Back to Journals » Substance Abuse and Rehabilitation » Volume 7
Systematic review and meta-analysis of Internet interventions for smoking cessation among adults
Authors Graham A, Carpenter K, Cha S, Cole S, Jacobs M, Raskob M, Cole-Lewis H
Received 1 December 2015
Accepted for publication 12 February 2016
Published 18 May 2016 Volume 2016:7 Pages 55—69
DOI https://doi.org/10.2147/SAR.S101660
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Li-Tzy Wu
Amanda L Graham,1,2 Kelly M Carpenter,3 Sarah Cha,1 Sam Cole,3 Megan A Jacobs,1 Margaret Raskob,3 Heather Cole-Lewis,4,5
1Schroeder Institute for Tobacco Research and Policy Studies, Truth Initiative, Washington, DC, 2Department of Oncology, Georgetown University Medical Center/Cancer Prevention and Control Program, Lombardi Comprehensive Cancer Center, Washington, DC, 3Alere Wellbeing, Seattle, WA, 4Johnson & Johnson Health and Wellness Solutions, Inc., New Brunswick, NJ, 5ICF International, Rockville, MD, USA
Background: The aim of this systematic review was to determine the effectiveness of Internet interventions in promoting smoking cessation among adult tobacco users relative to other forms of intervention recommended in treatment guidelines.
Methods: This review followed Cochrane Collaboration guidelines for systematic reviews. Combinations of “Internet,” “web-based,”and “smoking cessation intervention” and related keywords were used in both automated and manual searches. We included randomized trials published from January 1990 through to April 2015. A modified version of the Cochrane risk of bias assessment tool was used. We calculated risk ratios (RRs) for each study. Meta-analysis was conducted using random-effects method to pool RRs. Presentation of results follows the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.
Results: Forty randomized trials involving 98,530 participants were included. Most trials had a low risk of bias in most domains. Pooled results comparing Internet interventions to assessment-only/waitlist control were significant (RR 1.60, 95% confidence interval [CI] 1.15–2.21, I2=51.7%; four studies). Pooled results of largely static Internet interventions compared to print materials were not significant (RR 0.83, 95% CI 0.63–1.10, I2=0%; two studies), whereas comparisons of interactive Internet interventions to print materials were significant (RR 2.10, 95% CI 1.25–3.52, I2=41.6%; two studies). No significant effects were observed in pooled results of Internet interventions compared to face-to-face counseling (RR 1.35, 95% CI 0.97–1.87, I2=0%; four studies) or to telephone counseling (RR 0.95, 95% CI 0.79–1.13, I2=0%; two studies). The majority of trials compared different Internet interventions; pooled results from 15 such trials (24 comparisons) found a significant effect in favor of experimental Internet interventions (RR 1.16, 95% CI 1.03–1.31, I2=76.7%).
Conclusion: Internet interventions are superior to other broad reach cessation interventions (ie, print materials), equivalent to other currently recommended treatment modes (telephone and in-person counseling), and they have an important role to play in the arsenal of tobacco-dependence treatments.
Keywords: systematic review, meta-analysis, Internet, smoking cessation, tobacco control
Background
Health care around the globe is being transformed to deliver care and services in ways that are less costly and more convenient for both providers and patients.1,2 At the center of this transformation are digital health interventions facilitated by the Internet. Internet interventions can reach large numbers of people who may not otherwise access preventive and clinical health care services and engage them with convenient, accessible, multimedia interventions that can be used flexibly, often anonymously, for as long as the user desires.3 Personalized and individually tailored treatment can be delivered via the Internet in ways that mimic many of the aspects of face-to-face clinical interventions.4 Importantly, whereas ongoing clinical intervention within the health care delivery system is often prohibitively expensive and unsustainable, treatment via Internet interventions is scalable, sustainable, and cost-efficient.5
Internet interventions for tobacco cessation may have an important role to play in improving individual, community, and population health. Tobacco use remains the leading cause of preventable death worldwide.6 It is widely accepted that comprehensive tobacco control measures are needed to reduce tobacco use, including efforts to provide tobacco-dependence treatments on a population-wide basis.7 Cessation treatment guidelines recommend screening for tobacco use in health care settings, behavioral counseling delivered via individual, group, or telephone counseling, and pharmacotherapy.8–10 However, these approaches may not reach a majority of smokers. For example, in the USA, only 20.9% of tobacco users are counseled about tobacco use and only 7.6% are advised to use pharmacotherapy by a health care provider.11 Residents throughout the USA and Canada have access to quitline services, but uptake is <10% of smokers each year.12 The use of cessation medication widely varies even when it is free or inexpensive to access.13–16 To curb the tobacco use epidemic and avert the enormous toll projected from tobacco, additional interventions are needed to complement the existing arsenal of tobacco treatment strategies.
Internet interventions for smoking cessation are currently offered around the world by a broad range of national, regional, and local government entities, as well as commercial and nonprofit organizations.17 Hundreds of thousands of smokers register on web-based cessation programs each year, whether through programs offered by quitlines,18 employers, and health plans,19 or on publicly available, high-volume web-based cessation programs around the globe.20–22 The Internet is the first place many people turn to for information and assistance with health-related concerns,23 and it has been estimated that millions of smokers look online for quit smoking assistance each year.24,25
However, despite the provision of Internet cessation interventions by a broad range of stakeholders around the globe, and the demonstrated uptake of Internet cessation interventions among smokers, tobacco-dependence treatment guidelines have noted their promise but have stopped short of including them as a recommended treatment strategy, instead calling for more research on their effectiveness.8–10,26 A recent review of reviews conducted by Patnode et al9 considered evidence from six systematic reviews, drawing primarily on the most recent 2013 review by Civljak et al.27 They concluded that Internet-based behavioral interventions for smoking cessation have high potential applicability to primary care within the USA but that evidence on the use of Internet interventions was limited and not definitive.
The objective of this systematic review was to determine the effectiveness of Internet interventions in promoting smoking cessation among adult tobacco users, considering the numerous studies published since the 2013 review by Civljak et al.27 We were particularly interested in studies comparing Internet interventions to other forms of treatment that have been recommended in treatment guidelines to better understand whether there is a role in comprehensive tobacco control for Internet-based approaches. We examined whether Internet interventions are more effective in promoting abstinence compared to: 1) assessment-only/waitlist control, 2) print materials, 3) face-to-face counseling, 4) telephone counseling, and 5) static/generic websites.
