Back to Journals » Cancer Management and Research » Volume 11
Synchronous Neoplastic Lesions In Referred Patients With Colorectal Cancer: A Retrospective Cohort Study
Authors Li S, Zhu K, Yu W, Wang Y, Wang T, Guo S, Teng G, Guo J
Received 31 August 2019
Accepted for publication 6 November 2019
Published 26 November 2019 Volume 2019:11 Pages 9951—9959
DOI https://doi.org/10.2147/CMAR.S229376
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
Shuai Li,1 Kongxi Zhu,1 Weihua Yu,1 Yunxia Wang,1 Teng Wang,1 Shuang Guo,2 Guoxin Teng,3 Jianqiang Guo1
1Department of Gastroenterology, The Second Hospital of Shandong University, Shandong, People’s Republic of China; 2Department of Digestive Endoscopy Center, The Second Hospital of Shandong University, Shandong, People’s Republic of China; 3Department of Pathology, The Second Hospital of Shandong University, Shandong, People’s Republic of China
Correspondence: Jianqiang Guo
Department of Gastroenterology, The Second Hospital of Shandong University, Jinan, Shandong, People’s Republic of China
Tel +860531-85875454
Email [email protected]
Background: Synchronous neoplastic lesions are usually present in patients with colorectal cancer (CRC) at diagnosis or postoperative follow-up endoscopy. However, few studies have been published about the clinicopathological features of synchronous lesions, especially those of synchronous advanced neoplasia. This study aimed to describe synchronous lesions in patients with CRC because this knowledge may be useful for preventing the development of metachronous cancer.
Material and methods: We retrospectively reviewed 261 primary CRC cases with synchronous lesions referred to our hospital during a 4-year period. Personal history, habits, family history, characteristics of index cancer, and synchronous lesions were assessed.
Results: In total, the 261 patients with CRC had 812 synchronous adenomas and 146 advanced neoplasia. Diminutive, small, and large polyps made up 66.7%, 20.2%, and 13.1% of all lesions, respectively; 9.3% of diminutive and small adenomas were advanced neoplasia, and 45.2% of synchronous advanced lesions were subcentimeter polyps. Both synchronous non-advanced lesions and advanced lesions developed most frequently in the distal colon, followed by the proximal colon, and were least frequently found in the rectum (P < 0.001). Older age (P = 0.04) and male gender (P = 0.001) were associated with the presence of advanced neoplasia in CRC cases with synchronous neoplastic lesions. Meanwhile, the use of aspirin may be associated with a lower incidence of advanced neoplasia (P = 0.04).
Conclusion: Patients diagnosed with CRC require detailed clearing of the remainder of the colon at baseline coloscopy or postoperative follow-up examination, and we should take a more cautious approach to synchronous subcentimeter polyps in this group of patients.
Keywords: colorectal cancer, synchronous neoplastic lesions, advanced neoplasia, subcentimeter polyps
Background
Colorectal cancer (CRC) is the third most common cancer and the fourth most common cause of death by cancer globally.1,2 In China, it is the fifth leading cause of death due to carcinoma, accounting for approximately 376,000 new cases and 191,000 deaths per year. Systematic screening and surveillance in asymptomatic patients can reduce the incidence and mortality of CRC. During the past 20 years, the incidence of CRC has stabilized or started to decrease in several high-income countries (e.g., USA and eastern Europe),3,4 probably because of the common use of colonoscopy for screening tests. However, rapid increases still occur in areas with poor healthcare (e.g., Central and South America and the remote countryside in China).3,5
CRC often develops over more than 10 years. Conventional adenomas and sessile serrated polyps are the most common forms of cancer precursor lesions,1,6 following a multistep process of cancerous sequence. Detection and removal of these precursor lesions may prevent the development of cancer and reduce mortality.7 Synchronous neoplastic lesions, such as dysplastic adenoma and intramucosal carcinoma, are usually present when patients undergo preoperative colonoscopy examination or postoperative review within 6 months. This situation, called tumor multiplicity, is highly common. Synchronous lesions may be due to an interaction of numerous risk factors, including male gender, advanced age, medical history of colon polyps, tumor location, and tumor stage.8,9 Usually, the risk of developing a metachronous CRC in the five years following curative resection of the tumor is about 2–12%.10 However, CRC patients with synchronous neoplastic lesions are at increased risk of developing metachronous adenomas or cancer compared to those without synchronous lesions. Nevertheless, few studies have been published on the clinicopathological features of synchronous lesions, especially those of synchronous advanced neoplasia. The primary aim of the current study was to describe synchronous neoplastic lesions and advanced neoplasia in patients with CRC based on histological classification, size, and location. The secondary aim was to determine whether there were any risk factors for the development of advanced histology.
