Back to Journals » Drug Design, Development and Therapy » Volume 13
Synthesis, structure elucidation, and antifungal potential of certain new benzodioxole–imidazole molecular hybrids bearing ester functionalities
Authors Al-Wabli RI , Al-Ghamdi AR, Ghabbour HA, Al-Agamy MH , Attia MI
Received 21 December 2018
Accepted for publication 29 January 2019
Published 26 February 2019 Volume 2019:13 Pages 775—789
DOI https://doi.org/10.2147/DDDT.S199135
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Reem I Al-Wabli,1 Alwah R Al-Ghamdi,1 Hazem A Ghabbour,2 Mohamed H Al-Agamy,3,4 Mohamed I Attia1,5
1Department of Pharmaceutical Chemistry, College of Pharmacy, King Saud University, Riyadh 11451, Saudi Arabia; 2Department of Medicinal Chemistry, Faculty of Pharmacy, Mansoura University, Mansoura 35516, Egypt; 3Department of Pharmaceutics, College of Pharmacy, King Saud University, Riyadh 11451, Saudi Arabia; 4Microbiology and Immunology Department, Faculty of Pharmacy, Al-Azhar University, Cairo 11884, Egypt; 5Medicinal and Pharmaceutical Chemistry Department, Pharmaceutical and Drug Industries Research Division, National Research Centre, Dokki, Giza 12622, Egypt
Background: The incidence of fungal infections is a growing serious global health burden. There is an urgent medical demand to acquire new antifungal drug-like compounds having azole nuclei to get rid of the drawbacks of the currently available azole antifungal agents.
Methods: The target compounds 5a-r were synthesized in a four-step reaction sequence using the appropriate acetophenone derivative as a starting material. The antifungal potential of the title compounds was assessed using DIZ and MIC assays according to the reported standard procedures.
Results: The newly synthesized oximino esters 5a-r were identified with the aid of various spectroscopic approaches. Their assigned chemical structures were confirmed via single-crystal X-ray structure of compound 5o. The molecular structure of compound 5o was crystallized in the triclinic, P-1, a=9.898 (3) Å, b=10.433 (3) Å, c=11.677 (4) Å, α=86.886 (6)°, β=87.071 (7)°, γ=64.385 (6)°, V=1,085.2 (6) Å3, Z=2. The synthesized compounds 5a-r were in vitro evaluated for antifungal potential against four fungal strains. Compounds 5l and 5m bearing a trifluoromethylphenyl moiety showed the best anti-Candida albicans activity with minimum inhibitory concentration (MIC) value of 0.148 µmol/mL, while compound 5b displayed the best activity toward Candida tropicalis with MIC value of 0.289 µmol/mL. Compounds 5o and 5l were the most active congeners against Candida parapsilosis and Aspergillus niger, respectively.
Conclusion: Single-crystal X-ray analysis of compound 5o confirmed without doubt the assigned chemical structures of the title compounds as well as confirmed the (E)-configuration of their oximino group. Compounds 5b, 5l, 5m, and 5o emerged as the most active compounds against the tested fungi and they could be considered as new antifungal lead candidates.
Keywords: crystal structure, imidazole, benzodioxole, ester, antifungal
Introduction
The incidence of fungal infections is a growing serious health burden worldwide, especially, in industrialized countries. Immunocompromised individuals are particularly more prone to life-threatening fungal infections caused by opportunistic fungi. In addition, the use of anticancer drugs and immunosupressant agents increases the incidence rate of serious fungal infections. The emergence of drug resistance and toxicity of the currently available antifungal agents (flucytosine, amphotericin B, and ketoconazole) have reinforced the demand for the development of new antifungal candidates with improved potency and safety profiles.1,2
Azoles are successfully used for the treatment of serious fungal infections owing to their favorable pharmacokinetic parameters, high therapeutic index, and wide antifungal spectrum.3 They inhibit the activity of lanosterol 14α-demethylase (CYP51) leading to prevention of ergosterol biosynthesis and hence the inability of fungi to grow normally.4 Imidazole and triazole heterocylic rings constitute essential pharmacophore fragments of the clinically used antifungal azoles. Fluconazole and itraconazole are potent triazole-bearing antifungal drugs; however, the former drug is not effective against invasive aspergillosis and the latter one suffers from poor aqueous solubility and oral bioavailability.5,6 Therefore, there is an urgent medical need to develop new antifungal drug-like candidates bearing azole nuclei to overcome the drawbacks of the currently available azole antifungal agents.
Most of the available azole antifungal agents bear an ethyl spacer which separates the azole moiety from an aromatic pharmacophore fragment. However, a propyl spacer separating the azole pharmacophore from the aromatic part occurred in a limited number of antifungal candidates.7–9 Accordingly, it was of our interest to report the synthesis of certain imidazole-based surrogates bearing a propyl spacer to be evaluated as new antifungal candidates. In addition, the title compounds 5a-r bear 1,3-benzodioxole aromatic fragments which might augment their antifungal potential.10,11 The assigned chemical structures of the target compounds 5a-r were thoroughly characterized using different spectroscopic techniques. Moreover, the configuration of the imine functionality of the title compounds 5a-r were examined with the aid of single crystal X-ray analysis of compound 5o as a representative example of the title compounds 5a-r.
Materials and methods
General
The uncorrected melting points of the synthesized compounds were measured using a Gallenkamp melting point device. A Perkin Elmer FT-IR Spectrum BX device was used to record the infrared (IR) spectra (as KBr disks). The nuclear magnetic resonance (NMR) spectra of the synthesized compounds were measured after dissolving the test samples in DMSO-d6 and the measurements were achieved at 500 MHz for 1H and 125.76 MHz for 13C on Bruker NMR spectrometer at the Research Center, College of Pharmacy, King Saud University, Saudi Arabia. Chemical shifts are articulated in δ-values (ppm) compared to tetramethylsilane as an internal standard. Elemental analyses of the target compounds were executed at the Microanalysis Laboratory, Cairo University, Cairo, Egypt, and the results agreed favorably with the proposed structure within ±0.4% of the theoretical values (Table S1). Agilent Quadrupole 6120 LC/MS was utilized to record the mass spectra of the synthesized compounds with the aid of electrospray ionization (ESI) source. Silica gel thin layer chromatography plates with fluorescent indicator at 254 nm were acquired from Merck and visualization was accomplished by illumination with a UV light source (254 nm).
Chemistry
Synthesis of 1-(2H-1,3-benzodioxol-5-yl)-3-(1H-imidazol-1-yl)propan-1-one (3)
Compound 3 was prepared according to the previously reported procedure and its spectral data are consistent with reported ones.12
Synthesis of (1E)-1-(2H-1,3-benzodioxol-5-yl)-N-hydroxy-3-(1H-imidazol-1-yl) propan-1-imine (4)
Oxime 4 was synthesized from ketone 3 through adopting the reported method and its spectral data are consistent with the previously reported ones.13
General procedure for the synthesis of the target oximino esters 5a-r
Method A
N,N′-Carbonyldiimidazole (0.32 g, 2.0 mmol) was added to a stirred solution of the appropriate carboxylic acid (2.0 mmol) in tetrahydrofuran (THF) (10 mL). Oxime 4 (0.5 g, 2.0 mmol) was added to the stirred reaction mixture and stirring was continued for further 18 hours at room temperature. THF was evaporated under vacuum and ethyl acetate (30 mL) was added to the residue and the organic phase was washed successively with water (2×20 mL), 10% NaHCO3 solution (2×15 mL), and water (2×15 mL). The organic layer was separated, dried (Na2SO4), and evaporated under reduced pressure. The corresponding oximino esters 5a-d, 5f, 5g, 5i, 5l, 5m, and 5p-r were purified either by recrystallization from ethanol (for solids) or column chromatography (for oils).