Methods
Design
This study is a systematic review of randomized controlled trials following Cochrane methodological guidance.28 The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist and flow diagram are used as aids in the reporting of this systematic review. A structured approach was used to build the eligibility criteria.29
Eligibility criteria
Participants: We included studies conducted with adults aged ≥18 years regardless of sex; studies with children were included, provided that outcome data for adults were reported separately. We included studies that involved participants who were current smokers at the outset of the trial. We included studies with recent quitters as long as abstinence was reported for current smokers. Smokeless tobacco studies were excluded.
Interventions: For the purposes of this review and borrowing from published definitions, we define Internet smoking cessation interventions as being primarily composed of directive information and support services delivered via the Internet with the goal of supporting the user in trying to quit tobacco.30 Internet interventions are largely self-guided, at least partially automated, and take advantage of the interactive nature of the Internet.31 The basis of such programs is typically behaviorally or cognitive-behaviorally based treatments that have been operationalized and transformed for delivery via the Internet.32 For the purposes of this review, we exclude mHealth interventions, such as text messaging or interventions delivered solely through a mobile device.33 mHealth interventions were excluded in order to decrease heterogeneity in the interventions assessed in this review and to be consistent with previous reviews of Internet interventions. Nonetheless, excluding interventions delivered solely through a mobile device does not exclude Internet smoking cessation interventions that may have been accessed through a mobile web browser.
Comparisons: There were no exclusion criteria for comparison interventions.
Outcomes: Studies that reported abstinence from smoking with at least a 1-month follow-up as the primary outcome were included. Measures of abstinence included 7-day point prevalence abstinence, 30-day point prevalence abstinence, repeated point abstinence, continuous abstinence, sustained abstinence, and prolonged abstinence. Self-reported and biochemically verified metrics of abstinence were included.
Report characteristics and study design: We included English-language quantitative studies that employed a randomized design published since 1990. We excluded cohort studies, qualitative studies, editorials, letters, and commentaries, studies where we could not identify a full text, and articles that did not report the minimum information required.
Information sources and search strategy
We employed a mixed automated and manual search strategy. We conducted a comprehensive literature search using the following bibliographic databases: PubMed, CINAHL Plus with Full Text, Cochrane Library, PsycINFO, EMBASE, Web of Science, Communication and Mass Media Complete, Global Health, Grey Literature Report, IEEE Xplore, and Google Scholar. We used a combination of the constructs “Internet,” “web-based,” and “smoking cessation intervention” and related keywords to ensure broad coverage of published studies. Search terms were intentionally broad to ensure that all relevant articles would be captured (Supplementary materials, Appendix 1). Our search covered English language papers published between January 1990 and April 2015. We also reviewed the reference lists of included manuscripts and previous systematic reviews. All other databases were searched with free text terms reflecting inclusion criteria. Citations were compiled in EndNote (EndNote Version X6; Thomson Reuters, Philadelphia, PA, USA) and imported and de-duplicated in EPPI-Reviewer 4.0 (University College London, Institute of Education, University of London, UK).34
Study selection
The title and abstract of identified citations were screened for eligibility by two reviewers. Items were included at this stage if they appeared to meet inclusion criteria based on information in the title and abstract and were excluded only if clearly ineligible. When only the study title was available, the presence of keywords in the title related to “an Internet intervention” and “smoking cessation” warranted full-text review. Next, we obtained the full texts of citations considered as potentially eligible. Two reviewers independently screened the full text for eligibility using a standardized and pilot-tested screening process. Discrepancies were resolved by the team. Finally, reference lists of previous reviews and recent publications were checked.
Data collection process and data items
Eligible studies were coded to capture both substantive and methodological characteristics. The coding focused on the following features of the studies: identifying information, funding source, design, aims and objectives, variables related to the characteristics of participants, the nature of the intervention and its implementation, the nature of the comparison condition(s) and their implementation, analytical methods, follow-up duration and rates, and outcome measurements. In addition, we extracted information about strategies used to promote engagement/adherence in accordance with the study by Alkhaldi et al.35 We included selected items from the CONSORT-EHEALTH checklist relevant to the reporting of eHealth trials36 (eg, intervention access, level of human involvement, and engagement strategies).
The data abstraction form was pilot tested on a purposive sample of eligible studies.22,37–41 Reviewers were retrained on coding items that showed discrepancies during this process, and the coding scheme was adapted. This process was repeated until a high level of consistency was achieved. The remaining studies were coded by a single reviewer and reviewed by a second reviewer. Discrepancies were resolved by group discussion.
Risk of bias in individual studies
We used the Cochrane Collaboration tool for assessing the risk of bias.28 As in the systematic review by Mathieu et al,42 we elected not to consider reporting bias since few studies prospectively registered their protocols. Following Civljak et al,27 we did not assess participant blinding due to the inherent difficulty in blinding participants to behavioral interventions. Reviewers’ judgments regarding the risk of bias for each criterion were rated as low, high, or unclear. We computed graphic representations of potential bias within and across studies using EPPI-Reviewer 4.0 software.
Data analysis
The majority of studies reported cessation outcomes at multiple endpoints using multiple metrics (eg, 7-day abstinence, 30-day abstinence). When the authors specified a primary outcome (eg, used for power analyses), we selected it for analysis; if a primary outcome was not explicitly stated, we included the longest endpoint and most conservative metric of abstinence. We conducted an intention-to-treat (ITT) analysis, including all participants as randomized in the denominator; individuals lost to follow-up were counted as smokers. Abstinence rates were summarized as risk ratios (RRs) and 95% confidence intervals (CIs) using the ITT principle, which were calculated as: ([number of quitters: intervention arm]/[number randomized: intervention arm])/([number of quitters: control arm]/[number randomized: control arm]). We display descriptive data alongside RRs with 95% CIs in forest plots.
We assessed statistical heterogeneity using the I2 statistic, which assesses the proportion of the variation between studies due to heterogeneity rather than to chance.43 I2 ranges from 0% to 100%, with 0% indicating no observed heterogeneity and larger values showing increasing heterogeneity. The importance of the observed value of I2 depends on the magnitude and direction of effects as well as the strength of evidence for heterogeneity (eg, P-value from the chi-squared test). I2 values of 25%, 50%, and 75% approximately correspond to low, moderate, and high levels of statistical heterogeneity, respectively.43
We judged random-effects meta-analysis to be appropriate for all five comparisons of interest. In each of these comparisons, we pooled the weighted average of RRs using a random-effects model and 95% CI. To be conservative, we excluded trials with less than a 3-month follow-up, those that were feasibility studies, and those with very low follow-up rates.
