Back to Journals » Clinical Interventions in Aging » Volume 16
Systemic Immune-Inflammation Index Predicts 3-Month Functional Outcome in Acute Ischemic Stroke Patients Treated with Intravenous Thrombolysis
Authors Weng Y, Zeng T, Huang H, Ren J, Wang J, Yang C, Pan W, Hu J, Sun F, Zhou X, Qiu H, Gao Y, Gao B, Chi L, Chen G
Received 17 March 2021
Accepted for publication 29 April 2021
Published 20 May 2021 Volume 2021:16 Pages 877—886
DOI https://doi.org/10.2147/CIA.S311047
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Zhi-Ying Wu
Yiyun Weng,1,* Tian Zeng,2,3,* Honghao Huang,2,3,* Junli Ren,2,3 Jianing Wang,2,3 Chenguang Yang,2,3 Wenjing Pan,2,3 Jingyu Hu,2,3 Fangyue Sun,2,3 Xinbo Zhou,2,3 Haojie Qiu,2,3 Yufan Gao,2,3 Beibei Gao,4 Lifen Chi,3 Guangyong Chen3
1Department of Neurology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, People’s Republic of China; 2School of the First Clinical Medical Sciences, Wenzhou Medical University, Wenzhou, People’s Republic of China; 3Department of Neurology, The Third Affiliated Hospital of Wenzhou Medical University, Wenzhou, People’s Republic of China; 4Department of Internal Medicine, The Third Affiliated Hospital of Wenzhou Medical University, Wenzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Guangyong Chen
Department of Neurology, The Third Affiliated Hospital of Wenzhou Medical University, No. 108 Wansong Road, Wenzhou, 325000, Zhejiang, People’s Republic of China
Tel +86-13777770788
Fax +86-577-65623263
Email [email protected]
Lifen Chi
Department of Neurology, The Third Affiliated Hospital of Wenzhou Medical University, Wenzhou, People’s Republic of China
Email [email protected]
Background and Purpose: Systemic immune-inflammation index (SII), a novel inflammation index derived from counts of circulating platelets, neutrophils and lymphocytes, has been studied in developing incident cancer. However, the clinical value of SII in acute ischemic stroke (AIS) patients had not been further investigated. Therefore, we aimed to explore the association between SII and severity of stroke as well as 3-month outcome of AIS patients.
Methods: A total of 216 AIS patients receiving intravenous thrombolysis (IVT) and 875 healthy controls (HCs) were retrospectively recruited. Blood samples were collected within 24h after admission. Severity of stroke was assessed by the National Institute of Health stroke scale (NIHSS) scores on admission and poor 3-month functional outcome was defined as Modified Rankin Scale (mRS) > 2.
Results: SII levels in AIS patients were higher than in HCs. The cut-off value of SII is 545.14× 109/L. Patients with SII > 545.14× 109/L had higher NIHSS scores (median: 5 vs 9, p < 0.001), a positive correlation between SII and NIHSS was observed (rs = 0.305, p < 0.001). Multivariate logistic regression analyses showed that high SII was one of the independent risk factors for poor prognosis at 3 months of AIS patients (OR = 3.953, 95% CI = 1.702– 9.179, p = 0.001). The addition of SII to the conventional prognostic model improved the reclassification (but not discrimination) of the functional outcome (net reclassification index 39.3%, p = 0.007).
Conclusion: SII is correlated with stroke severity at admission and can be a novel prognostic biomarker for AIS patients treated with IVT.
