assessments of function and well-being, as previously used in the Kivitz et al study. Safety was also assessed in a similar fashion to previous studies with adverse effects being monitored for in each of the groups.
Those who remained in the trial to its completion were to undergo 64 weeks of additional treatment in an extension study. A total of 849 patients were randomized 2:1 (20 mg:10 mg). Baseline demographics of the patients included were nonheterogeneous among the two study arms. The study was terminated early, but prior to its termination, the two groups received an average of 270 days (10 mg) and 259 days (20 mg) of treatment.
Either dose of tanezumab was associated with a pain improvement compared to those who had been in the naproxen, 5 mg tanezumab, or placebo group initially. There was no further improvement of score seen in those who had previously taken tanezumab at the 10 or 20 mg dose. There was no statistically significant difference in efficacy between the 10 and 20 mg arms of the extension studies. Secondary markers of well-being were sustained in those taking tanezumab, but there was no further improvement in the secondary trial.
The trial was discontinued for safety reasons at the sponsors’ request. The reason for early termination was related to a partial clinical hold enacted by the FDA over joint safety. Tanezumab at the 20 mg dose was associated with more adverse outcomes than the 10 mg dose, (70.2% and 61.7% respectively). Parasthesias and neurological sequelae were again the most common complaints. Most adverse effects were considered non-serious, but serious adverse effects occurred in 4.7% (10 mg) and 4.6% (20 mg). The most serious adverse effect was OA requiring urgent joint replacement (six patients total; four patients took 20 mg and two took 10 mg tanezumab). In addition, nine patients required less urgent joint replacement. Histological examination of the bone samples suggested simple OA or RPOA as opposed to primary ON. Three patients died in the study, all of whom were taking the 20 mg dose of tanezumab; however, these deaths were adjudicated and were not attributed to the study drug.20
The study team determined that tanezumab is an effective treatment for lower back pain and safety was similar to previous tanezumab trials. The 10 mg dose of tanezumab seemed to be better tolerated that 20 mg doses, but both dosages showed improvement in pain scores and global functional assessments. They concluded that this may be an efficacious treatment of back pain, while also acknowledging safety concerns.20
A 2014 meta analysis by Leite et al summarized anti-NGF trials in lower back pain.21 Four trials were included in the analysis, including the two studies discussed previously. This study determined that the studies by Katz et al and Kivitz et al were the two highest quality studies and the greatest in size, guiding the discussion on anti-NGF therapy. The other two trials were of study drugs REGN475 and fulranumab. The conclusion of the meta-analysis was that tanezumab showed a low to moderate effect for pain relief in this cohort and low evidence for global functional improvement of lower back pain-related disability.21 It suggested that while the findings may be statistically significant, they might lack in clinical significance. The authors proposed that the rate of adverse effects in these studies were unacceptable for the general population, and that its use for lower back pain was not recommended.21
Patient perspectives
In the study by Katz et al, most patients reported an improvement of their back pain from baseline with the use of tanezumab. In addition, patients taking the study drug rated their back pain more favorably than patients taking both naproxen and placebo. At the 6-week mark in this study, a larger percentage of patients in the tanezumab group (78.8%) classified their back pain as either “very good” or “good” compared to the placebo (71.0%) and naproxen groups (65.3%). However, this was not measured to be statistically significant. Also, at week 6 more patients in the tanezumab group (83.1%) gave the study medication a rating of “good” or “excellent” opposed to those receiving placebo (58.1%) or naproxen (54.7%). This trend was seen for all time points, but was only statistically significant at week 6.18