Results
Study selection
Figure 1 shows the study flow. A total of 80 records were reviewed for eligibility, and 37 were excluded (Supplementary materials, Appendix 2) for the following reasons: full text was not available (n=3), intervention did not meet the definition of “Internet-based” (n=9), record was not a published study (n=2), not a randomized design (n=11), secondary analysis of outcome data presented elsewhere (n=2), smoking outcomes not reported (n=8), and we were unable to calculate abstinence rates using available data (n=2). Forty individual trials with a total of 98,530 participants were included (described in 43 reports) and are listed in Supplementary materials (Appendix 3).
  | Figure 1 PRISMA flow diagram. |
Risk of bias within and across studies
Overall, the studies included in this review had low risk of bias in most or all areas assessed. Figure 2A displays the risk of bias summary for individual studies, and Figure 2B displays the risk of bias graph. Details regarding the assessment of risk for each individual study are noted in Supplementary materials (Appendix 4).
  | Figure 2 (A) Risk of bias summary; (B) risk of bias graph. |
Selection bias: Most studies used an automated randomization strategy that was considered low risk. Allocation concealment was not often described, but when studies were automated, we judged allocation concealment bias risk as low. There was a higher risk when personnel were involved in randomization and allocation. For example, Emmons et al44 used personnel to randomize the participants and allocation concealment was not described. The study by Haug et al45 was conducted in inpatient rehabilitation centers, and participants were randomized by the week of their admission. We judged there to be a risk of selection bias since personnel would know ahead of time which participants would be in each group. Similarly, the study by Shuter et al46 was judged to be at high risk since clinic personnel were involved in allocation and were not blinded.
Performance and detection bias: We evaluated the included studies with regard to personnel and their ability to influence outcomes and found most studies to be at low risk for performance bias. Most studies were conducted on the Internet with no personnel involvement in the delivery of the intervention. When personnel did have opportunities to influence participants (eg, in a clinic setting), risk was judged as unclear when interactions with personnel were not described. There were two trials in which performance bias was judged as high risk. In the study by Swan et al,47 quitline counselors were not blind to condition but interacted with all participants. The study by Mehring et al48 was also judged to be at high risk for performance bias as unblinded personnel interacted with all participants. Most trials conducted outcome assessments via the Internet with no risk of detection bias. Some trials, however, had assessors to collect the outcome data by phone for at least some participants. A few studies did not describe their assessors as blinded, leading to ratings of unclear bias. No studies were believed to be at high risk of detection bias.
Attrition bias: Attrition is a particular challenge in web-based studies.49 We focused on two elements when rating attrition bias: reporting outcomes and differential attrition by study arm. Reporting results with all randomized participants included and missing participants identified as smoking can protect against an overly optimistic interpretation of study findings that can occur when only responders are considered. With the exception of the studies by Bricker et al50 and Humfleet et al,51 all studies provided this type of ITT analysis. Only half of included studies reported significance testing of attrition rates. Studies that did not report attrition by study arm or that did not report whether significance testing of attrition rates across arms was conducted were rated as unclear with regard to attrition bias37,38,51–60 unless they had very high follow-up rates (eg, 96% and 99% follow-up in the study by Shuter et al).46 Studies that reported significantly different attrition rates across arms were judged as high risk.48,61–65 Studies with low follow-up rates but equivalent attrition across study arms were not rated as high risk, as has been done in previous reviews.27 Attrition varied greatly from study to study, with follow-up rates ranging from >95% in the study by Shuter et al46 to follow-up rates approximately 5% in Mañanes and Vallejo.64
Study characteristics
Supplementary materials (Appendix 4) details the characteristics of included studies with regard to participants, interventions, comparison, outcomes, study design, risk of bias notations, and other characteristics relevant to this review.
Recruitment strategies
Most studies recruited participants via the Internet using a variety of strategies, including search engine advertising,37,53,55,66–68 online ads,39,50,55,59,62,69–71 and social media.39,50 Five trials recruited new registered users on the website being evaluated.38,56,63,64,72 Other reactive recruitment sources included newspapers and magazines,39,47,52,62 print advertisements such as flyers, posters, and billboards,52,62,73 radio and television advertisements,39,50,52 and other paid advertising campaigns (unspecified).22,74 Several trials recruited through listservs, health plans, or survey panels.40,41,47,54,61,65,73,75–77 Other proactive recruitment sources included health facilities or clinics,44–48,51,58,60,74 quitlines,47,75 and worksites.57 Several studies used a combination of recruitment strategies.
Participants
Average age in most studies was mid-30s to late 40s; four studies explicitly focused on young adults and recruited participants who were 18–30 years of age.40,61,76,78 The majority of studies enrolled a higher proportion of women; several trials recruited an equal number of men and women.64,69,70 Trials with a higher proportion of men46,51,53,55,57,58,68,76 recruited from sources where men were more likely to be represented (eg, workplace for operating engineers and human immunodeficiency virus [HIV] clinic). The only trial to enroll only women focused on cessation among pregnant smokers.59 Approximately one-third of studies did not provide details about race; among those that did, the majority had primarily White participants. Some studies targeted specific racial or ethnic groups.55,64 Participants were most likely to have at least some college education; a majority of participants in three studies had a high school degree or less.39,51,57
Intervention elements
The flexibility of the Internet allows for web-based cessation treatments to take myriad forms. In fact, this is one of the clearest findings from this review: there is currently no single or core web-based cessation treatment. The following groupings highlight common features among this diverse landscape.
Static web interventions: Ten trials22,37,44,45,50,53,62,68–70 included stand-alone static web components as part of the intervention condition. Static content was generally informational and non-tailored and contained content comparable to a printed cessation guide. Included in this category are static interventions in which the intervention is fully available and those that deliver intervention components over time. In some studies, static content was paired with additional features such as tailored feedback reports, text messaging, and/or social support.
Tailored feedback: Tailored feedback consists of advice or information provided to users based on responses to one or more assessments. Eight studies22,37,44,45,56,62,63,75 examined interventions consisting largely of a feedback report. Tailoring was often performed on the basis of participants’ responses to an initial assessment and/or on the basis of participants’ stage of quitting. The form of tailored messages, however, varied greatly. In the study by Wangberg et al,22 participants could receive up to 150 tailored emails over 6–12 months with tailoring on multiple factors. In contrast, Etter63 provided participants with a single tailored letter, six to nine pages in length, based on a 62-item questionnaire.