Method B
4-Dimethylaminopyridine (400 mg) was added to a stirred solution of the appropriate carboxylic acid (7 mmol) and N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDCI·HCl, 1.39 g, 7.3 mmol) in dichloromethane (75 mL). Oxime 4 (1.79 g, 6.9 mmol) was added to the stirred reaction mixture and stirring was continued for further 18 hours at room temperature. The reaction mixture was washed successively with water (2×20 mL), 10% NaHCO3 solution (2×15 mL), and water (2×15 mL). The organic layer was separated, dried (Na2SO4), and evaporated under reduced pressure. The respective crude oximino esters 5e, 5h, 5j, 5k, 5n, and 5o were purified by recrystallization from ethanol.
({[(1E)–1-(1,3-Benzodioxol-5-yl)–3-(1H-imidazol-1yl)propylidene]amino}oxy)(phenyl)methanone (5a). Yield 0.28 g (28%); white powder, mp 130°C–132°C; IR (KBr): ν (cm−1) 3,099, 2,887, 1,739 (C=O), 1,600 (C=N), 1,504, 1,452, 1,232, 744; 1H-NMR (CDCl3): δ (ppm) 3.44 (t, J = 7.0 Hz, 2H, −CH2−CH2−N), 4.36 (t, J = 7.0 Hz, 2H, −CH2−CH2−N), 6.05 (s, 2H, −O−CH2−O−), 6.84 (d, J = 8.0 Hz, 1H, Ar-H), 6.95 (s, 1H, −N−CH=CH−N=), 7.07 (s, 1H, −N−CH=CH−N=), 7.14 (d, J = 7.0 Hz, 1H, Ar-H), 7.32 (s, 1H, Ar-H), 7.52–7.55 (m, 2H, Ar-H), 7.64–7.66 (m, 1H, Ar-H), 7.83 (s, 1H, −N−CH=N−), 8.05 (d, J = 7.5 Hz, 2H, Ar-H); 13C-NMR (CDCl3): δ (ppm) 30.7 (−CH2−CH2−N), 44.2 (−CH2−CH2−N), 101.8 (−O−CH2−O−), 107.2, 108.5 (Ar-CH), 119.1 (−N−CH=CH−N=), 122.1, 126.8, 128.7, 128.8, 129.5, 133.7 (Ar-CH, Ar–C, −N−CH=CH−N=), 136.8 (−N−CH=N−), 148.5, 150.5 (Ar-C), 162.6, 163.4 (C=N, C=O); MS m/z (ESI): 364.1 [M + H]+.
({[(1E)–1-(1,3-Benzodioxol-5-yl)–3-(1H-imidazol-1yl)propylidene]amino}oxy)(4-bromophenyl)methanone (5b). Yield 0.34 g (34%); pale yellow powder, mp 92°C–94°C; IR (KBr): ν (cm−1) 3,107, 2,914, 1,726 (C=O), 1,589 (C=N), 1,504, 1,442, 1,265, 748; 1H-NMR (CDCl3): δ (ppm) 3.43 (t, J = 7.0 Hz, 2H, −CH2−CH2−N), 4.34 (t, J = 7.0 Hz, 2H, −CH2−CH2−N), 6.06 (s, 2H, −O−CH2−O−), 6.85 (d, J = 8.0 Hz, 1H, Ar-H), 6.93 (s, 1H, −N−CH=CH−N=), 7.05 (s, 1H, −N−CH=CH−N=), 7.14 (d, J = 8.0 Hz, 1H, Ar-H), 7.31 (d, J = 1.0 Hz, 1H, Ar-H), 7.67 (d, J = 8.5 Hz, 2H, Ar-H), 7.82 (s, 1H, −N−CH=N−), 7.87 (d, J = 8.5 Hz, 2H, Ar-H); 13C-NMR (CDCl3): δ (ppm) 30.6 (−CH2−CH2−N), 44.2 (−CH2−CH2−N), 101.9 (−O−CH2−O−), 107.2, 108.5 (Ar-CH), 119.1 (−N−CH=CH−N=), 122.1, 126.5, 127.5, 128.7, 128.9, 131.0, 132.2 (Ar-CH, Ar–C, −N−CH=CH−N=), 136.8 (−N−CH=N−), 148.6, 150.6 (Ar-C), 162.8, 162.9 (C=N, C=O); MS m/z (ESI): 442.0 [M + H]+, 444.0 [(M + 2)+ H]+, 445.0 [(M + 3)+ H]+.
({[(1E)–1-(1,3-Benzodioxol-5-yl)–3-(1H-imidazol-1yl)propylidene]amino}oxy)(2-chlorophenyl)methanone (5c). Yield 0.53 g (53%); pale yellow viscous oil; IR (KBr): ν (cm−1) 3,016, 2,937, 1,739 (C=O), 1,604 (C=N), 1,510, 1,446, 1,253, 754; 1H-NMR (CDCl3): δ (ppm) 3.35 (t, J = 6.6 Hz, 2H, −CH2−CH2−N), 4.23 (t, J = 6.6 Hz, 2H, −CH2−CH2−N), 6.07 (s, 2H, −O−CH2−O−), 6.81 (d, J = 7.8 Hz, 1H, Ar-H), 6.86 (s, 1H, −N−CH=CH−N=), 6.98 (s, 1H, −N−CH=CH−N=), 7.09 (d, J = 7.8 Hz, 1H, Ar-H), 7.26 (s, 1H, Ar-H), 7.40 (s, 1H, Ar-H), 7.42 (s, 1H, −N−CH=N−), 7.48–7.50 (m, 2H, Ar-H), 7.79 (d, J = 7.2 Hz, 1H, Ar-H); 13C-NMR (CDCl3): δ (ppm) 31.0 (−CH2−CH2−N), 43.9 (−CH2−CH2−N), 101.8 (−O−CH2−O−), 107.2, 108.5 (Ar-CH), 118.7 (−N−CH=CH−N=), 122.1, 126.5, 127.0, 129.4, 129.8, 130.9, 131.7, 132.9, 133.1 (Ar-CH, Ar–C, −N−CH=CH−N=), 136.8 (−N−CH=N−), 148.5, 150.5 (Ar-C), 162.8, 163.3 (C=N, C=O); MS m/z (ESI): 398.1 [M + H]+, 399.1 [(M + 1)+ H]+, 400.1 [(M + 2)+ H]+.