Keywords: systemic immune-inflammation index, ischemic stroke, inflammation, prognosis
Introduction
Stroke has a high morbidity and mortality worldwide and is the leading cause of death and disability in China.1 Among all incident strokes, ischemic stroke accounts for about 70%.2 Though intravenous alteplase can improve functional outcome when administered within 4.5 hours after stroke onset and is considered as a first-line agent in acute ischemic stroke (AIS) patients,3 nearly half of patients tend to have poor function outcomes after the therapy of intravenous thrombolysis (IVT). Thus, it is still of vital importance to establish accurate prognostic models of the functional outcome in patients after ischemic stroke.4
Inflammation has been demonstrated to play an important role in the pathogenesis of stroke.5 Ischemic milieu causes local immune responses and produces inflammatory cytokines, which increases the permeability of blood-brain barrier (BBB). It should be noticed that IVT with alteplase might alter leucocyte function or migration and promote neutrophil degranulation, inducing the disruption of BBB.6,7 The necrosis of vascular endothelial cells drives leukocyte entry into the infarct site, later molecules on the leukocyte surface and endogenous alarming molecules contribute to the infiltration of neutrophils, macrophages and other leukocytes, resulting in acute inflammatory response.8,9 Meanwhile, after the disruption of BBB, microglia is activated by necrotic neurons and releases inflammatory mediators, which jointly accelerates the inflammatory cascades and eventually leads to serious brain lesions and neurological deficits.7
Systemic immune-inflammation index (SII), a relatively novel inflammatory index combining peripheral lymphocytes, neutrophils, and platelet counts, has been studied as a marker for developing incident cancer.10 It was also evaluated as a predictor of survival in patients with diseases like gastric cancer,11 osteosarcoma,12 colorectal cancer.13 Several studies have studied SII in patients with stroke. In our single-center retrospective cohort study, we aimed to systematically explore the association between SII with the severity of ischemic stroke and its prognostic value in AIS patients treated with r-tPA IVT.
Materials and Methods
Study Population
This retrospective study consecutively included 365 AIS patients who were treated with IVT at the Third Affiliated Hospital of Wenzhou Medical University from February 2016 to April 2019. 875 healthy controls (HCs) that were free of any disease as shown by physical examination were also recruited.
Exclusion criteria for AIS patients were as follows: (1) patients treated with bridging therapy; (2) with cancer; (3) with infection; (4) with severe hepatic or renal dysfunction; (5) with autoimmune diseases; (6) with missing baseline data and follow-up data. Overall, 216 patients were included in our study. Figure 1 presents the selection of patients in a flow chart.
 |
Figure 1 Flow chart for patients’ selection. |
This study was approved by the Ethics Committee of the Third Affiliated Hospital of Wenzhou Medical University (YJ2020034) and was carried out in accordance with the Declaration of Helsinki. Because this study was retrospectively designed, the ethics committee granted a waiver of the requirement for informed consent for analyzing existing data in accordance with the national legislation and the institutional requirements. For the purpose of privacy protection, personal identification information of enrolled participants was anonymized and replaced with a coding system in this study.
Data Collection
Demographic and baseline data of patients with age, sex, smoking, hypertension, diabetes, hyperlipidemia, atrial fibrillation (AF), coronary artery disease (CAD), and prior stroke were obtained from medical records. The severity of ischemic stroke was assessed by the National Institutes of Health Stroke Scale (NIHSS) scores on admission and stroke subtypes were evaluated by experienced clinicians. Severe stroke was defined as NIHSS > 10.14
Blood samples were collected within the first 24 hours. The platelets, neutrophils and lymphocytes were counted using XT-1800i (Sysmex, Kobe, Japan). SII was calculated as platelet × neutrophil/lymphocyte. 3-month modified Rankin scale (mRS) was collected by two trained physicians through phone interview and the favorable prognosis was defined as mRS ≤ 2.15 As for HCs, information was obtained from their examination reports.
Statistical Analysis
Kolmogorov–Smirnov test was used to check the distribution normality. We used mean ± standard deviation (SD) to describe continuous variables with normal distribution while median (interquartile range [IQR]) to describe continuous variables with non-normal distribution. One-way analysis of variance and Mann–Whitney U-test were carried out to analyze the intergroup difference of continuous variables. Categorical variables were described as percentage numbers and analyzed by χ2 test. The relationship between SII and NIHSS scores was evaluated using the Spearman correlation test. To balance the difference in the baseline data between the patients and HCs, propensity score matching (PSM) with a match tolerance set at 0.1 was carried out. Age, sex, hypertension, diabetes and hyperlipidemia were matched in the comparison of HCs and AIS patients.
Univariate logistic regression was first performed to find out potential predictors and variables with p < 0.1 were considered as confounding factors. Multivariate logistic regression analysis included these variables to distinguish whether SII was an independent predictor of poor outcome at 3 months. The area under the receiver operating characteristic curve (ROC) was used to calculate the cut-off value. C-statistic, the continuous net reclassification index (NRI) and integrated discrimination improvement (IDI) were calculated to compare the predictive ability of 2 models. One is a conventional model including stroke severity by NIHSS scores and age, which were considered as crucial predictors of outcome while the other included SII in addition.16,17 All these analyses were processed using SPSS Statistics 25.0 software (IBM Analytics) and R (version 4.0.3). A value of p < 0.05 was considered to be statistically significant.