Interactive/tailored web intervention: The majority of studies evaluated the effectiveness of interactive web interventions. Interactivity was defined as any part of a web intervention that solicited/required user input and included features such as exercises, quizzes, cost calculators, tailored messages, quit planning tools, training in coping strategies, and self-monitoring. A minority of the interactive interventions offered tailored content and/or guided users through the intervention based on information provided by the participant (eg, as in the study by Wangberg et al).22
Coaching analogs and social support: A number of trials included social support resources such as peers, coaches, or counselors. The most common form of social support was the provision of an asynchronous discussion forum. Eight trials22,37,44,45,53,67,68,77 included a discussion forum, either moderated by a peer or an expert, in at least some of the study arms. Seven trials included access to live coaching or counseling either via telephone, face-to-face counseling, or SMS text or email.38,48,52,57,66,77,78 Two studies evaluated other methods of accessing social support.40,56
Other adjunctive components: Four trials described the use of SMS text messaging as part of the intervention.48,62,69,70 The two trials by Brendryen et al and Brendryen and Kraft69,70 also included interactive voice response calls. The studies by Muñoz et al37,68 and Leykin et al53 included an online eight-module cognitive-behavioral mood management component in some arms. The study by Simmons et al76 included videos and the ability to create video content.
Medication: Several studies provided pharmacotherapy along with the web-based intervention. Nicotine replacement therapy (NRT) was the most common form of pharmacotherapy and was included in seven trials.38,41,44,46,51,57,70 Medication treatment ranged from a 2-week starter kit used in the study by Fraser et al38 to a 10-week starter kit used in the study by Strecher et al.41 Two trials47,58 included 12 weeks of varenicline. The study by Japuntich et al52 included a 9-week course of bupropion.
Comparison arms
Six studies involved a no-treatment control condition. The studies by Elfeddali et al62 and Haug et al45 involved assessment-only control conditions. Smit et al39 compared a fully automated, tailored Internet intervention to a no-treatment control. In a cluster randomized trial by Pisinger et al,60 participants randomized to the control arm received usual care by their general practitioner. Swartz et al73 tested a tailored, video-based Internet site in worksites against a waitlist control. Participants randomized to the control arm had access to the intervention after 90 days. Oenema et al65 studied a multiple behavior change Internet intervention that addressed saturated fat intake, physical activity, and smoking. Smokers were encouraged to complete the smoking module first, which was interactive and included tailored feedback. Participants randomized to the control arm had access to the intervention after completing the posttreatment assessment at 1 month.
Five studies involved self-help print materials.44,51,55,69,70 Three studies compared largely static Internet interventions to self-help print materials, and two compared interactive Internet interventions to print materials. Emmons et al44 adapted Partnership for Health-2, a smoking cessation intervention for cancer survivors, for delivery via the Internet and compared it to a print version of the program. The print arm received a series of manuals designed to be interactive (eg, worksheets and personalized content). McDonnell et al55 compared a static website composed of six sequential sections with a printed version of the same program. Humfleet et al51 compared a static Internet intervention to a printed self-help guide among patients in an HIV clinic. Two studies by Brendryen et al and Brendryen and Kraft69,70 evaluated the effect of an interactive, multimodal (Internet, email, SMS, interactive voice response) cessation intervention against self-help print materials. All participants in the study by Brendryen and Kraft70 received NRT.
Seven studies compared Internet interventions to face-to-face advice or counseling, either in individual or group format. The study by Dezee et al58 involved in-person counseling (four 1.5-hour classes) and standard-dose varenicline. The control condition in the study by Japuntich et al52 consisted of 9 weeks of twice-daily bupropion sustained release, three brief individual counseling sessions, and five follow-up visits. In a cluster randomized trial conducted by Mehring et al48 within primary care clinics, participants randomized to the control arm received usual care and advice from their practitioner. A pilot study by Shuter et al46 among HIV clinic patients randomized participants in the control arm to standard care, defined as brief advice to quit and self-help brochure. All subjects were offered nicotine patches. An experimental study by Simmons et al76 included a group-based intervention as one of the controls. In small groups, students reviewed paper versions of the content from the Internet intervention and discussed it during a group discussion. In the study by Humfleet et al,51 the control arm received six sessions of 40–60-minute in-person counseling plus NRT. In a cluster randomized trial by Pisinger et al,60 general practitioners provided brief cessation counseling and referred smokers to five sessions of group-based counseling.
Two studies involved telephone counseling as a comparison condition.47,57 In the study by Choi et al,57 participants were encouraged to call a toll-free telephone quitline and use NRT. A three-arm trial by Swan et al47 compared proactive telephone counseling, an interactive website based on the same program, and a combined phone + Internet intervention. All participants received varenicline.
Twenty-three trials compared an interactive and/or tailored Internet intervention with a less intensive, static or generic Internet intervention.22,37,38,40,41,49,53,54,56,59,61,63,64,66–68,71,72,74–78 Two of these studies examined the active ingredients of an Internet intervention using a factorial design.41,54 The remainder of the trials employed two- or three-arm randomized designs to examine the comparative effectiveness of different Internet interventions.
Outcome measures
The primary endpoints differed widely among the studies, ranging from 1 or 2 months post randomization56,59,65 to 15 months post randomization.44 Most studies used self-reported abstinence measures (ie, 7-day, 30-day abstinence) as the primary outcome abstinence metric. A small number of studies used more conservative metrics of self-reported abstinence.56,62,72,74 Humfleet et al,51 Japuntich et al,52 Shuter et al,46 Simmons et al,76 and Dezee et al58 collected carbon monoxide samples; Mehring et al48 collected urine cotinine; and Pisinger et al60 confirmed smoking status via urine cotinine through mailed urine samples. In each of these trials, clinic-based or local recruitment/intervention made biochemical verification feasible.
Effects of interventions
Internet interventions compared to an assessment-only or waitlist control
Prior to analysis, we pooled the intervention arms used in the study by Elfeddali et al62 using data from Sample 1 as reported in the manuscript. We excluded the study by Oenema et al65 from this analysis since the primary outcome was measured at 1 month post randomization and was based on self-reported abstinence in response to “Do you smoke?” We excluded the study by Pisinger et al60 because of the low usage of Internet intervention. Pooled results from the four studies included Internet interventions (Figure 3) demonstrated a statistically significant effect in favor of the intervention (RR 1.60, 95% CI 1.15–2.21). However, results should be interpreted with caution as statistical heterogeneity was high (I2=51.7%). In addition, the study by Elfeddali et al62 was at high risk of attrition bias since follow-up attrition was higher in both intervention arms, though this likely resulted in an underestimate of the potential intervention effect under ITT analysis.
  | Figure 3 Internet interventions compared to assessment-only/waitlist control. |
Static Internet interventions compared to self-help print materials
The study by Humfleet et al51 was excluded since we could not calculate ITT abstinence rates based on data presented in the manuscript. Pooled results from the studies by Emmons et al44 and McDonnell et al55 (Figure 4) found a nonsignificant effect in favor of the print materials (RR 0.83, 95% CI 0.63–1.10). Both studies included in this analysis44,55 were at low risk of bias and had no statistical heterogeneity (I2=0%).