({[(1E)–1-(1,3-Benzodioxol-5-yl)–3-(1H-imidazol-1yl)propylidene]amino}oxy)(3-chlorophenyl)methanone (5d). Yield 0.39 g (39%); white powder, mp 118°C–121°C; IR (KBr): ν (cm−1) 3,064, 2,922, 1,735 (C=O), 16,24 (C=N), 1,575, 1,504, 1,232, 736; 1H-NMR (CDCl3): δ (ppm) = 3.40 (t, J = 7.0 Hz, 2H, −CH2−CH2−N), 4.30 (t, J = 7.0 Hz, 2H, −CH2−CH2−N), 6.06 (s, 2H, −O−CH2−O−), 6.85 (d, J = 8.0 Hz, 1H, Ar-H), 6.94 (s, 1H, −N−CH=CH−N=), 7.05 (s, 1H, −N−CH=CH−N=), 7.13 (dd, J = 1.5, 8.0 Hz, 1H, Ar-H), 7.29–7.30 (m, 1H, Ar-H), 7.46–7.49 (m, 2H, −N−CH=N−, Ar-H), 7.63 (dd, J = 0.9, 7.9 Hz, 1H, Ar-H), 7.91 (d, J = 7.7 Hz, 1H, Ar-H), 8.01 (s, 1H, Ar-H); 13C-NMR (CDCl3): δ (ppm) 30.7 (−CH2−CH2−N), 43.9 (−CH2−CH2−N), 101.9 (−O−CH2−O−), 107.2, 108.5 (Ar-CH), 118.8 (−N−CH=CH−N=), 122.1, 126.5, 127.7, 129.6, 130.0, 130.1, 130.4, 133.7, 134.9 (Ar-CH, Ar–C, −N−CH=CH−N=), 136.9 (−N−CH=N−), 148.6, 150.6 (Ar-C), 162.3, 163.1 (C=N, C=O); MS m/z (ESI): 398.1 [M + H]+, 399.1 [(M + 1)+ H]+, 400.1 [(M + 2)+ H]+.
({[(1E)–1-(1,3-Benzodioxol-5-yl)–3-(1H-imidazol-1yl)propylidene]amino}oxy)(4-chlorophenyl)methanone (5e). Yield 0.37 g (37%); white powder, mp 103°C–105°C; IR (KBr): ν (cm−1) 3,111, 2,912, 1,730 (C=O), 1,589 (C=N), 1,504, 1,444, 1,255, 750; 1H-NMR (CDCl3): δ (ppm) 3.43 (t, J = 6.5 Hz, 2H, −CH2−CH2−N), 4.34 (t, J = 6.5 Hz, 2H, −CH2−CH2−N), 6.05 (s, 2H, −O−CH2−O−), 6.84 (d, J = 8.5 Hz, 1H, Ar-H), 6.93 (s, 1H, −N−CH=CH−N=), 7.05 (s, 1H, −N−CH=CH−N=), 7.14 (dd, J = 1.5, 8.5 Hz, 1H, Ar-H), 7.29 (s, 1H, Ar-H), 7.48 (d, J = 8.5 Hz, 2H, Ar-H), 7.83 (s, 1H, −N−CH=N−), 7.94 (d, J = 8.5 Hz, 2H, Ar-H); 13C-NMR (CDCl3): δ (ppm) 30.6 (−CH2−CH2−N), 44.2 (−CH2−CH2−N), 101.9 (−O−CH2−O−), 107.2, 108.5 (Ar-CH), 119.1 (−N−CH=CH−N=), 122.1, 126.5, 127.0, 128.6, 129.2, 130.9 (Ar-CH, Ar–C, −N−CH=CH−N=), 136.8 (−N−CH=N−), 140.2, 148.5, 150.6 (Ar-C), 162.6, 162.8 (C=N, C=O); MS m/z (ESI): 398.1 [M + H]+, 399.1 [(M + 1)+ H]+, 400.1 [(M + 2)+ H]+.
({[(1E)–1-(1,3-Benzodioxol-5-yl)–3-(1H-imidazol-1-yl)propylidene]amino}oxy)(3-fluorophenyl)methanone (5f). Yield 0.4 g (40%); white powder, mp 135°C–137°C; IR (KBr): ν (cm−1) 3,007, 1,741 (C=O), 1,703 (C=N), 1,589, 1,506, 1,267, 746; 1H-NMR (DMSO-d6): δ (ppm) 3.47 (t, J = 6.0 Hz, 2H, −CH2−CH2−N), 4.27 (t, J = 6.1 Hz, 2H, −CH2−CH2−N), 6.14 (s, 2H, −O−CH2−O−), 6.78 (s, 1H, −N−CH=CH−N=), 7.04 (d, J = 8.6 Hz, 1H, Ar-H), 7.16 (s, 1H, −N−CH=CH−N=), 7.32 (s, 2H, Ar-H), 7.55 (s, 1H, −N−CH=N−), 7.60–7.67 (m, 2H, Ar-H), 7.77 (d, J = 8.8 Hz, 1H, Ar-H), 7.88 (d, J = 7.4 Hz, 1H, Ar-H); 13C-NMR (DMSO-d6): δ (ppm) 30.4 (−CH2−CH2−N), 43.7 (−CH2−CH2−N), 102.3 (−O−CH2−O−), 107.4, 108.9 (Ar-CH), 116.4, 119.8, 121.2, 121.3, 122.9, 126.1, 126.2, 127.1, 128.9 (Ar-CH, Ar–C, −N−CH=CH−N=, −N−CH=CH−N=), 137.6 (−N−CH=N−), 148.3, 150.3, 162.3, 163.5, 164.7 (Ar-C, C=N, C=O); MS m/z (ESI): 382.1 [M + H]+, 383.1 [(M + 1)+ H]+.
({[(1E)–1-(1,3-Benzodioxol-5-yl)–3-(1H-imidazol-1-yl)propylidene]amino}oxy)(4-fluorophenyl)methanone (5g). Yield 0.35 g (35%); white powder, mp 75°C–77°C; IR (KBr): ν (cm−1) 3,107, 2,920, 1,739 (C=O), 1,602 (C=N), 1,583, 1,506, 1,246, 756; 1H-NMR (CDCl3): δ (ppm) 3.40 (t, J = 6.9 Hz, 2H, −CH2−CH2−N), 4.30 (t, J = 6.9 Hz, 2H, −CH2−CH2−N), 6.05 (s, 2H, −O−CH2−O−), 6.85 (d, J = 8.1 Hz, 1H, Ar-H), 6.92 (s, 1H, −N−CH=CH−N=), 7.04 (s, 1H, −N−CH=CH−N=), 7.14 (dd, J = 1.5, 8.1 Hz, 1H, Ar-H), 7.18–7.22 (m, 2H, Ar-H), 7.28–7.29 (m, 1H, Ar-H), 7.57 (s, 1H, −N−CH=N−), 8.03–8.06 (m, 2H, Ar-H); 13C-NMR (CDCl3): δ (ppm) 30.7 (−CH2−CH2−N), 43.9 (−CH2−CH2−N), 101.9 (−O−CH2−O−), 107.2, 108.5 (Ar-CH), 116.1 (d, J C-3′, F& C-5′, F = 22.1 Hz, C-3′ and C-5′), 118.9 (−N−CH=CH−N=), 122.1, 124.9, 126.7, 129.7 (Ar-CH, Ar–C, −N−CH=CH−N=), 132.2 (d, J C-2′, F& C-6′, F = 9.4 Hz, C-2′and C-6′), 136.9 (−N−CH=N−), 148.5, 150.5, 162.6, 162.8, (Ar-C, C=N, C=O), 166.1 (d, J C-4′, F = 255.8 Hz, C-4′); MS m/z (ESI): 382.1 [M + H]+, 383.1 [(M + 1)+ H]+.