In the Kivitiz et al trial, the overall incidence of early withdrawal was lower in all groups treated with tanezumab compared to those receiving naproxen or placebo. The incidence of premature discontinuation was highest in the placebo group (43.8%) and lowest in the tanezumab 10 mg group (34.2%). The most cited reason for early withdrawal was lack of efficacy. Highest withdrawal rates were seen with the placebo group (25.7%) and lowest in those taking tanezumab 20 mg (11.5%). Patients receiving 20 mg tanezumab (9.5%) had the highest rate of adverse events and study termination because of these particular effects. Those receiving naproxen had the lowest rate (3.4%) of discontinuation due to adverse events. All other groups had similar rates of withdrawal due to adverse events.19
Conclusion
Chronic lower back pain affects a significant proportion of the population, which often results in a significant disease burden, lost productivity, and reduced quality of life. Treating chronic lower back pain can often prove difficult for providers, so there is an urgent need for effective pharmacological agents. The use of both intravenous and subcutaneous tanezumab has shown promise in the treatment of chronic lower back pain. There are few studies that have examined the efficacy of tanezumab in chronic lower back pain, likely because it is a relatively new agent and there have been several partial clinical holds placed on the drug.22 One particular clinical hold was enacted by the FDA because there was a concern for the development of ON with tanezumab use. After closer investigation, no evidence was found associating the use of tanezumab with an increased risk of ON. Tanezumab was associated with an increased risk of RPOA, especially when higher doses were used and when coadministered with NSAIDs.17 Tanezumab was also associated with a higher risk of adverse effects including myalgia, arthralgia, and abnormal peripheral nerve sensations, but this was not statistically significant, and there was no increase in serious adverse events.22
A single dose of tanezumab may provide analgesic effects for up to 12 weeks.18 Doses of 10 mg and 20 mg were shown to provide prolonged analgesic effectiveness in addition to improvements in physical functioning.21 There appears to be no measurable difference in efficacy between 10 mg and 20 mg doses; however, 10 mg tanezumab appears to be better tolerated than the 20 mg dose.20 In the studies examined, there was also no statistically significant difference in improvement of chronic lower back pain between 5 mg tanezumab, naproxen, and placebo.19 However, there is evidence that 5 mg of tanezumab can provide measurable improvements in physical functioning and pain in patients with OA.23 A meta-analysis found a small to moderate analgesic effect for chronic lower back pain as well as a small increase in functional improvement when compared to placebo.21
More studies are needed to better characterize the analgesic effects, the impact on quality of life, and the safety profile of tanezumab. There are currently two Phase III randomized controlled trials underway to further investigate the efficacy and safety of tanezumab in the treatment of chronic lower back pain. Estimated enrollments for these trials are 390 and 1,800 patients and both plan to administer either 5 or 10 mg of tanezumab subcutaneously every 8 weeks over a 56-week period.24,25 The completion of these studies and hopefully other trials to come will help to determine the role of tanezumab in the treatment of chronic lower back pain.
Tanezumab is a relatively new therapeutic agent for back pain, which may have some positive benefit in the treatment of chronic LBP. While there is a small statistically significant clinical benefit observed in trials of the drug, there are also problematic large numbers of adverse effects associated with its use. Due to the high rates of adverse effects and lack of long-term data, more evidence is needed before a therapeutic role can be clearly defined. Monoclonal antibodies against neurotrophic agents represent a new class of drug that warrants further exploration of its use as analgesic modality for patients with chronic low lack pain.
Acknowledgments
The authors acknowledge the recent passing of Dr Phillip J Kadowitz, PhD, Professor at Tulane School of Medicine, Department of Pharmacology, and Adjunct Professor at the Louisiana State University Health Science Center, Department of Anesthesiology, for his lifelong teaching of medical students, graduate students, and residents over a 50 plus year career. One of his PhD graduate students was Dr Alan D Kaye, MD, PhD.
Disclosure
Dr Kaye is a speaker for Depomed, Inc, and Merck. Dr Urman has received research funding from Cara Pharmaceuticals, Mallinckrodt, Merck, and Medtronic. The other authors report no conflicts of interest in this work.