Materials And Methods
A retrospective evaluation was conducted in 578 consecutive patients who underwent resection for primary CRC in the Second Hospital of Shandong University (Shandong, China) between January 2014 and December 2017. A review of the database was approved by the medical ethics committee on June 16, 2018. The methods were carried out in accordance with the relevant guidelines and regulations. All personal information had already been anonymized and de-identified to protect patient privacy. For these reasons, the study protocol was exempted from the need for informed consent from its participants. This study was conducted in accordance with the Declaration of Helsinki. All pathological sections were reviewed and confirmed by a single pathologist to avoid inter-observer differences. The exclusion criteria were patients in whom CRC occurred in the context of familial adenomatous polyposis, inflammatory bowel disease, or Lynch syndrome, those who were lost to follow-up, and those with incomplete clinical data. In addition, patients in whom bowel preparation was poor and not adequate to detect lesions > 5 mm during coloscopy examination were also excluded. CRC cases without synchronous neoplastic lesions were registered but not enrolled in our study.
Patients enrolled were stratified into two groups depending on whether they presented synchronous advanced neoplasia. Synchronous neoplastic lesions were defined as the presence of adenomatous polyps, serrated polyps, and colorectal carcinomas on preoperative colonoscopy examination or postoperative review within 6 months. Particularly, in patients with synchronous colorectal cancer, which means more than one primary colorectal tumor was detected in a single patient at diagnosis, the most advanced/deepest tumor was considered as the index cancer and other lower-stage tumors were labeled as synchronous lesions. Hyperplastic polyps, which has an extremely low risk potential of developing CRC, and other non-adenomatous/non-serrated lesions were not considered in our study. Advanced adenoma was defined as an adenoma ≥ 10 mm in size, villous histology, high-grade dysplasia, or any combinations thereof. Advanced neoplasia included advanced adenomas and cancers.11,12
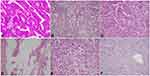
Family history and clinicopathological information of each patient were obtained from treatment records. It is important to note that in synchronous CRC, the clinicopathological characteristics of the index cancer were used in the analysis of each patient. Personal parameters recorded included: a) age; b) gender; c) history of smoking and alcohol consumption; d) tumor markers; e) the use of aspirin at a frequency of at least 2 times a week for 1 year; f) fecal occult-blood tests (FOBT) using immunohistochemical methods; g) medical history of first-degree relatives (FDR); h) operation method, from which we selected for patients who had undergone radical resection of the index cancer; i) location of index cancer, namely the proximal colon, distal colon, or rectum, which were defined similarly to other studies13-15 with the cecum, ascending colon, hepatic flexure, and transverse colon defined as the proximal colon and the splenic flexure, descending colon, and sigmoid colon classified as the distal colon; j) size of index cancer according to the maximum diameter of the tumor which, to facilitate statistical analysis, were grouped into < 50 mm and ≥ 50 mm; k) tumor stage, according to the Union for International Cancer Control tumor-node-metastasis classification;16 and l) grade of tumor differentiation (Figure 1), namely well-differentiated adenocarcinoma (Well), moderately differentiated adenocarcinoma (Mod), poorly differentiated adenocarcinoma (Poor), mucinous adenocarcinoma (Muc), signet-ring cell carcinoma (Srcc), or squamous cell carcinoma (Scc); and (m) size of synchronous neoplastic lesions (Figure 2), namely diminutive (1 to 5 mm in diameter), small (6 to 9 mm), or large (≥ 10 mm).
 |
Figure 2 (A) White light image of diminutive polyp (3 mm in diameter); (B) White light image of small polyp (8mm in diameter); (C) White light image of large polyp (15 mm in diameter). |
Statistical Analysis
Statistical analysis was performed using SPSS version 25 (IBM, New York, NY). The quantitative variables were compared using the Kruskal-Wallis tests, while qualitative variables were compared by means of the Chi-square and Fisher’s exact tests. A P < 0.05 was considered statistically significant.