({[(1E)–1-(1,3-Benzodioxol-5-yl)–3-(1H-imidazol-1-yl)propylidene]amino}oxy)(4-methoxyphenyl)methanone (5h). Yield 0.63 g (63%); white powder, mp 147°C–149°C; IR (KBr): ν (cm−1) 3,015, 2,966, 1,722 (C=O), 1,604 (C=N), 1,506, 1,440, 1,259, 740; 1H-NMR (CDCl3): δ (ppm) 3.41 (t, J = 6.5 Hz, 2H, −CH2−CH2−N), 3.90 (s, 3H, −OCH3), 4.34 (t, J = 6.5 Hz, 2H, −CH2−CH2−N), 6.03 (s, 2H, −O−CH2−O−), 6.82 (d, J = 8.0 Hz, 1H, Ar-H), 6.95 (s, 1H, −N−CH=CH−N=), 6.98 (d, J = 8.5 Hz, 2H, Ar-H), 7.05 (s, 1H, −N−CH=CH−N=), 7.12 (dd, J = 1.5, 8.0 Hz, 1H, Ar-H), 7.30 (s, 1H, Ar-H), 7.79 (s, 1H, −N−CH=N−), 7.97 (d, J = 9.0 Hz, 2H, Ar-H.); 13C-NMR (CDCl3): δ (ppm) 30.7 (−CH2−CH2−N), 44.2 (−CH2−CH2−N), 55.6 (−OCH3), 101.8 (−O−CH2−O−), 107.1, 108.4, 114.1 (Ar-CH), 119.1 (−N−CH=CH−N=), 120.7, 122.0, 126.9, 128.7, 131.6 (Ar-CH, Ar–C, −N−CH=CH−N=), 136.8 (−N−CH=N−), 148.6, 150.4, 162.2 (Ar-C), 163.2, 163.9 (C=N, C=O); MS m/z (ESI): 394.1 [M + H]+, 416.1 [M + 23]+.
({[(1E)–1-(1,3-Benzodioxol-5-yl)–3-(1H-imidazol-1-yl)propylidene]amino}oxy)(2-methylphenyl)methanone (5i). Yield 0.4 g (40%); pale yellow viscous oil; IR (KBr): ν (cm−1) 3,014, 2,966, 1,747 (C=O), 1,600 (C=N), 1,506, 1,444, 12,32, 710; 1H-NMR (CDCl3): δ (ppm) 2.64 (s, 3H, CH3), 3.35 (t, J = 6.6 Hz, 2H, −CH2−CH2−N), 4.24 (t, J = 6.6 Hz, 2H, −CH2−CH2−N), 6.02 (s, 2H, −O−CH2−O−), 6.82 (d, J = 8.4 Hz, 1H, Ar-H), 6.87 (s, 1H, −N−CH=CH−N=), 7.05 (s, 1H, −N−CH=CH−N=), 7.11 (d, J = 7.8 Hz, 1H, Ar-H), 7.22–7.26 (m, 1H, Ar-H.), 7.30–7.32 (m, 2H, Ar-H.), 7.46 (t, J = 7.2 Hz, 1H, Ar-H.), 7.56 (s, 1H, −N−CH=N−), 7.77 (d, J = 7.8 Hz, 1H, Ar-H); 13C-NMR (CDCl3): δ (ppm) 21.8 (CH3), 30.7 (−CH2−CH2−N), 44.0 (−CH2−CH2−N), 101.8 (−O−CH2−O−), 107.1, 108.4 (Ar-CH), 118.8 (−N−CH= CH−N=), 122.0, 125.9, 126.8, 128.0, 129.2, 129.7, 130.8, 132.0 (Ar-CH, Ar–C, −N−CH=CH−N=), 136.8 (−N−CH=N−), 140.8, 148.5, 150.4 (Ar-C), 162.1, 164.2 (C=N, C=O); MS m/z (ESI): 378.1 [M + H]+.
({[(1E)–1-(1,3-Benzodioxol-5-yl)–3-(1H-imidazol-1-yl)propylidene]amino}oxy)(3-methylphenyl)methanone (5j). Yield 0.36 g (36%); white powder, mp 103°C–105°C; IR (KBr): ν (cm−1) 3,107, 2,978, 1,743 (C=O), 1,610 (C=N), 1,575, 1,502, 1,269, 720; 1H-NMR (CDCl3): δ (ppm) 2.45 (s, 3H, CH3), 3.42 (t, J = 7.0 Hz, 2H, −CH2−CH2−N), 4.34 (t, J = 7.0 Hz, 2H, −CH2−CH2−N), 6.03 (s, 2H, −O−CH2−O−), 6.83 (d, J = 8.0 Hz, 1H, Ar-H), 6.96 (s, 1H, −N−CH=CH−N=), 7.05 (s, 1H, −N−CH=CH−N=), 7.14 (d, J = 8.0 Hz, 1H, Ar-H), 7.30 (d, J = 1.5 Hz, 1H, Ar-H), 7.40–7.44 (m, 2H, Ar-H.), 7.74 (s, 1H, −N−CH=N−), 7.81 (d, J = 7.0 Hz, 1H, Ar-H), 7.85 (s, 1H, Ar-H); 13C-NMR (CDCl3): δ (ppm) 21.4 (CH3), 30.7 (−CH2−CH2−N), 44.2 (−CH2−CH2−N), 101.8 (−O−CH2−O−), 107.2, 108.5 (Ar-CH), 119.1 (−N−CH=CH−N=), 122.1, 126.6, 126.8, 128.6, 128.7, 128.8, 130.1, 134.5 (Ar-CH, Ar–C, −N−CH=CH−N=), 136.8 (−N−CH=N−), 138.7, 148.5, 150.5 (Ar-C), 162.6, 163.6 (C=N, C=O); MS m/z (ESI): 378.1 [M + H]+.