References
Centers for Disease Control and Prevention (CDC). Prevalence of disabilities and associated health conditions among adults – United States, 1999. MMWR Morb Mortal Wkly Rep. 2001;50(7):120–125. | ||
Simon J, McAuliffe M, Shamim F, Vuong N, Tahaei A. Discogenic low back pain. Phys Med Rehabil Clin N Am. 2014;25(2):305–317. | ||
GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545–1602. | ||
Frymoyer JW, editor. The Adult Spine: Principles and Practice. Philadelphia, PA: Lippincott-Raven; 1997. | ||
Croft P, Rigby AS, Boswell R, Schollum J, Silman A. The prevalence of chronic widespread pain in the general population. J Rheumatol. 1993;20(4):710–713. | ||
Rubin DI. Epidemiology and risk factors for spine pain. Neurol Clin. 2007;25(2):353–371. | ||
Jarvik JG, Deyo RA. Diagnostic evaluation of low back pain with emphasis on imaging. Ann Intern Med. 2002;137(7):586–597. | ||
Lane NE, Schnitzer TJ, Birbara CA, Mokhtarani M, Shelton DL, Smith MD, Brown MT. Tanezumab for the treatment of pain from osteoarthritis of the knee. N Engl J Med. 2010;363(16):1521–1531. | ||
Abdiche YN, Malashock DS, Pons J. Probing the binding mechanism and affinity of tanezumab, a recombinant humanized anti-NGF monoclonal antibody, using a repertoire of biosensors. Protein Sci. 2008;17(8):1326–1335. | ||
Halliday DA, Zettler C, Rush RA, Scicchitano R, McNeil JD. Elevated nerve growth factor levels in the synovial fluid of patients with inflammatory joint disease. Neurochem Res. 1998;23(6):919–922. | ||
Zhao L, Ren TH, Wang DD. Clinical pharmacology considerations in biologics development. Acta Pharmacol Sin. 2012;33(11):1339–1347. | ||
Jonsson EN, Xie R, Marshall SF, Arends RH. Population pharmacokinetics of tanezumab in phase 3 clinical trials for osteoarthritis pain. Br J Clin Pharmacol. 2016;81(4):688–699. | ||
Paul WE. Immunology: structure and function. In: Paul WE. Fundamental immunology. 4th ed. Philadelphia, PA: Lippencott-Raven; 1999. | ||
Akilesh S, Huber TB, Wu H, et al. Podocytes use FcRn to clear IgG from the glomerular basement membrane. Proc Natl Acad Sci U S A. 2008;105(3):967–972. | ||
Wang DD, Zhang S, Zhao H, Men AY, Parivar K. Fixed dosing versus body size-based dosing of monoclonal antibodies in adult clinical trials. J Clin Pharmacol. 2009;49(9):1012–1024. | ||
United States Food and Drug Administration. Tanezumab Arthritis Advisory Committee Briefing Document. US FDA; 2016. Available from: http://www.fda.gov/downloads/committeesmeetingmaterials/drugs/arthritisadvisorycommittee/ucm295205.pdf. Accessed February 4, 2018. | ||
Hochberg MC, Tive LA, Abramson SB, et al. When is osteonecrosis not osteonecrosis?: adjudication of reported serious adverse joint events in the Tanezumab Clinical Development Program. Arthritis Rheumatol. 2016;68(2):382–391. | ||
Katz N, Borenstein DG, Birbara C, Bramson C, Nemeth MA, Smith MD, Brown MT. Efficacy and safety of tanezumab in the treatment of chronic low back pain. Pain. 2011;152(10):2248–2258. | ||
Kivitz AJ, Gimbel JS, Bramson C, et al. Efficacy and safety of tanezumab versus naproxen in the treatment of chronic low back pain. Pain. 2013;154(7):1009–1021. | ||
Gimbel JS, Kivitz AJ, Bramson C, et al. Long-term safety and effectiveness of tanezumab as treatment for chronic low back pain. Pain. 2014;155(9):1793–1801. | ||
Leite VF, Buehler AM, El Abd O, et al. Anti-nerve growth factor in the treatment of low back pain and radiculopathy: a systematic review and a meta-analysis. Pain Physician. 2014;17(1):E45–E60. | ||
Knezevic NN, Mandalia S, Raasch J, Knezevic I, Candido KD. Treatment of chronic low back pain – new approaches on the horizon. J Pain Res. 2017;10:1111–1123. | ||
Bannwarth B, Kostine M. Targeting nerve growth factor (NGF) for pain management: what does the future hold for NGF antagonists? Drugs. 2014;74(6):619–626. | ||
Pfizer. A phase 3 study of tanezumab for chronic low back pain (TANGO). Available from: https://Clinicaltrials.gov/ct2/show/NCT02528253. NLM identifier: NCT02528253. Accessed February 4, 2018. | ||
Pfizer. Long term safety and efficacy study of tanezumab in Japanese adult subjects with chronic low back pain (TANGO). Available from: https://clinicaltrials.gov/ct2/show/record/NCT02725411?term=tanezumab&cond=Chronic+Low-back+Pain&rank=1. NLM identifier: NCT02725411. Accessed February 4, 2018. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