Results
During the 4-year period, 578 consecutive patients with primary CRC were identified in the database. A total of 151 patients were excluded for known familial adenomatous polyposis (n = 3), inflammatory bowel disease (n = 2), being lost to follow-up (n = 42), incomplete clinic data (n = 51), or poor bowel preparation (n = 53). In addition, 166 patients without synchronous neoplastic lesions were registered but not enrolled in the study. Thus, 261 patients constituted the basis of this study, of whom advanced neoplasia was documented in 87 patients (33.3%) (Figure 3). The presence of synchronous colorectal cancer was confirmed in 20 patients (7.7% of the total; 16 with 2 tumors, 2 with 3 tumors, and 2 with 4 tumors). The clinicopathological characteristics of the patients enrolled are detailed in Table 1. The compared analysis showed that older age (P = 0.04; odds ratio (OR), 2.75; 95% confidence interval (CI): 1.02–7.46) and male gender (P = 0.001; OR, 2.92; 95% CI: 1.50–5.70) were associated with the presence of advanced histology in CRC cases with synchronous neoplastic lesions. Meanwhile, the use of aspirin may be associated with a lower incidence of advanced neoplasia (P = 0.04; OR, 0.40; 95% CI: 0.17–0.96). No significant differences were observed with other variables, such as history of smoking and alcohol consumption, FOBT, and size of index cancer (Table 2).
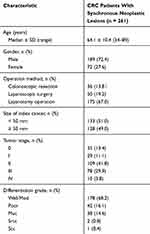 |
Table 1 Clinicopathological Characteristics Of Colorectal Cancer Patients With Synchronous Neoplastic Lesions Included In The Study |
 |
Table 2 Clinicopathological Characteristics Of Synchronous Advanced Neoplasia And Non-Advanced Neoplasia Groups |
 |
Figure 3 Patient flowchart. |
A total of 812 synchronous lesions (with an average of 3.1 per patient) were recorded, as shown in Table 3 with histological classification, size, and location. Specially, 571 adenomatous polyps accounted for 70.3% of all lesions, while serrated polyps and synchronous carcinomas accounted for 26.5% and 3.2%, respectively. When classified by size, diminutive polyps made up 66.7% of all lesions and small and large lesions made up 20.2% and 13.1%, respectively. Table 4 shows the characteristics and location of 146 synchronous advanced neoplasia (with an average of 0.6 per patient). There were 120 advanced adenomas, of which adenomas ≥10 mm in size, villous histology, and high-grade dysplasia comprised 62.3%, 21.9%, and 32.2%, respectively. In total, 23 well-/moderately differentiated adenocarcinomas and 3 poorly differentiated adenocarcinomas were confirmed by histology, and no mucinous or signet-ring cell features were found in synchronous carcinomas.
 |
Table 3 Histological Classification, Size, And Location Of 812 Synchronous Neoplastic Lesions |
 |
Table 4 Characteristics And Location Of 146 Synchronous Advanced Neoplasia |
At last, we tried to identify the number of synchronous lesions per patient in different locations of the bowel (Tables 5 and 6). The results revealed that both synchronous non-advanced lesions and advanced lesions developed most frequently in the distal colon, followed by the proximal colon, and were least frequently found in the rectum (P < .001). When comparing the locations of the index cancer, a similar conclusion was drawn.