({[(1E)–1-(1,3-Benzodioxol-5-yl)–3-(1H-imidazol-1-yl)propylidene]amino}oxy)(4-methylphenyl)methanone (5k). Yield 0.3 g (30%); pale yellow powder, mp 73°C–76°C; IR (KBr): ν (cm−1) 3,107, 2,920, 1,722 (C=O), 1,612 (C=N), 1,581, 1,504, 1,265, 742; 1H-NMR (CDCl3): δ (ppm) 2.41 (s, 3H, CH3), 3.41 (t, J = 6.5 Hz, 2H, −CH2−CH2−N), 4.33 (t, J = 7.0 Hz, 2H, −CH2−CH2−N), 6.04 (s, 2H, −O−CH2−O−), 6.84 (d, J = 8.0 Hz, 1H, Ar-H), 6.95 (s, 1H, −N−CH=CH−N=), 7.05 (s, 1H, −N−CH=CH−N=), 7.13 (d, J = 7.5 Hz, 1H, Ar-H), 7.30 (s, 1H, Ar-H), 7.32 (d, J = 8.0 Hz, 2H, Ar-H.), 7.67 (s, 1H, −N−CH=N−), 7.93 (d, J = 8.0 Hz, 2H, Ar-H); 13C-NMR (CDCl3): δ (ppm) 21.8 (CH3), 30.8 (−CH2−CH2−N), 44.1 (−CH2−CH2−N), 101.7 (−O−CH2−O−), 107.2, 108.5 (Ar-CH), 118.9 (−N−CH=CH−N=), 122.0, 125.8 (Ar-CH), 126.9, 129.2, 129.5, 129.6 (Ar–C, −N−CH=CH−N=), 136.9 (−N−CH=N−), 144.6, 148.5, 150.4 (Ar-C), 162.4, 163.5 (C=N, C=O); MS m/z (ESI): 378.1 [M + H]+.
({[(1E)–1-(1,3-Benzodioxol-5-yl)–3-(1H-imidazol-1-yl)propylidene]amino}oxy)[(3-(trifluoromethyl)phenyl)]methanone (5l). Yield 0.38 g (38%); white powder, mp 129°C–131°C; IR (KBr): ν (cm−1) 3,089, 1,753 (C=O), 1,612 (C=N), 1,579, 1,500, 1,228, 744; 1H-NMR (DMSO-d6): δ (ppm) 3.48 (t, J = 6.4 Hz, 2H, −CH2−CH2−N), 4.29 (t, J = 6.4 Hz, 2H, −CH2−CH2−N), 6.14 (s, 2H, −O−CH2−O−), 6.77 (s, 1H, −N−CH=CH−N=), 7.04 (d, J = 8.6 Hz, 1H, Ar-H), 7.16 (s, 1H, −N−CH=CH−N=), 7.34–7.36 (m, 2H, Ar-H), 7.54 (s, 1H, −N−CH=N−), 7.84–7.87 (m, 1H, Ar-H), 8.11 (d, J = 7.5 Hz, 1H, Ar-H), 8.24 (s, 1H, Ar-H), 8.31 (d, J = 7.6 Hz, 1H, Ar-H); 13C-NMR (DMSO-d6): δ (ppm) 30.5 (−CH2−CH2−N), 43.8 (−CH2−CH2−N), 102.3 (−O−CH2−O−), 107.4, 108.9 (Ar-CH), 119.8 (−N−CH=CH−N=), 122.9, 126.1, 126.2, 127.0, 128.9, 130.0, 130.7, 130.8, 130.9, 133.8 (Ar-CH, Ar–C, −N−CH=CH−N=), 137.6, 148.4, 150.4 (−N−CH=N−, Ar-C), 162.2, 164.8 (C=N, C=O); MS m/z (ESI): 432.1 [M + H]+, 433.1 [(M + 1)+ H]+.
({[(1E)–1-(1,3-Benzodioxol-5-yl)–3-(1H-imidazol-1-yl)propylidene]amino}oxy)[(4-(trifluoromethyl)phenyl)]methanone (5m). Yield 0.35 g (35%); white powder, mp 136°C–138°C; IR (KBr): ν (cm−1) 3,026, 2,939, 1,741 (C=O), 1,624 (C=N), 1,577, 1,510, 1,251, 742; 1H-NMR (DMSO-d6): δ (ppm) 3.48 (t, J = 6.3 Hz, 2H, −CH2−CH2−N), 4.28 (t, J = 6.4 Hz, 2H, −CH2−CH2−N), 6.14 (s, 2H, −O−CH2−O−), 6.79 (s, 1H, −N−CH=CH−N=), 7.04 (d, J = 8.5 Hz, 1H, Ar-H), 7.16 (s, 1H, −N−CH=CH−N=), 7.32–7.34 (m, 2H, Ar-H), 7.56 (s, 1H, −N−CH=N−), 7.96 (d, J = 8.0 Hz, 2H, Ar-H.), 8.22 (d, J = 7.9 Hz, 2H, Ar-H); 13C-NMR (DMSO-d6): δ (ppm) 30.4 (−CH2−CH2−N), 43.6 (−CH2−CH2−N), 102.3 (−O−CH2−O−), 107.4, 108.9 (Ar-CH), 119.9 (−N−CH=CH−N=), 122.9, 126.3, 126.4, 127.0, 128.9, 130.8, 132.7, 133.5 (Ar-CH, Ar–C, −N−CH=CH−N=), 137.7 (−N−CH=N−), 148.4, 150.3 (Ar-C), 162.3, 164.8 (C=N, C=O); MS m/z (ESI): 432.1 [M + H]+, 433.1 [(M + 1)+ H]+.
({[(1E)–1-(1,3-Benzodioxol-5-yl)–3-(1H-imidazol-1-yl)propylidene]amino}oxy)(3,4-dichlorophenyl)methanone (5n). Yield 0.48 g (48%); white powder, mp 180°C–183°C; IR (KBr): ν (cm−1) 3,088, 2,958, 1,737 (C=O), 1,573 (C=N), 1,506, 1,469, 1,230, 748; 1H-NMR (CDCl3): δ (ppm) 3.38 (t, J = 6.5 Hz, 2H, −CH2−CH2−N), 4.29 (t, J = 7.0 Hz, 2H, −CH2−CH2−N), 6.07 (s, 2H, −O−CH2−O−), 6.86 (d, J = 8.0 Hz, 1H, Ar-H), 6.93 (s, 1H, −N−CH=CH−N=), 7.05 (s, 1H, −N−CH=CH−N=), 7.15 (dd, J = 1.0, 8.0 Hz, 1H, Ar-H), 7.30 (s, 1H, Ar-H), 7.53 (s, 1H, −N−CH=N−), 7.60 (d, J = 8.0 Hz, 1H, Ar-H.), 7.82 (dd, J = 1.5, 8.0 Hz, 1H, Ar-H), 8.08 (d, J = 1.5 Hz, 1H, Ar-H); 13C-NMR (CDCl3): δ (ppm) 30.7 (−CH2−CH2−N), 43.9 (−CH2−CH2−N), 101.9 (−O−CH2−O−), 107.2, 108.6 (Ar-CH), 118.8 (−N−CH=CH−N=), 122.2, 126.3, 128.5, 128.6, 130.0, 131.0, 131.4, 133.4 (Ar-CH, Ar–C, −N−CH=CH−N=), 136.9 (−N−CH=N−), 138.4, 148.6, 150.7 (Ar-C), 161.7, 163.2 (C=N, C=O); MS m/z (ESI): 432.0 [M + H]+, 433.0 [(M + 1)+ H]+, 434.0 [(M + 2)+ H]+.