Results
Baseline Clinical Characteristics of the Study Subjects
Our study had enrolled 216 AIS patients and 875 HCs. The baseline characteristics are displayed in Table 1. Before PSM, there were significant differences in age, neutrophil, lymphocyte, platelet, history of hypertension, diabetes and hyperlipidemia between AIS patients and HCs. The average level of SII in AIS patients was significantly higher (p < 0.001) than HCs’. In addition, after matching of age, sex, hypertension, diabetes and hyperlipidemia, the SII levels in AIS patients were still higher than those in HCs (p < 0.001).
 |
Table 1 Baseline Clinical Characteristics of AIS Patients and Healthy Controls |
Associations Between SII Level and the Clinical Status of Ischemic Stroke Patients
According to the analysis of ROC, the SII cut-off value that best-distinguished 3-month poor outcome was 545.14×109/L with a sensitivity of 82.8% and a specificity of 50.7%. The area under curve (AUC) was 0.678 (95% CI = 0.612–0.740, p < 0.001) (Figure 2). In order to gain a further understanding of the associations between SII and the clinical status of ischemic stroke patients, 216 AIS patients were divided into two groups based on ROC cut-off values of SII (SII > 545.14×109/L, n = 87; SII ≤ 545.14×109/L, n = 129). The median value of SII in the two groups was, respectively, 884.58 and 357.78. In the high SII group, baseline NIHSS score and neutrophil counts were significantly higher, while lymphocyte, platelet counts and the percentage of favorable functional outcome at 90 days were significantly lower than the low SII group (Table 2). Spearman correlation analysis showed that SII was positively correlated with baseline NIHSS score (rs = 0.305, p < 0.001). Since the correlation was weak, both linear and non-linear regression are conducted in Figure 3A. Besides, patients with baseline NIHSS score ≤ 10 had a significant reduction in the SII level compared with those with NIHSS > 10 (p = 0.001, 631.26 ± 333.45 vs 810.57 ± 349.06) (Figure 3B).
 |
Table 2 Comparisons of Baseline Characteristics and Outcomes Between SII Groups |
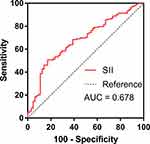 |
Figure 2 Receiver operator characteristic curves for the prediction of 3-month poor outcome using systemic immune-inflammation index (SII). |
High SII Predicting Poor 3-Month Outcome in Ischemic Stroke Patients
As shown in the univariate regression analyses, age, current smoking, AF, prior stroke, baseline NIHSS, and high SII were significantly associated with the poor outcome at three months. To figure out whether high SII was an independent prognostic indicator for poor outcome at three months, multivariate logistic regression was then performed. In model 1, after adjusting for potential confounders mentioned above, high SII remained independently associated with poor 3-month function outcome (OR = 3.925, 95% CI = 1.694–9.096, p = 0.001) (Table 3). Hypertension and diabetes are two widely accepted independent risk factors for the prognosis of AIS, but in our study, the p values of hypertension and diabetes were higher than 0.1 in the univariate regression analyses (p = 0.115, p = 0.893). Considering the impacts the two factors may cast on the result, we further included them into model 1, establishing model 2. The results of model 2 revealed that the further adjustment slightly strengthened the association between SII and the functional outcome in relative (OR = 3.953, 95% CI = 1.702–9.179, p = 0.001). Meanwhile, Figure 4 shows that patients with high SII had an increased 3-month poor outcome (41.09% vs 12.64%, p < 0.001). Furthermore, we assessed whether adding SII to a conventional model could improve the predictive ability of 3-month outcome. In terms of discrimination, C-statistic is considered to be more important than NRI. Significant improvements in discriminatory ability in NRI (continuous NRI = 39.3%, p = 0.007) and IDI (3.70%, p = 0.008) can be observed (Table 4).
 |
Table 3 Univariate and Multivariate Logistic Regression Analysis for 3-month Poor Outcome |
 |
Table 4 Reclassification and Discrimination Statistics for Poor Functional Outcomes by SII at 3 Months |
 |
Figure 4 mRS distribution at 3 months for high SII group vs low SII group. mRS, modified Rankin Scale; SII, systemic immune-inflammation index. |
Discussion
Although several researches have studied SII in AIS, no comprehensive studies were reported to the best of our knowledge. In this study, we found that AIS patients tended to have higher SII compared with the HCs. Higher SII was correlated with severe stroke. Multivariate logistic regression analysis demonstrated that SII level was an independent predictor of poor outcome at 3 months. We are going to elucidate leading scholar’s research results and possible explanations for our results in the following text.