 |
Table 5 Comparison Of The Location And Number Of Synchronous Non-Advanced Neoplasia Lesions Among Index Cancers With Different Locations |
 |
Table 6 Comparison Of The Location And Number Of Synchronous Advanced Neoplasia Lesions Among Index Cancers With Different Locations |
Discussion
There is a high probability of synchronous neoplastic lesions, including conventional adenomas, sessile serrated polyps, and less frequently carcinomas, in CRC patients when a tumor is diagnosed.9,17 It has been postulated that the presence of synchronous lesions indicates a personal predisposition to tumor multiplicity and a high risk of developing metachronous CRC. In surveillance studies, patients with advanced neoplasia are more likely to develop interval advanced lesions and cancer compared with patients without advanced lesions at baseline colonoscopy.18 A study including 7863 individuals diagnosed with CRC revealed individuals diagnosed with CRC in the proximal colon and those with synchronous CRC were associated with an increased risk of metachronous CRC.19 Two previous studies revealed that the incidence of synchronous neoplastic lesions in patients with CRC were 33.0%9 and 54.5%.8 In our study, the incidence rate was 61.1% (261/427), higher than those in previous studies. One possible explanation is that endoscopic techniques have advanced dramatically in recent years; accordingly, the adenoma detection rate has had a remarkable increase. The higher incidence rate reported in our study may also be attributed to the differences in genetic background, including race. The cohort in this study was mostly of Chinese ethnicity. The 4-year study also included 36 patients who underwent endoscopic submucosal dissection (ESD) for early CRC; most of the tumors were stage 0 and well- or moderately differentiated. To our knowledge, the present study constitutes the first investigation specifically enrolling patients with early CRC who had undergone ESD surgery to evaluate synchronous neoplastic lesions. In the series by Pinol et al, mucinous histological type and stage II were linked to tumor multiplicity. Similarly, our results revealed a higher proportion of mucinous histological type and stage II tumors than other subtypes in patients with CRC.1,2
Size has long been recognized as one of the most important markers of the potential of the adenoma to develop into cancer.20 Adenomas are considered advanced on the basis of size alone if they are 10 mm or more in diameter (also called large adenomas). Although diminutive (1–5 mm) and small (6–9 mm) polyps comprise 90% of detected lesions, there is evidence that more than 99% of diminutive and small adenomas are benign.20 The risk of showing features of advanced lesions such as villous histology and high-grade dysplasia within diminutive and small polyps is low and has been shown in recent studies to be between 0–4.3% and 1.1–13.6%, respectively.21 In view of that, Lieberman DA et al have suggested using size as an alternative marker for advanced adenomas to the exclusion of subcentimeter polyps.22 The American Society for Gastrointestinal Endoscopy (ASGE) Technology Committee proposed the approach of documenting diminutive polyps with a high-resolution photograph, followed by a resect-and-discard or diagnose-and-leave strategy.23 A previous study involving 13,992 asymptomatic adults who underwent screening colonoscopy revealed that diminutive polyps and small polyps made up 62.6% and 20.0% of all polyps, of which advanced adenomas account for 1.1% and 1.3%, respectively.18 Our cohort indicated diminutive polyps made up 66.7% of all polyps, of which 5.0% were advanced neoplasia, and included 4 cancers. Small polyps made up 20.2% of all polyps, of which 23.8% were advanced adenomas, and included 7 cancers. In all, 9.3% of diminutive and small adenomas were advanced neoplasia, and 45.2% of synchronous advanced lesions were subcentimeter polyps. In other words, diminutive and small polyps in patients with CRC would harbor neoplasia more likely to progress to malignancy compared to the general population. Therefore, we hold the opinion that the strategy of dealing with diminutive and small polyps in patients diagnosed with CRC deserves further study.