({[(1E)–1-(1,3-Benzodioxol-5-yl)–3-(1H-imidazol-1-yl)propylidene]amino}oxy)(3,4,5-trimethoxyphenyl)methanone (5o). Yield 0.65 g (65%); white powder, mp 178°C–180°C; IR (KBr): ν (cm−1) 3,109, 2,941, 1,739 (C=O), 1,583 (C=N), 1,502, 1,462, 1,240, 736; 1H-NMR (CDCl3): δ (ppm) 3.40 (t, J = 7.0 Hz, 2H, −CH2−CH2−N), 3.94 (s, 6H, 2 x −OCH3), 3.95 (s, 3H, −OCH3), 4.30 (t, J = 7.0 Hz, 2H, −CH2−CH2−N), 6.05 (s, 2H, −O−CH2−O−), 6.85 (d, J = 8.0 Hz, 1H, Ar-H), 6.93 (s, 1H, −N−CH=CH−N=), 7.04 (s, 1H, −N−CH=CH−N=), 7.12 (dd, J = 1.5, 8.0 Hz, 1H, Ar-H), 7.28–7.29 (m, 3H, Ar-H), 7.51 (s, 1H, −N−CH=N−); 13C-NMR (CDCl3): δ (ppm) 30.8 (−CH2−CH2−N), 43.9 (−CH2−CH2−N), 56.4, 61.0 (−OCH3), 101.9 (−O−CH2−O−), 106.9, 107.2, 108.5 (Ar-CH), 118.7 (−N−CH=CH−N=), 122.1, 123.5, 126.7, 130.0 (Ar-CH, Ar–C, −N−CH=CH−N=), 136.8 (−N−CH=N−), 143.0, 148.5, 150.5, 153.2 (Ar-C), 162.8, 163.2 (C=N, C=O); MS m/z (ESI): 454.2 [M + H]+.
({[(1E)–1-(1,3-Benzodioxol-5-yl)–3-(1H-imidazol-1-yl)propylidene]amino}oxy)(pyridine-4-yl)methanone (5p). Yield 0.44 g (44%); white powder, mp 175°C–177°C; IR (KBr): ν (cm−1) 3,145, 2,914, 1,747 (C=O), 1,589 (C=N), 1,504, 1,442, 1,230, 729; 1H-NMR (CDCl3): δ (ppm) 3.48 (t, J = 6.5 Hz, 2H, −CH2−CH2−N), 4.28 (t, J = 6.5 Hz, 2H, −CH2−CH2−N), 6.14 (s, 2H, −O−CH2−O−), 6.77 (s, 1H, −N−CH=CH−N=), 7.03 (d, J = 8.5 Hz, 1H, Ar-H), 7.16 (s, 1H, −N−CH=CH−N=), 7.32 (s, 1H, Ar-H), 7.33 (s, 1H, Ar-H), 7.55 (s, 1H, −N−CH=N−), 7.91 (dd, J = 1.5, 4.5 Hz, 2H, pyridine-H), 8.86 (dd, J = 1.5, 4.5 Hz, 2H, pyridine-H); 13C-NMR (CDCl3): δ (ppm) 30.4 (−CH2−CH2−N), 43.6 (−CH2−CH2−N), 102.3 (−O−CH2−O−), 107.4, 108.9 (Ar-CH), 119.9 (−N−CH=CH−N=), 123.0, 123.1, 126.9 (Ar-CH), 128.9 (−N−CH=CH−N=), 136.2 (−N−CH=N−), 137.7, 148.3, 150.4,151.3 (Ar-C), 162.2, 165.1 (C=N, C=O); MS m/z (ESI): 365.1 [M + H]+.
({[(1E)–1-(1,3-Benzodioxol-5-yl)–3-(1H-imidazol-1-yl)propylidene]amino}oxy)(thiophen-2-yl)methanone (5q). Yield 0.28 g (28%); white powder, mp 149°C–151°C; IR (KBr): ν (cm−1) 3,099, 2,889, 1,734 (C=O), 1,577 (C=N), 1,504, 1,452, 1,246, 729; 1H-NMR (CDCl3): δ (ppm) 3.42 (t, J = 6.5 Hz, 2H, −CH2−CH2−N), 4.27 (t, J = 6.5 Hz, 2H, −CH2−CH2−N), 6.13 (s, 2H, −O−CH2−O−), 6.82 (s, 1H, −N−CH=CH−N=), 7.02 (d, J = 8.5 Hz, 1H, Ar-H), 7.18 (s, 1H, −N−CH=CH−N=), 7.29–7.33 (m, 3H, Ar-H, thiophene-H), 7.58 (s, 1H, −N−CH=N−), 7.97 (dd, J = 1.0, 3.5 Hz, 1H, thiophene-H), 8.08 (dd, J = 1.0, 4.5 Hz, 1H, thiophene-H); 13C-NMR (CDCl3): δ (ppm) 30.5 (−CH2−CH2−N), 43.5 (−CH2−CH2−N), 102.3 (−O−CH2−O−), 107.4, 108.9 (Ar-CH), 119.8 (−N−CH=CH−N=), 122.9, 127.1, 128.9, 129.1 (Ar-CH), 131.3 (−N−CH=CH−N=), 135.1, 135.3, (Ar-C) 137.6, (−N−CH=N−) 148.3, 150.2 (Ar-C), 159.0, 163.9 (C=N, C=O); MS m/z (ESI): 370.1 [M + H]+.
({[(1E)–1-(1,3-Benzodioxol-5-yl)–3-(1H-imidazol-1-yl)propylidene]amino}oxy)(thiophen-3-yl)methanone (5r). Yield 0.43 g (43%); white powder, mp 138°C–140°C; IR (KBr): ν (cm−1) 3,144, 2,887, 1,739 (C=O), 1,577 (C=N), 1,504, 1,450, 1,230, 727; 1H-NMR (CDCl3): δ (ppm) 3.44 (t, J = 6.5 Hz, 2H, −CH2−CH2−N), 4.26 (t, J = 6.5 Hz, 2H, −CH2−CH2−N), 6.13 (s, 2H, −O−CH2−O−), 6.79 (s, 1H, −N−CH=CH−N=), 7.01 (d, J = 8.5 Hz, 1H, Ar-H), 7.16 (s, 1H, −N−CH=CH−N=), 7.28–7.29 (m, 2H, Ar-H), 7.56 (s, 1H, −N−CH=N−), 7.57 (d, J = 5.5 Hz, 1H, thiophene-H), 7.75 (dd, J = 3.0, 5.0 Hz, 1H, thiophene-H), 8.53 (d, J = 1.5 Hz, 1H, thiophene-H); 13C-NMR (CDCl3): δ (ppm) 30.3 (−CH2−CH2−N), 43.6 (−CH2−CH2−N), 102.2 (−O−CH2−O−), 107.4, 108.9 (Ar-CH), 119.9 (−N−CH=CH−N=), 122.8, 127.3, 127.9, 128.4 (Ar-CH), 128.9 (−N−CH=CH−N=), 131.4, 135.1, (Ar-C) 137.7, (−N−CH=N−), 148.3, 150.1 (Ar-C), 159.5, 163.9 (C=N, C=O); MS m/z (ESI): 370.1 [M + H]+.