It is already known that inflammation is implicated in the pathogenesis of stroke.18 Various inflammatory-based scores, such as neutrophil–lymphocyte ratio (NLR), platelet-to-neutrophil ratio (PNR) and platelet-to-lymphocyte ratio (PLR) have been proved to be correlated with ischemic stroke. The cohort study and meta-analysis of Wang et al revealed that NLR was positively associated with the risk of Hemorrhagic Transformation and 3-month death after AIS.19 Elevated PLR was reported to predict the development of depression after stroke.20 Chen et al have found in their research that lower PNR level was associated with poor 3 months outcomes and suggested that PNR level might be an autocephaly protective predictor of 90-days outcome in AIS patients.21 SII, combining platelet counts and leukocytes subpopulations, represented the systemic immune-inflammation status and was proved in many studies to be related to major adverse events.22 In the following sections, we are going to discuss the influence platelets, neutrophils and lymphocytes have on stroke and the interaction mechanism of them.
Neutrophils are associated with stroke severity and function outcome in AIS patients as evidenced by previous studies. They are among the first innate immune cells to respond to brain ischemia and have a deleterious effect on AIS.23 Infiltrating neutrophils can release a number of pro-inflammatory mediators, such as matrix metalloproteinases (MMPs), thus aggravating brain inflammation.24 Besides, based on previous studies, the damage of neutrophil homeostasis was associated with stroke severity by affecting systemic inflammation and the BBB.25 Lymphocytes were found in studies on animals to be able to coordinate inflammatory responses.26 However, the number of different T cells is so huge that the role of lymphocytes in AIS becomes quite complicated.27 T lymphocytes have been discovered to play either a beneficial or harmful role in AIS. For example, natural killer (NK) cells exacerbate the brain injury by catalyzing neuronal death.28 Recent studies suggest that subtypes of T cells like γδ T cells that independent of antigen activation also play deleterious roles in stroke by producing pro-inflammatory cytokines like IL-17, while Treg cells seem to be beneficial through the release of anti-inflammatory cytokines like IL-10.29 As for platelet, when AIS occurs, the excessive activation and aggregation of it may result in thrombosis and vessel occlusion.30
Leukocytes and platelets accumulate at regions of cerebral hemorrhage and their interaction connects the thrombotic and inflammatory responses.31 Studies have already shown that these interactions are mediated by platelet P-selectin and GPIb-a binding neutrophil P-selectin glycoprotein-1 and MAC-1 (CD11b/CD18).32 A recent experiment on mice proved that necrotic platelets will interact with neutrophils, thus exacerbating brain injury during ischemic stroke by controlling cyclophilin D, a mediator of necrosis.32 Apart from that, due to the activation of the hypothalamic-pituitary-adrenal axis caused by stress from the excessive activation and accumulation of platelets, lymphocyte concentration may be relatively reduced.33 There are also studies revealing that neutrophils can trigger lymphocyte apoptosis by release inflammatory cytokines.34 The inflammatory response in AIS is complex.
In conclusion, SII is a relatively integrated index and the mechanisms mentioned above might be potential therapeutic targets. Our study proved that SII is correlated with stroke severity as well as an independent prognostic indicator for poor outcome at 3 months. In addition, adding SII to the conventional prognostic model could improve the risk reclassification of the functional outcome. Furthermore, SII can be easily obtained and calculated from blood routine examinations. Therefore, we believe that SII may be a potential prognostic assessment tool in the clinical practice.
The limitations of present study are listed as follows: First, our study is a single-center study with a relatively small sample size, which may cause selection bias and inaccuracy to some degree. Secondly, we have only investigated into SII values on admission, while the dynamic variability over time should also be assessed and studied. Thirdly, we tried to reduce the impact of confounding factors on outcomes, but confounding factors still could not be completely ruled out in the multiple logistic regression analysis. Finally, further study is needed to testify the relationship between SII and long-term prognosis for AIS patients.
Conclusion
The SII level was found higher in AIS patients than HCs. Our study had revealed its correlation with the severity of ischemic stroke at admission. We also demonstrated that SII, a relatively more comprehensive inflammatory index, could be a potential prognostic indicator for AIS patients undergoing IVT.