It is well-known that most CRCs develop from the distal colon and rectum (78.5% of CRCs were located in the distal colon and rectum in the present study). Nevertheless, that does not mean we can neglect the detection of lesions in the proximal colon when a tumor is diagnosed in the distal colon or rectum. From the present study, 29.9% of adenomatous polyps and 59.5% of serrated polyps were found in the proximal colon. Meanwhile, 40.1% of synchronous non-advanced neoplasia and 25.3% of advanced neoplasia were located in the same segment of the bowel. When comparing the number of synchronous lesions per patient in different locations of the bowel, the results revealed that both synchronous non-advanced lesions and advanced lesions developed most frequently in the distal colon, followed by the proximal colon and were least frequently found in the rectum. However, the lesions in the proximal colon are often overlooked or missed by the endoscopic physician. Several studies have showed that patients who develop cancer after colonoscopy are more likely to have cancer in the proximal colon than in the distal colon.24,25 This could be due to poor bowel preparation, failure to fully examine the proximal colon, features of serrated polyps (flat or oblong, often covered with mucus and are thus difficult to see), the skill of the endoscopist, and variable quality of colonoscopy.11
Older age and male gender have been confirmed as risk factors accounting for most cases of CRC.1,20 A research enrolling 3558 persons who underwent post-mortem examination between 1985 and 2004 revealed that the prevalence of adenomas was 1.72% for people in the third decade of life; this was increased to 3.59% for people in their fifth decade, with the incidence increasing sharply after 50 years of age.26 Several studies also showed that male gender is a risk factor for the development of synchronous lesions and increases the risk of malignant features of these lesions.8,9,20 In the compared analysis, both older age and male gender were more common in patients with synchronous advanced neoplasia. In contrast, the use of aspirin seemed like a preventive factor of forestalling the development of advanced polyps in patients with CRC. One plausible explanation is that aspirin could inhibit cyclooxygenase-2 and induce apoptosis in adenomatous tissue.27 In a series of studies, RCTs designed to examine the impact of aspirin on the development and recurrence of colorectal adenomas, have provided the efficacy of aspirin chemoprevention. Four trials repport long-term daily aspirin treatment can reduce the risk of recurrent adenoma and advanced adenoma among individuals with a high risk of sporadic CRC.28–31 However, the the Japan Colorectal Aspirin Polyps Prevention (J-CAPP) trial showed that no overall association between aspirin treatment and adenoma recurrence, but provided evidence of an interaction between treatment effects and smoking status.32
The main limitation of the current study was the likelihood of population bias. Being a single-center study, the population may be biased with respect to genetic background, which may be particularly important for the incidence of synchronous neoplastic lesions. Secondly, we did not record in detail the information on the quality of bowel preparation, as well as the exact withdrawal time for each patient. Finally, we did not account for a family history of polyps. As the veracity of family histories of polyps in our treatment records was likely to be low, we decided to focus only on family histories of CRC in FDR.
Conclusion
In summary, the risk of diminutive and small polyps progressing to malignancy in patients with CRC was likely to be higher than that of the general population. We should take a more cautious approach to synchronous subcentimeter polyps. A high proportion of synchronous neoplastic lesions and advanced neoplasia developed in the proximal colon. Patients diagnosed with CRC require detailed clearing of the remainder of the colon at baseline coloscopy or postoperative follow-up examination. Older age and male gender were associated with an increased risk of synchronous advanced neoplasia in patients with CRC, while the use of aspirin seemed to be a preventive factor.
Acknowledgements
We thank all the clinicians who enrolled patients and participated in the clinical study, in particular the coordinators Lan Liu and Liting Zhang. We also would like to thank Editage for English language editing.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383(9927):1490–1502. doi:10.1016/S0140-6736(13)61649-9
2. Talbot R, Kirkham S. Colorectal cancer. Lancet. 2010;376(9738):330. doi:10.1016/S0140-6736(10)61182-8
3. Center MM, Jemal A, Smith RA, Ward E. Worldwide variations in colorectal cancer. CA Cancer J Clin. 2009;59(6):366–378. doi:10.3322/caac.20038
4. Bosetti C, Levi F, Rosato V, et al. Recent trends in colorectal cancer mortality in Europe. Int J Cancer. 2011;129(1):180–191. doi:10.1002/ijc.v129.1
5. Guo P, Huang ZL, Yu P, Li K. Trends in cancer mortality in China: an update. Ann Oncol. 2012;23(10):2755–2762. doi:10.1093/annonc/mds069
6. Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 2007;50(1):113–130. doi:10.1111/his.2007.50.issue-1
7. Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366(8):687–696. doi:10.1056/NEJMoa1100370