Crystal structure determination of compound 5o
Compound 5o was obtained as single crystals by slow evaporation from ethanol solution of the pure compound at room temperature. Data were collected on a Bruker APEX-II D8 Venture area diffractometer, equipped with graphite monochromatic Mo Kα radiation, λ=0.71073 Å at 293 (2) K. Cell refinement and data reduction were carried out by Bruker SAINT. SHELXT14 was used to solve the structure. The final refinement was carried out by full-matrix least-squares techniques with anisotropic thermal data for non-hydrogen atoms on F. CCDC 1875757 contains the supplementary crystallographic data for this compound and can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Antifungal activity of the title compounds 5a-r
The in vitro antifungal potential of the oximino esters 5a-r was examined with the aid of diameter of the inhibition zone (DIZ) and minimum inhibitory concentration (MIC) assays according to literature methods.12 The detailed experimental procedures are provided under the “Antifungal activity” section of in the Supplementary materials.
Results and discussion
Chemistry
The adopted synthetic pathway to prepare the title compounds 5a-r is portrayed in Scheme 1. Thus, the reaction sequence was commenced via conducting a Mannich reaction on the commercially available 1,3-benzodioxole derivative 1 to achieve oxime 3 in a three-step reaction sequence according to the reported methods.13 Compound 3 was subjected to esterification with the appropriate carboxylic acid under mild conditions to furnish the target oximino esters 5a-r in acceptable yields.
Crystal structure of compound 5o
The crystallographic data and refinement information of compound 5o, C23H24N4O6, are summarized in Table 1. The selected bond lengths and bond angles for compound 5o are presented in Table 2. The asymmetric unit contains one independent molecule as shown in Figure 1. The 1,3-benzodioxole plane makes dihedral angles 5.69° and 77.56° with trimethoxyphenyl ring and imidazole ring, respectively. All the bond lengths and angles are in normal ranges.15 The molecules are packed together in the crystal structure by three non-classical hydrogen bonds along the b axis as shown in Table 3 and Figure 2.
  | Table 1 The crystallographic data and refinement information of compound 5o |
  | Table 2 Selected geometric parameters (Å, °) of compound 5o |
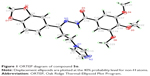  | Figure 1 ORTEP diagram of compound 5o. |
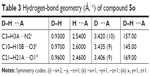  | Table 3 Hydrogen-bond geometry (Å, °) of compound 5o |
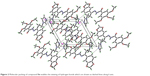  | Figure 2 Molecular packing of compound 5o enables the viewing of hydrogen bonds which are drawn as dashed lines along b axis. |
Antifungal activity of the target oximino esters 5a-r
The antifungal activity of the synthesized oximino esters 5a-r is presented in Table 4. Compounds 5a, 5g, 5j, 5l, and 5m showed the best antifungal activity against the tested Candida albicans strain in the DIZ assay with DIZ value of 15 mm being about 1.2-fold less potent than fluconazole. Whereas, compounds 5a, 5b, 5d-f, and 5k-r were the most active oximino esters against the tested Candida tropicalis strain in the DIZ assay with DIZ values equal to or more than 20 mm being nearly equipotent with fluconazole. Regarding the tested Candida parapsilosis and Aspergillus niger strains, compounds 5a-r displayed moderate activity in the DIZ assay with DIZ values ranging from 11 to 23 mm, being nearly equipotent with fluconazole. Moreover, compounds 5l and 5m (bearing trifluoromethylphenyl moiety) were the most active equipotent congeners in the MIC assay against the tested C. albicans strain with MIC value of 0.148 μmol/mL, being about threefold less potent than fluconazole whereas, compound 5b, bearing the 4-bromophenyl fragment, was the most active oximino ester toward C. tropicalis with MIC value of 0.289 μmol/mL, being about six times less potent than fluconazole, followed by the equipotent candidates 5l and 5m which displayed MIC value of 0.297 μmol/mL. Compound 5o incorporating 3,4,5-trimethoxyphenyl moiety exhibited the best activity against the tested C. parapsilosis with MIC value of 0.141 μmol/mL, being nearly threefold less potent than fluconazole. The oximino ester 5l was the most active anti-A. niger compound with MIC value of 0.297 μmol/mL, being equipotent with compound 5m. Furthermore, compounds 5h, 5i, 5n, and 5q manifested the weakest antifungal activity among the synthesized oximino esters 5a-r toward C. tropicalis, C. parapsilosis, C. albicans, and A. niger, respectively, with MIC values equal to or more than 1.18 μmol/mL. In summary, it seems that substitution with the 3- or 4-trifluoromethyl moiety (compounds 5l and 5m) is favored for the antifungal potential of the prepared oximino esters 5a-r, particularly toward C. albicans and A. niger. On the other hand, the best anti-C. tropicalis and anti-C. parapsilosis activity was achieved with 4-bromophenyl (compound 5b) and 3,4,5-trimethoxyphenyl (compound 5o) moieties, respectively. Regarding the heteroaryl-bearing oximino esters (compounds 5p-r), the thiophene-bearing compound 5r is the best heteroaryl-bearing oximino ester against both C. tropicalis (MIC value =0.347 μmol/mL) and A. niger (MIC value =0.693 μmol/mL), and the pyridine-bearing compound, 5p, is the best heteroaryl-bearing oximino ester against C. parapsilosis (MIC value =0.176 μmol/mL). The weak to moderate antifungal activity of the synthesized compounds 5a-r as compared to the reference antifungal drugs might be attributed to either their poor pharmacokinetic properties or improper interaction with their target fungal protein.
Conclusion
The synthesis and spectroscopic identification of certain new oximino esters 5a-r bearing imidazole and 1,3-benzodioxole fragments have been reported. Single-crystal X-ray analysis of compound 5o confirmed without doubt the assigned chemical structures of the title compounds as well as confirmed the (E)-configuration of their oximino group. The antifungal potentials of the title compounds 5a-r have been examined in vitro against four fungal strains using DIZ and MIC assays. It seems that substitution with the 3- or 4-trifluoromethyl moiety (compounds 5l and 5m) is favored for the antifungal potential of the prepared oximino esters 5a-r, particularly toward C. albicans and A. niger. On the other hand, the best anti-C. tropicalis and anti-C. parapsilosis activity was achieved with 4-bromophenyl (compound 5b) and 3,4,5-trimethoxyphenyl (compound 5o) moieties, respectively. The results of the current investigation could support the development of new antifungal lead candidates.
Supporting materials
The details of the experimental methods which were adopted for the antifungal evaluation of the prepared compounds and representative NMR spectra (Figures S1–S6) of the target compounds are provided as Supplementary materials.
Acknowledgment
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the Research Group Project no RGP-196.
Disclosure
The authors report no conflicts of interest in this work.