Data Sharing Statement
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval
This study was approved by the Ethics Committee of the Third Affiliated Hospital of Wenzhou Medical University (YJ2020034) and was carried out in accordance with the Declaration of Helsinki.
Consent to Participate
Because this study was retrospectively designed, the ethics committee granted a waiver of the requirement for informed consent for analyzing existing data in accordance with the national legislation and the institutional requirements. For the purpose of privacy protection, personal identification information of enrolled participants was anonymized and replaced with a coding system in this study.
Author Contributions
Conception and study design: LC and GC; data acquisition: YW, TZ, HH, JR, JW, CY, WP, JH, FS, XZ, HQ, YG and BG; data analysis and interpretation: YW, TZ, HH and JR; drafting the article: YW, TZ and HH; revising the article: LC, GC, YW, TZ, HH, JR, JW, CY, WP, JH, FS, XZ, HQ, YG and BG. All authors have made substantial contributions to conception and study design, acquisition of data, or analysis and interpretation of data; have taken part in drafting or critically revised it critically for important intellectual content; agreed to submit to the current journal; reviewed and agreed on all versions to be published; agreed to take responsibility and be accountable for the contents of the article.
Funding
The funding agencies had no further role in study design, data collection and analysis, decision to publish, or preparation of the article.
Disclosure
Yiyun Weng, Tian Zeng and Honghao Huang are co-first authors for this study. We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
References
1. Feigin V, Nguyen G, Cercy K, et al. Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N Engl J Med. 2018;379:2429–2437.
2. Phipps M, Cronin CJB. Management of acute ischemic stroke. BMJ. 2020;368:l6983. doi:10.1136/bmj.l6983
3. Powers WJ. Acute ischemic stroke. N Engl J Med. 2020;383:252–260. doi:10.1056/NEJMcp1917030
4. Jampathong N, Laopaiboon M, Rattanakanokchai S, et al. Prognostic models for complete recovery in ischemic stroke: a systematic review and meta-analysis. BMC Neurol. 2018;18:26. doi:10.1186/s12883-018-1032-5
5. Kim J, Park J, Chang J, et al. Inflammation after ischemic stroke: the role of leukocytes and glial cells. Exp Neurobiol. 2016;25:241–251. doi:10.5607/en.2016.25.5.241
6. Cuadrado E, Ortega L, Hernández-Guillamon M, et al. Tissue plasminogen activator (t-PA) promotes neutrophil degranulation and MMP-9 release. J Leukocyte Biol. 2008;84:207–214. doi:10.1189/jlb.0907606
7. Jian Z, Liu R, Zhu X, et al. The involvement and therapy target of immune cells after ischemic stroke. Front Immunol. 2019;10:2167.
8. Tsuyama J, Nakamura A, Ooboshi H, et al. Pivotal role of innate myeloid cells in cerebral post-ischemic sterile inflammation. Semin Immunopathol. 2018;40:523–538. doi:10.1007/s00281-018-0707-8
9. Malone K, Amu S, Moore A, et al. The immune system and stroke: from current targets to future therapy. Immunol Cell Biol. 2019;97:5–16. doi:10.1111/imcb.12191
10. Fest J, Ruiter R, Mulder M, et al. The systemic immune-inflammation index is associated with an increased risk of incident cancer-A population-based cohort study. Int J Cancer. 2020;146:692–698. doi:10.1002/ijc.32303
11. Wang K, Diao F, Ye Z, et al. Prognostic value of systemic immune-inflammation index in patients with gastric cancer. Cancer Commun. . 2017;36:75.
12. Huang X, Hu H, Zhang W, et al. Prognostic value of prognostic nutritional index and systemic immune-inflammation index in patients with osteosarcoma. J Cell Physiol. 2019;234:18408–18414. doi:10.1002/jcp.28476
13. Dong M, Shi Y, Yang J, et al. Prognostic and clinicopathological significance of systemic immune-inflammation index in colorectal cancer: a meta-analysis. Ther Adv Med Oncol. 2020;12:1758835920937425.