Interactive Internet interventions compared to self-help print materials
  | Figure 4 Static Internet interventions compared to self-help print materials. |
Pooled results from the two studies by Brendryen et al and Brendryen and Kraft69,70 (Figure 5) found a statistically significant effect in favor of the interactive Internet intervention (RR 2.10, 95% CI 1.25–3.52). Statistical heterogeneity was low (I2=41.6%), and both studies were at low risk of bias.
  | Figure 5 Interactive Internet interventions compared to self-help print materials. |
Internet interventions compared to face-to-face counseling
The study by Humfleet et al51 was excluded from this analysis since we could not calculate ITT abstinence rates based on data presented in the manuscript. The study by Pisinger et al60 was excluded because of the low usage of the Internet intervention; only 15.8% of participants randomized to this arm accessed the program. The study by Mehring et al48 was excluded because of the high risk of attrition bias: response rates were 85% and 59% for control and intervention, respectively. Pooled results from four studies46,52,58,76 (Figure 6) found a nonsignificant effect in favor of Internet interventions (RR 1.35, 95% CI 0.97–1.87, I2=0%).
  | Figure 6 Internet interventions compared to face-to-face intervention. |
Internet interventions compared to telephone counseling
Prior to the analysis, we combined the two arms from the study by Swan et al47 that involved Internet treatment (Web, Phone + Web) and compared it to telephone counseling. Pooled results from the studies by Choi et al57 and Swan et al47 (Figure 7) found a nonsignificant effect in favor of telephone counseling (RR 0.95, 95% CI 0.79–1.13, I2=0%).
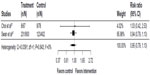  | Figure 7 Internet interventions compared to telephone counseling. |
Internet interventions compared to other websites
Twenty-three trials compared Internet interventions to other web-based interventions. Excluded from this analysis were the studies by Bricker et al50 and Berg et al61 (pilot studies focused on feasibility, no power analysis was conducted); Etter,63 Herbec et al,59 and Strecher et al56 (primary outcome assessed at < 3 months); Leykin et al53 (low rates of follow-up and high risk for attrition bias); Mañanes and Vallejo64 (very low follow-up rates <5%); and Strecher et al41 (fractional factorial design precludes reporting of main effects). Pooled results for the remaining 15 trials (24 comparisons) found a significant effect in favor of Internet interventions compared to other websites (RR 1.16, 95% CI 1.03–1.31, I2=76.7%). The forest plot is presented in Figure 8. Two trials with college students in the study by An et al40,78 showed significant effects for the two experimental conditions over the control condition, and for a personally tailored website with peer coaching over the personally tailored website alone. In the study by Stanczyk et al,74 there was a significant effect of the computer-tailored video-based website over a static website with general information. Of the remaining 19 comparisons, eleven showed nonsignificant effects in favor of the experimental condition, with RRs at or below 1.34. In the study by McClure et al,54 three of the four comparisons favored the more intensive/experimental factor over the control. Nonsignificant effects in favor of the control arm were observed in the studies by Graham et al,66 Mason et al,72 McClure et al54 (email factor), Muñoz et al68 (static website + email vs static website), Muñoz et al37 (study 3 and study 4), Stoddard et al,77 and Wangberg et al.22
  | Figure 8 Internet interventions compared to other websites. |
Discussion
The goal of this systematic review was to evaluate the literature regarding the effectiveness of Internet cessation interventions, with particular reference to other cessation interventions supported by treatment guidelines. We reviewed 40 randomized trials that included 98,530 participants published from January 1990 through April 2015. These studies varied considerably with regard to the intervention features, comparison conditions, participant characteristics, and cessation outcome. However, grouping studies by the nature of the comparison condition and by intervention type yielded the following findings: 1) Internet interventions outperformed assessment-only/waitlist controls, 2) largely static Internet interventions were equivalent to self-help print materials, 3) interactive Internet interventions outperformed self-help print materials, 4) Internet interventions appeared equivalent to counseling delivered via face-to-face and telephone interventions, and 5) Internet interventions outperformed a range of website controls, although statistical and clinical heterogeneity were high.
The finding that interactive Internet interventions outperformed print materials but largely static interventions did not is consistent with previous reviews,27 with a larger number of studies included herein. Delivery of static content via the Internet may increase its reach, but engagement with static content online would be expected to be comparable to print content, which has not been shown to significantly increase abstinence rates.8 Self-assessments, self-monitoring, quitting-specific exercises, games, and social communications are now commonplace elements of online interactivity and are expected by end users. We note that the interventions included in these analyses were all published in the past 5 years, well past the advent of Web 2.0 social technologies and the proliferation of interactive components enabled by using JavaScript, Adobe Flash, and other languages that allow for a robust web experience. Results highlight the fact that the true potential of the Internet in promoting cessation is not exemplified by largely static interventions.
Our analyses did not detect significant differences between Internet interventions and face-to-face or telephone counseling. These findings are comparable to the results reported in the study by Civljak et al,27 but with a larger number of trials. The studies of face-to-face counseling involved a range of counseling formats, including brief advice in the study by Shuter et al,46 individual counseling in the study by Japuntich et al,52 and group interventions in the studies by Dezee et al58 and Simmons et al.76 Effect sizes favored Internet interventions in each of these comparisons, but did not reach statistical significance. In comparisons of Internet interventions with telephone counseling, Choi et al57 found a nonsignificant effect in favor of the Internet intervention, whereas Swan et al47 found a nonsignificant effect in favor of telephone counseling. The equivalence of Internet interventions to these approaches lends support to the notion that Internet interventions may belong alongside face-to-face and telephone counseling interventions in tobacco treatment guidelines.
The largest group of studies compared two or more Internet interventions. More than two-thirds of comparisons in this category favored the experimental condition, perhaps signaling significant progress in the development of modern, engaging, rigorous, and effective Internet cessation interventions. Identifying the active ingredients of Internet interventions is an important area for future research, yet only three studies used factorial designs to compare specific features in order to optimize the effectiveness of Internet interventions.38,41,54 Given the efficiency of this type of design and the speed with which it can advance the science of Internet interventions, more research of this type is needed.
Our results may not be as conservative as those reported in the study by Civljak et al.27 Our use of the primary outcome specified in each trial rather than the longest available follow-up is methodologically sound given that many analyses of longer-term follow-up may have been underpowered; however, it could result in more optimistic, shorter-term outcomes than those presented elsewhere. In addition, our coverage of adherence and engagement is limited based on the nature and scope of this review. A large and growing number of studies point to engagement as a critical element in promoting abstinence.79 We gathered information on engagement strategies for descriptive purposes but did not examine this as a moderating variable. Future reviews are encouraged to consider this important aspect of effectiveness.