References
Terra L, Abreu PA, Teixeira Valãria L, et al. Mycoses and antifungals: reviewing the basis of a current problem that still is a biotechnological target for marine products. Front Mar Sci. 2014;1:12. | ||
Altıntop MD, Atlı Özlem, Ilgın S, Demirel R, Özdemir A, Kaplancıklı ZA. Synthesis and biological evaluation of new naphthalene substituted thiosemicarbazone derivatives as potent antifungal and anticancer agents. Eur J Med Chem. 2016;108:406–414. | ||
Jiang Z, Wang Y, Wang W, et al. Discovery of highly potent triazole antifungal derivatives by heterocycle-benzene bioisosteric replacement. Eur J Med Chem. 2013:64:16–22. | ||
Hof H. A new, broad-spectrum azole antifungal: posaconazole – mechanisms of action and resistance, spectrum of activity. Mycoses. 2006;49(s1):2–6. | ||
Corrêa JCR, Salgado HRN. Review of fluconazole properties and analytical methods for its determination. Crit Rev Anal Chem. 2011;41(2):124–132. | ||
Kale P, Johnson LB. Second-generation azole antifungal agents. Drugs Today. 2005;41(2):91–106. | ||
Aboul-Enein MN, El-Azzouny AAE-S, Attia MI, Saleh OA, Kansoh AL. Synthesis and anti-Candida potential of certain novel 1-[(3-substituted-3-phenyl)propyl]-1H-imidazoles. Arch Pharm. 2011;344(12):794–801. | ||
Roman G, Mareş M, Năstasă V. A novel antifungal agent with broad spectrum: 1-(4-biphenylyl)-3-(1 H-imidazol-1-yl)-1-propanone. Arch Pharm. 2013;346(2):110–118. | ||
Attia MI, Radwan AA, Zakaria AS, Almutairi MS, Ghoneim SW. 1-Aryl-3-(1H-imidazol-1-yl)propan-1-ol esters: synthesis, anti-Candida potential and molecular modeling studies. Chem Cent J. 2013;7(1):168. | ||
Leite ACL, da Silva KP, de Souza IA, de Araújo JM, Brondani DJ. Synthesis, antitumour and antimicrobial activities of new peptidyl derivatives containing the 1,3-benzodioxole system. Eur J Med Chem. 2004;39(12):1059–1065. | ||
Attia MI, El-Brollosy NR, Kansoh AK, et al. Synthesis, single crystal X-ray structure, and antimicrobial activity of 6-(1,3-benzodioxol-5-ylmethyl)-5-ethyl-2-{[2-(morpholin-4-yl)ethyl]sulfanyl} pyrimidin-4(3H)-one. J Chem. 2014. | ||
Al-Wabli RI, Al-Ghamdi AR, Primsa IP, et al. (2 E)-2-[1-(1,3-Benzodioxol-5-yl)-3-(1 H-imidazol-1-yl)propylidene]-N-(4-methoxyphenyl) hydrazinecarboxamide: Synthesis, crystal structure, vibrational analysis, DFT computations, molecular docking and antifungal activity. J Mol Struct. 2018;1166:121–130. | ||
Al-Wabli R, Al-Ghamdi A, Ghabbour H, et al. Synthesis, X-ray single crystal structure, molecular docking and DFT computations on N-[(1E)-1-(2H-1,3-benzodioxol-5-yl)-3-(1H-imidazol-1-yl)propylidene]-hydroxylamine: A new potential antifungal agent precursor. Molecules. 2017;22(3):373. | ||
Sheldrick GM. A short history of SHELX. Acta Crystallogr A Found Crystallogr. 2008;64(1):112–122. | ||
Allen FH, Kennard O, Watson DG, Brammer L, Orpen AG, Taylor R. Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. bond lengths in organic compounds. J Chem Soc. 1987;2(12):S1–S19. |
Supplementary materials
Antifungal activity
Materials
The reference standard antifungal drug, ketoconazole, was purchased from Sigma-Aldrich Co. (St Louis, MO, USA). Liquid RPMI 1640 medium supplemented with L-glutamine was obtained from Gibco-BRL, Life Technologies (Paisley, Scotland). Sabouraud Dextrose Agar (SDA) was obtained from Merck Co. (Darmstadt, Germany). Dimethyl sulfoxide (100%) was used to dissolve ketoconazole, and/or the tested compound 4 to give an initial concentration of 2,048 mg/L.
Organisms
The used fungal strains are Candida albicans (ATCC 90028), Candida tropicalis (ATCC 66029), Candida parapsilosis (ATCC 22019) and Aspergillus niger (ATCC 16404).
Preparation of fungal inocula
The inocula of the standard mold Aspergillus niger strain have been prepared by removing the sporulated A. niger from the Sabouraud Dextrose agar slant with a microbiological loop and the spores have been suspended in 10 mL of sterile water. The suspension has been filtered through sterile gauze to remove hyphae. The resulting suspension of conidia has been vigorously mixed using a vortex. The suspension has been adjusted to 1×105 CFU/mL using spectrophotometer. This fungal suspension has been diluted 1:5 with RPMI medium to obtain suspensions having 2× of the required final concentration. This conidial suspension had a final concentration of 1×104 CFU/mL when mixed with the tested solution of compound 4. On the other hand, the inocula of the standard yeast strains of C. albicans, C. tropicalis and C. parapsilosis have been prepared by suspending five representative colonies, obtained from 24 to 48 h culture on Sabauraud Dextrose agar medium, in sterile distilled water. The final inoculum concentration must be between 0.5×105 and 2.5×105 CFU/mL.
Preparation of the tested compound solution
Briefly, a twofold dilution series of the tested compounds has been prepared in a double strength RPMI 1640 culture medium. Ten serial dilutions were prepared to give concentrations ranged from 1,024 mg/L to 2 mg/L.
Antifungal susceptibility studies
Minimum Inhibitory Concentrations (MICs) have been determined by broth microdilution testing as described previously by EUCAST.1 The experiment was carried out in duplicate. Briefly, one mL of RPMI 1640 medium from each of the bottle containing the corresponding concentration of the tested compounds has been transferred into sterile 7 mL Sterilin tubes (Thermo Fisher Scientific, Waltham, MA, USA). The RPMI 1640 medium containing 1,024 mg/L of the tested compounds has been dispensed to tube 1, the medium containing 512 mg/L has been dispensed to tube 2, the medium containing 256 mg/L has been dispensed to tube 3 and so on to tube 10 for the medium containing 2 mg/L of the tested compounds. One mL of the medium has been dispensed in tubes 11 (positive control) and 12 (negative control). One mL of the diluted inoculum suspension has transferred to each tube except tube 12 to bring the tested compounds dilutions to the required final test concentrations. The tubes were incubated at 35°C for 72 h. The MICs of the tested compounds were determined visually by recording the degree of growth inhibition in each tube. The microanalysis results (Table S1) of the target compounds 5a-r agreed favorably with the proposed structures within ±0.4% of the theoretical values.
  | Table S1 Microanalysis data of the title compounds 5a–r |
  | Figure S1 1H NMR spectrum of compound 5d. |
  | Figure S2 13C NMR spectrum of compound 5d. |
  | Figure S3 1H NMR spectrum of compound 5e. |
  | Figure S4 13C NMR spectrum of compound 5e. |
  | Figure S5 1H NMR spectrum of compound 5k. |
  | Figure S6 13C NMR spectrum of compound 5k. |
Reference
Rodriguez-Tudela JL, Arendrup MC, Barchiesi F, et al. EUCAST Definitive Document EDef 7.1: method for the determination of broth dilution MICs of antifungal agents for fermentative yeasts: Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). Clin Microbiol Infect. 2008;14:398–405. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