14. Pendlebury S, Rothwell P. Incidence and prevalence of dementia associated with transient ischaemic attack and stroke: analysis of the population-based Oxford Vascular Study. Lancet Neurol. 2019;18:248–258. doi:10.1016/S1474-4422(18)30442-3
15. Khatri P, Abruzzo T, Yeatts S, et al. Good clinical outcome after ischemic stroke with successful revascularization is time-dependent. Neurology. 2009;73:1066–1072. doi:10.1212/WNL.0b013e3181b9c847
16. Rost N, Bottle A, Lee J, et al. Stroke severity is a crucial predictor of outcome: an international prospective validation study. J Am Heart Assoc. 2016;5. doi:10.1161/JAHA.115.002433
17. Swarowska M, Ferens A, Pera J, et al. Can prediction of functional outcome after stroke be improved by adding fibrinogen to prognostic model? J Stroke Cerebrovasc Dis. 2016;25:2752–2755. doi:10.1016/j.jstrokecerebrovasdis.2016.07.029
18. Parikh N, Merkler A, Iadecola CJS. Inflammation, autoimmunity, infection, and stroke: epidemiology and lessons from therapeutic intervention. Stroke. 2020;51:711–718. doi:10.1161/STROKEAHA.119.024157
19. Wang L, Song Q, Wang C, et al. Neutrophil to lymphocyte ratio predicts poor outcomes after acute ischemic stroke: a cohort study and systematic review. J Neurol Sci. 2019;406:116445.
20. Huang G, Chen H, Wang Q, et al. High platelet-to-lymphocyte ratio are associated with post-stroke depression. J Affect Disord. 2019;246:105–111. doi:10.1016/j.jad.2018.12.012
21. Jin P, Li X, Chen J, et al. Platelet-to-neutrophil ratio is a prognostic marker for 90-days outcome in acute ischemic stroke. J Clin Neurosci. 2019;63:110–115. doi:10.1016/j.jocn.2019.01.028
22. Yang Y, Wu C, Hsu P, et al. Systemic immune-inflammation index (SII) predicted clinical outcome in patients with coronary artery disease. Eur J Clin Investig. 2020;50:e13230.
23. Anrather J, Iadecola CJ. Inflammation and stroke: an overview. Neurotherapeutics. 2016;13:661–670. doi:10.1007/s13311-016-0483-x
24. Iadecola C, Anrather JJ. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796–808. doi:10.1038/nm.2399
25. Weisenburger-Lile D, Dong Y, Yger M, et al. Harmful neutrophil subsets in patients with ischemic stroke: association with disease severity. Neurol Neuroimmunol Neuroinflam. 2019;6:e571. doi:10.1212/NXI.0000000000000571
26. Jander S, Kraemer M, Schroeter M, et al. Lymphocytic infiltration and expression of intercellular adhesion molecule-1 in photochemically induced ischemia of the rat cortex. J Cerebral Blood Flow Metabol. 1995;15:42–51. doi:10.1038/jcbfm.1995.5
27. Petrone A, Eisenman R, Steele K, et al. Temporal dynamics of peripheral neutrophil and lymphocytes following acute ischemic stroke. Neurolog Sci. 2019;40:1877–1885. doi:10.1007/s10072-019-03919-y
28. Gan Y, Liu Q, Wu W, et al. Ischemic neurons recruit natural killer cells that accelerate brain infarction. Proc Natl Acad Sci USA. 2014;111:2704–2709. doi:10.1073/pnas.1315943111
29. Gill D, Veltkamp RJ. Dynamics of T cell responses after stroke. Curr Opin Pharmacol. 2016;26:26–32. doi:10.1016/j.coph.2015.09.009
30. Chen Y, Xiao Y, Lin Z, et al. The role of circulating platelets microparticles and platelet parameters in acute ischemic stroke patients. J Stroke Cerebrovascular Dis. 2015;24:2313–2320. doi:10.1016/j.jstrokecerebrovasdis.2015.06.018
31. Simon D, Chen Z, Xu H, et al. Platelet glycoprotein ibalpha is a counterreceptor for the leukocyte integrin Mac-1 (CD11b/CD18). J Exp Med. 2000;192:193–204. doi:10.1084/jem.192.2.193
32. Denorme F, Manne B, Portier I, et al. Platelet necrosis mediates ischemic stroke outcome in mice. Blood. 2020;135:429–440. doi:10.1182/blood.2019002124
33. Xu J, He X, Li Q, et al. Higher platelet-to-lymphocyte ratio is associated with worse outcomes after intravenous thrombolysis in acute ischaemic stroke. Front Neurol. 2019;10:1192.
34. Shantsila E, Lip GY. Stroke in atrial fibrillation and improving the identification of ‘high-risk’ patients: the crossroads of immunity and thrombosis. J Thromb Haemost. 2015;13:1968–1970
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