Conclusion
In summary, based on this review of >10 years of research on Internet cessation interventions, the field has advanced significantly with newer interventions incorporating more interactive and engaging features. Six previous reviews have reported a mixture of conclusions, some more encouraging than others. Our goal was to address a practical question of relevance to payers and other decision makers regarding the role of Internet interventions in comprehensive tobacco control. As noted by the US Preventive Services Task Force,26 “The best and most effective combinations [of cessation interventions] are those that are acceptable to and feasible for an individual patient.” Given the significant uptake of Internet interventions, their superiority to other broad reach cessation interventions (ie, print materials), and equivalence to other currently recommended treatment modes (telephone and in-person counseling), the results of this review suggest that Internet interventions have an important role to play in the arsenal of tobacco-dependence treatments.
Acknowledgments
The authors appreciate the contributions of Erik Augustson, PhD, MPH, who provided feedback on the study protocol. Support for the use of the EndNote X7.3 and EPPI-Reviewer 4.0 software was provided by Truth Initiative (formerly the American Legacy Foundation).
Disclosure
Amanda L Graham, Sarah Cha, and Megan A Jacobs are employees of Truth Initiative, a nonprofit public health foundation that runs BecomeAnEX.org, a web-based smoking cessation program. Kelly M Carpenter, Sam Cole, and Margaret Raskob are employees of Alere Wellbeing, a for-profit company that offers the Quit For Life comprehensive smoking cessation program which includes Web Coach®, a web-based smoking cessation intervention. Heather Cole-Lewis is an employee of Johnson & Johnson Health and Wellness Solutions, a for-profit company that offers Breathe®, a digital health coaching programs for smoking cessation. During the conduct of this study, Heather Cole-Lewis was an employee of ICF International which is a contractor to the National Cancer Institute (NCI) of the National Institutes of Health and supports Smokefree.gov, the NCI smoking cessation website. The authors report no other conflicts of interest in this work.
References
Deloitte [webpage on the Internet]. 2014 Global Health Care Outlook: Shared Challenges, Shared Opportunities. 2014. Available from: http://www2.deloitte.com/content/dam/Deloitte/global/Documents/Life-Sciences-Health-Care/dttl-lshc-2014-global-health-care-sector-report.pdf. Accessed February 20, 2016. (Archived by WebCite® at http://www.webcitation.org/6glLGbAV3). | |
Office of the National Coordinator for Health Information Technology. Connecting Health and Care for the Nation A Shared Nationwide Interoperability Roadmap, Draft Version 1.0. Office of the National Coordinator for Health Information Technology; Washington, DC, 2015. Available from: https://www.healthit.gov/sites/default/files/hie-interoperability/nationwide-interoperability-roadmap-final-version-1.0.pdf. Accessed November 14, 2015. (Archived by WebCite® at http://www.webcitation.org/6glLdmZCh). | |
Griffiths F, Lindenmeyer A, Powell J, Lowe P, Thorogood M. Why are health care interventions delivered over the Internet? A systematic review of the published literature. J Med Internet Res. 2006; 8(2):e10. | |
Etter JF. The Internet and the industrial revolution in smoking cessation counselling. Drug Alcohol Rev. 2006;25(1):79–84. | |
Murray E. Internet-delivered treatments for long-term conditions: strategies, efficiency and cost-effectiveness. Expert Rev Pharmacoecon Outcomes Res. 2008;8(3):261–272. | |
World Health Organization. WHO Report on the Global Tobacco Epidemic, 2008: The MPOWER Package. Geneva: World Health Organization; 2008. Available from: http://www.who.int/tobacco/mpower/2008/en/. Accessed November 02, 2015. (Archived by WebCite® at http://www.webcitation.org/6glLkPkaY) | |
World Health Organization [webpage on the Internet]. Tobacco Fact Sheet. 2009. Available from: http://www.who.int/nmh/publications/fact_sheet_tobacco_en.pdf. Accessed February 20, 2016. (Archived by WebCite® at http://www.webcitation.org/6glLMdeI2). | |
Fiore M, Jaén C, Baker T, Tobacco Use and Dependence Guideline Panel. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; 2008. | |
Patnode CD, Henderson JT, Thompson JH, Senger CA, Fortmann SP, Whitlock EP. Behavioral Counseling and Phamacotherapy Interventions for Tobacco Cessation in Adults, Including Pregnant Women: A Review for the U.S. Preventive Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality; 2015. | |
National Institute for Health and Care Excellence (NICE). Stop Smoking Services, NICE guidelines [PH10]. Available from: https://www.nice.org.uk/guidance/ph10. Accessed: 2016-03-27. Archived by WebCite® at http://www.webcitation.org/6gK0VbclF. London: National Institute for Health and Care Excellence; 2008. | |
Jamal A, Dube SR, Malarcher AM, Shaw L, Engstrom MC, Centers for Disease Control and Prevention (CDC). Tobacco use screening and counseling during physician office visits among adults – National Ambulatory Medical Care Survey and National Health Interview Survey, United States, 2005–2009. MMWR Morb Mortal Wkly Rep. 2012;61(suppl): 38–45. | |
North American Quitline Consortium. Results from the 2013 NAQC Annual Survey of Quitlines. Available from: http://c.ymcdn.com/sites/www.naquitline.org/resource/resmgr/Research/FINALNAQCFY13.pptx.pdf. North American Quitline Consortium; Phoenix, AZ, 2015. Accessed March 27, 2016. (Archived by WebCite® at http://www.webcitation.org/6gJzi3pzW). | |
Etter JF, Perneger TV. Attitudes toward nicotine replacement therapy in smokers and ex-smokers in the general public. Clin Pharmacol Ther. 2001;69:175–183. | |
Shiffman S, Brockwell SE, Pillitteri JL, Gitchell JG. Use of smoking-cessation treatments in the United States. Am J Prev Med. 2008;34(2):102–111. | |
Shin DW, Suh B, Chun S, et al. The prevalence of and factors associated with the use of smoking cessation medication in Korea: trend between 2005–2011. PLoS One. 2013;8(10):e74904. | |
Kotz D, Fidler J, West R. Factors associated with the use of aids to cessation in English smokers. Addiction. 2009;104(8):1403–1410. | |