8. Borda A, Martínez-Peñuela JM, Muñoz-Navas M, Prieto C, Betés M, Borda F. [Synchronous neoplastic lesions in colorectal cancer. An analysis of possible risk factors favouring presentation]. Rev Esp Enferm Dig. 2008;100(3):139–145. doi:10.4321/s1130-01082008000300003
9. Pinol V, Andreu M, Castells A, et al. Synchronous colorectal neoplasms in patients with colorectal cancer: predisposing individual and familial factors. Dis Colon Rectum. 2004;47(7):1192–1200. doi:10.1007/s10350-004-0562-7
10. Erenay FS, Alagoz O, Banerjee R, Cima RR. Estimating the unknown parameters of the natural history of metachronous colorectal cancer using discrete-event simulation. Med Decis Making. 2011;31(4):611–624. doi:10.1177/0272989X10391809
11. Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143(3):844–857. doi:10.1053/j.gastro.2012.06.001
12. Gao Q, Tsoi KK, Hirai HW, et al. Serrated polyps and the risk of synchronous colorectal advanced neoplasia: a systematic review and meta-analysis. Am J Gastroenterol. 2015;110(4):501–509. doi:10.1038/ajg.2015.49
13. Bufill JA. Colorectal cancer: evidence for distinct genetic categories based on proximal or distal tumor location. Ann Intern Med. 1990;113(10):779–788. doi:10.7326/0003-4819-113-10-779
14. Myer PA, Mannalithara A, Singh G, Ladabaum U. Proximal and distal colorectal cancer resection rates in the United States since widespread screening by colonoscopy. Gastroenterology. 2012;143(5):1227–1236. doi:10.1053/j.gastro.2012.07.107
15. Missiaglia E, Jacobs B, D’Ario G, et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol. 2014;25(10):1995–2001. doi:10.1093/annonc/mdu275
16. O’Sullivan B, Brierley J, Byrd D, et al. The TNM classification of malignant tumours-towards common understanding and reasonable expectations. Lancet Oncol. 2017;18(7):849–851. doi:10.1016/S1470-2045(17)30438-2
17. Imperiale TF, Wagner DR, Lin CY, Larkin GN, Rogge JD, Ransohoff DF. Risk of advanced proximal neoplasms in asymptomatic adults according to the distal colorectal findings. N Engl J Med. 2000;343(3):169–174. doi:10.1056/NEJM200007203430302
18. Lieberman D, Moravec M, Holub J, Michaels L, Eisen G. Polyp size and advanced histology in patients undergoing colonoscopy screening: implications for CT colonography. Gastroenterology. 2008;135(4):1100–1105. doi:10.1053/j.gastro.2008.06.083
19. Jayasekara H, Reece JC, Buchanan DD, et al. Risk factors for metachronous colorectal cancer following a primary colorectal cancer: a prospective cohort study. Int J Cancer. 2016;139(5):1081–1090. doi:10.1002/ijc.30153
20. Strum WB. Colorectal adenomas. N Engl J Med. 2016;374(11):1065–1075. doi:10.1056/NEJMra1513581
21. Vleugels JLA, Hazewinkel Y, Fockens P, Dekker E. Natural history of diminutive and small colorectal polyps: a systematic literature review. Gastrointest Endosc. 2017;85(6):1169–1176.e1. doi:10.1016/j.gie.2016.12.014
22. Lieberman DA, Holub JL, Morris CD, Logan J, Williams JL, Carney P. Low rate of large polyps (>9 mm) within 10 years after an adequate baseline colonoscopy with no polyps. Gastroenterology. 2014;147(2):343–350. doi:10.1053/j.gastro.2014.04.020
23. Abu Dayyeh BK, Thosani N; ASGE Technology Committee, et al. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. 2015;81(3):
24. Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150(1):1–8. doi:10.7326/0003-4819-150-1-200901060-00306
25. Brenner H, Chang-Claude J, Seiler CM, Rickert A, Hoffmeister M. Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Ann Intern Med. 2011;154(1):22–30. doi:10.7326/0003-4819-154-1-201101040-00004
26. Pendergrass CJ, Edelstein DL, Hylind LM, et al. Occurrence of colorectal adenomas in younger adults: an epidemiologic necropsy study. Clin Gastroenterol Hepatol. 2008;6(9):1011–1015. doi:10.1016/j.cgh.2008.03.022
27. Liao X, Lochhead P, Nishihara R, et al. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N Engl J Med. 2012;367(17):1596–1606. doi:10.1056/NEJMoa1207756
28. Baron JA, Cole BF, Sandler RS, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348(10):891–899. doi:10.1056/NEJMoa021735
29. Benamouzig R, Deyra J, Martin A, et al. Daily soluble aspirin and prevention of colorectal adenoma recurrence: one-year results of the APACC trial. Gastroenterology. 2003;125(2):328–336. doi:10.1016/S0016-5085(03)00887-4
30. Sandler RS, Halabi S, Baron JA, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 2003;348(10):883–890. doi:10.1056/NEJMoa021633
31. Logan RF, Grainge MJ, Shepherd VC, et al. Aspirin and folic acid for the prevention of recurrent colorectal adenomas. Gastroenterology. 2008;134(1):29–38. doi:10.1053/j.gastro.2007.10.014
32. Ishikawa H, Mutoh M, Suzuki S, et al. The preventive effects of low-dose enteric-coated aspirin tablets on the development of colorectal tumours in Asian patients: a randomised trial. Gut. 2014;63(11):1755–1759. doi:10.1136/gutjnl-2013-305827
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.