European Network of Quitlines [webpage on the Internet]. Guidelines to Best Practice for Smoking Cessation Websites. 2012. Available from: http://docplayer.net/6348332-European-network-of-quitlines-guidelines-to-best-practice-for-smoking-cessation-websites.html. Accessed March 27, 2016. Archived by WebCite® at http://www.webcitation.org/6gK1Olczc. | |
North American Quitline Consortium [webpage on the Internet]. Web-Based Services in the U.S. and Canada 2014. Available from: http://map.naquitline.org/reports/web/. Accessed February 20, 2016. (Archived by WebCite® at http://www.webcitation.org/6glMHeK5X). | |
Alere Wellbeing Inc [webpage on the Internet]. American Cancer Society Quit For Life Program. 2014. Available from: http://www.alerewellbeing.com/company/. Accessed February 20, 2016. (Archived by WebCite® at http://www.webcitation.org/6glMKLCOk). | |
Healthways [webpage on the Internet]. Healthways Tobacco Cessation: QuitNet Comprehensive Kicks Habit with Greater Support. 2011. Available from: http://docslide.us/documents/healthways-tobacco-cessation-quitnet-comprehensive-kicks-habits-with-greater-support.html. Accessed February 20, 2016. Archived at http://www.webcitation.org/6gJzVZEna. | |
van Mierlo T, Voci S, Lee S, Fournier R, Selby P. Superusers in social networks for smoking cessation: analysis of demographic characteristics and posting behavior from the Canadian Cancer Society’s smokers’ helpline online and StopSmokingCenter.net. J Med Internet Res. 2012;14(3):e66. | |
Wangberg SC, Nilsen O, Antypas K, Gram IT. Effect of tailoring in an Internet-based intervention for smoking cessation: randomized controlled trial. J Med Internet Res. 2011;13(4):e121. | |
Fox S. Health Topics. Available at http://www.pewinternet.org/Reports/2013/Health-online.aspx. Washington, DC: Pew Research Center; 2013. Accessed December 18, 2013. Archived by Webcitation.org: http://www.webcitation.org/6LxbeFGGv. | |
Cobb NK, Graham AL. Characterizing Internet searchers of smoking cessation information. J Med Internet Res. 2006;8(3):e17. | |
Eysenbach G, Kohler C. Health-related searches on the Internet. JAMA. 2004;291(24):2946. | |
Siu AL; U.S. Preventive Services Task Force. Behavioral and pharmacotherapy interventions for tobacco smoking cessation in adults, including pregnant women: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;163(8):622–634. | |
Civljak M, Stead LF, Hartmann-Boyce J, Sheikh A, Car J. Internet-based interventions for smoking cessation. Cochrane Database Syst Rev. 2013;7:CD007078. | |
Higgins J, Green S [webpage on the Internet]. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. 2011. Available from: http://handbook.cochrane.org/. Accessed February 20, 2016. (Archived by WebCite® at http://www.webcitation.org/ 6glMBOgSC). | |
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–e34. | |
Bock BC, Graham AL, Whiteley JA, Stoddard JL. A review of web-assisted tobacco interventions (WATIs). J Med Internet Res. 2008;10(5):e39. | |
Barak A, Klein B, Proudfoot JG. Defining Internet-supported therapeutic interventions. Ann Behav Med. 2009;38(1):4–17. | |
Ritterband LM, Thorndike FP, Cox DJ, Kovatchev BP, Gonder-Frederick LA. A behavior change model for Internet interventions. Ann Behav Med. 2009;38(1):18–27. | |
Riley WT, Rivera DE, Atienza AA, Nilsen W, Allison SM, Mermelstein R. Health behavior models in the age of mobile interventions: are our theories up to the task? Transl Behav Med. 2011;1(1):53–71. | |
Thomas J, Brunton J, Graziosi S. EPPI-Reviewer 4.0: Software for Research Synthesis. London: Social Science Research Unit, Institute of Education, University of London; 2010. | |
Alkhaldi G, Hamilton FL, Lau R, Webster R, Michie S, Murray E. The effectiveness of technology-based strategies to promote engagement with digital interventions: a systematic review protocol. JMIR Res Protoc. 2015;4(2):e47. | |
Eysenbach G, Group CE. CONSORT-EHEALTH: improving and standardizing evaluation reports of web-based and mobile health interventions. J Med Internet Res. 2011;13(4):e126. | |
Muñoz RF, Lenert LL, Delucchi K, et al. Toward evidence-based Internet interventions: a Spanish/English web site for international smoking cessation trials. Nicotine Tob Res. 2006;8(1):77–87. | |
Fraser D, Kobinsky K, Smith SS, Kramer J, Theobald WE, Baker TB. Five population-based interventions for smoking cessation: a MOST trial. Transl Behav Med. 2014;4(4):382–390. | |
Smit ES, Vries H, Hoving C. Effectiveness of a web-based multiple tailored smoking cessation program: a randomized controlled trial among Dutch adult smokers. J Med Internet Res. 2012;14(3):e82. | |
An LC, Demers MR, Kirch MA, et al. A randomized trial of an avatar-hosted multiple behavior change intervention for young adult smokers. J Natl Cancer Inst Monogr. 2013;2013(47):209–215. | |
Strecher VJ, McClure JB, Alexander GL, et al. Web-based smoking-cessation programs: results of a randomized trial. Am J Prev Med. 2008;34(5):373–381. | |
Mathieu E, McGeechan K, Barratt A, Herbert R. Internet-based randomized controlled trials: a systematic review. J Am Med Inform Assoc. 2013;20(3):568–576. | |
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. | |
Emmons KM, Puleo E, Sprunck-Harrild K, et al. Partnership for health-2, a web-based versus print smoking cessation intervention for childhood and young adult cancer survivors: randomized comparative effectiveness study. J Med Internet Res. 2013;15(11):e218. | |
Haug S, Meyer C, John U. Efficacy of an Internet program for smoking cessation during and after inpatient rehabilitation treatment: a quasi-randomized controlled trial. Addict Behav. 2011;36(12):1369–1372. | |
Shuter J, Morales DA, Considine-Dunn SE, An LC, Stanton CA. Feasibility and preliminary efficacy of a web-based smoking cessation intervention for HIV-infected smokers: a randomized controlled trial. J Acquir Immune Defic Syndr. 2014;67(1):59–66. | |
Swan GE, McClure JB, Jack LM, et al. Behavioral counseling and varenicline treatment for smoking cessation. Am J Prev Med. 2010;38(5):482–490. | |
Mehring M, Haag M, Linde K, Wagenpfeil S, Schneider A. Effects of a guided web-based smoking cessation program with telephone counseling: a cluster randomized controlled trial. J Med Internet Res. 2014;16(9):e218. | |
Murray E, Khadjesari Z, White IR, et al. Methodological challenges in online trials. J Med Internet Res. 2009;11(2):e9. | |
Bricker J, Wyszynski C, Comstock B, Heffner JL. Pilot randomized controlled trial of web-based acceptance and commitment therapy for smoking cessation. Nicotine Tob Res. 2013;15(10):1756–1764. | |
Humfleet GL, Hall SM, Delucchi KL, Dilley JW. A randomized clinical trial of smoking cessation treatments provided in HIV clinical care settings. Nicotine Tob Res. 2013;15(8):1436–1445. | |
Japuntich SJ, Zehner ME, Smith SS, et al. Smoking cessation via the Internet: a randomized clinical trial of an Internet intervention as adjuvant treatment in a smoking cessation intervention. Nicotine Tob Res. 2006;8(suppl 1):S59–S67. | |
Leykin Y, Aguilera A, Torres LD, Perez-Stable EJ, Muñoz RF. Interpreting the outcomes of automated Internet-based randomized trials: example of an International Smoking Cessation Study. J Med Internet Res. 2012;14(1):e5. | |
McClure JB, Peterson D, Derry H, et al. Exploring the “active ingredients” of an online smoking intervention: a randomized factorial trial. Nicotine Tob Res. 2014;16(8):1129–1139. | |
McDonnell DD, Kazinets G, Lee HJ, Moskowitz JM. An Internet-based smoking cessation program for Korean Americans: results from a randomized controlled trial. Nicotine Tob Res. 2011;13(5):336–343. | |
Strecher VJ, Shiffman S, West R. Randomized controlled trial of a web-based computer-tailored smoking cessation program as a supplement to nicotine patch therapy. Addiction. 2005;100(5):682–688. | |
Choi SH, Waltje AH, Ronis DL, et al. Web-enhanced tobacco tactics with telephone support versus 1-800-QUIT-NOW telephone line intervention for operating engineers: randomized controlled trial. J Med Internet Res. 2014;16(11):e255. | |
Dezee KJ, Wink JS, Cowan CM. Internet versus in-person counseling for patients taking varenicline for smoking cessation. Mil Med. 2013; 178(4):401–405. | |
Herbec A, Brown J, Tombor I, Michie S, West R. Pilot randomized controlled trial of an Internet-based smoking cessation intervention for pregnant smokers (‘MumsQuit’). Drug Alcohol Depend. 2014;140:130–136. | |
Pisinger C, Jorgensen MM, Moller NE, Dossing M, Jorgensen T. A cluster randomized trial in general practice with referral to a group-based or an Internet-based smoking cessation programme. J Public Health (Oxf). 2010;32(1):62–70. | |
Berg CJ, Stratton E, Sokol M, Santamaria A, Bryant L, Rodriguez R. Novel incentives and messaging in an online college smoking intervention. Am J Health Behav. 2014;38(5):668–680. | |
Elfeddali I, Bolman C, Candel M, Wiers RW, de Vries H. Preventing smoking relapse via web-based computer-tailored feedback: a randomized controlled trial. J Med Internet Res. 2012;14(4):87–102. | |
Etter JF. Comparing the efficacy of two Internet-based, computer-tailored smoking cessation programs: a randomized trial. J Med Internet Res. 2005;7(1):e2. | |
Mañanes G, Vallejo MA. Usage and effectiveness of a fully automated, open-access, Spanish web-based smoking cessation program: randomized controlled trial. J Med Internet Res. 2014;16(4):e111. | |
Oenema A, Brug J, Dijkstra A, de Weerdt I, de Vries H. Efficacy and use of an Internet-delivered computer-tailored lifestyle intervention, targeting saturated fat intake, physical activity and smoking cessation: a randomized controlled trial. Ann Behav Med. 2008;35(2):125–135. | |
Graham AL, Cobb NK, Papandonatos GD, et al. A randomized trial of Internet and telephone treatment for smoking cessation. Arch Intern Med. 2011;171(1):46–53. | |
McKay HG, Danaher BG, Seeley JR, Lichtenstein E, Gau JM. Comparing two web-based smoking cessation programs: randomized controlled trial. J Med Internet Res. 2008;10(5):e40. | |
Muñoz RF, Barrera AZ, Delucchi K, Penilla C, Torres LD, Perez-Stable EJ. International Spanish/English Internet smoking cessation trial yields 20% abstinence rates at 1 year. Nicotine Tob Res. 2009; 11(9):1025–1034. | |
Brendryen H, Drozd F, Kraft P. A digital smoking cessation program delivered through Internet and cell phone without nicotine replacement (happy ending): randomized controlled trial. J Med Internet Res. 2008;10(5):e51. | |
Brendryen H, Kraft P. Happy ending: a randomized controlled trial of a digital multi-media smoking cessation intervention. Addiction. 2008;103(3):478–484. | |
Rabius V, Pike KJ, Wiatrek D, McAlister AL. Comparing Internet assistance for smoking cessation: 13-month follow-up of a six-arm randomized controlled trial. J Med Internet Res. 2008;10(5):e45. | |
Mason D, Gilbert H, Sutton S. Effectiveness of web-based tailored smoking cessation advice reports (iQuit): a randomized trial. Addiction. 2012;107(12):2183–2190. | |
Swartz LH, Noell JW, Schroeder SW, Ary DV. A randomised control study of a fully automated Internet based smoking cessation programme. Tob Control. 2006;15(1):7–12. | |
Stanczyk NE, Smit ES, Schulz DN, et al. An economic evaluation of a video- and text-based computer-tailored intervention for smoking cessation: a cost-effectiveness and cost-utility analysis of a randomized controlled trial. PLoS One. 2014;9(10):e110117. | |
Borland R, Balmford J, Benda P. Population-level effects of automated smoking cessation help programs: a randomized controlled trial. Addiction. 2013;108(3):618–628. | |
Simmons VN, Heckman BW, Fink AC, Small BJ, Brandon TH. Efficacy of an experiential, dissonance-based smoking intervention for college students delivered via the Internet. J Consult Clin Psychol. 2013;81(5):810–820. | |
Stoddard JL, Augustson EM, Moser RP. Effect of adding a virtual community (bulletin board) to smokefree.gov: randomized controlled trial. J Med Internet Res. 2008;10(5):e53. | |
An LC, Klatt C, Perry CL, et al. The RealU online cessation intervention for college smokers: a randomized controlled trial. Prev Med. 2008;47(2):194–199. | |
Donkin L, Christensen H, Naismith SL, Neal B, Hickie IB, Glozier N. A systematic review of the impact of adherence on the effectiveness of e-therapies. J Med Internet Res. 2011;13(3):e52. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
