Back to Journals » Pharmacogenomics and Personalized Medicine » Volume 13
Susceptibility of PON1/PON2 Genetic Variations to Ischemic Stroke Risk in a Chinese Han Population
Authors Pan Y, He B , Sun H, Xu T, Pan B, Wang S, Mei Y
Received 3 August 2020
Accepted for publication 6 October 2020
Published 29 October 2020 Volume 2020:13 Pages 563—570
DOI https://doi.org/10.2147/PGPM.S275341
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Yuqin Pan,1,* Bangshun He,1,* Huiling Sun,1 Tao Xu,1 Bei Pan,1 Shukui Wang,1 Yanping Mei2
1General Clinical Research Center, Nanjing First Hospital, Nanjing Medical University, Nanjing, Jiangsu Province, 210006, People’s Republic of China; 2Department of Laboratory Medicine, Nanjing First Hospital, Nanjing Medical University, Nanjing 210006, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shukui Wang
General Clinical Research Center, Nanjing First Hospital, Nanjing Medical University, 68 Changle Road, Nanjing, Jiangsu 210006, People’s Republic of China
Email [email protected]
Yanping Mei
Department of Laboratory Medicine, Nanjing First Hospital, Nanjing Medical University, 68 Changle Road, Nanjing, Jiangsu 210006, People’s Republic of China
Email [email protected]
Background: Paraoxonases (PONs) are a family of orphan enzymes with multiple functions, including anti-inflammatory, antioxidative, antiatherogenic activities. Studies have suggested that genetic variations in PON1 and PON2 are associated with ischemic stroke (IS) risk; however, the conclusion remains unclear in the Chinese population.
Methods: To investigate the susceptibility of genetic variations in PON1 and PON2 to risk of IS and its subtypes, this case–control study was carried out on a Chinese population comprising 300 IS patients and 300 healthy controls. Genotypes of six genetic variations in PON1 and PON2 were identified with an improved multiplex ligase detection–reaction technique.
Results: PON1 rs662 was associated with increased risk of IS (CT vs. TT — ORadjusted 1.79, 95% CI 1.08– 2.97; p=0.025). Stratified analysis for patients by sex revealed that the significant association of PON1 rs662 with IS risk was maintained in the male cohort (CT vs. TT — ORadjusted 2.59, 95% CI 1.29– 5.21 [p=0.009]; CT/CC vs. TT — ORadjusted 2.03, 95% CI 1.05– 3.93 [p=0.036]), but not in the female cohort. Analysis according to IS subtype revealed that PON1 rs662 genetic variation was an increased risk in the subcohort of patients with large-artery atherosclerosis (CT/CC vs. TT — ORadjusted 2.31, 95% CI 1.09– 4.91; p=0.029), but not in patients with other types of IS.
Conclusion: This study suggested that PON1 rs662 presented a potential risk of IS, especially for males, and this association was more obvious for large-artery atherosclerosis.
Keywords: ischemic stroke, genetic variation, PON1, PON2
Introduction
Stroke has become one of the main causes of death and disability worldwide: >15 million people suffer ischemic stroke (IS), each year, causing 6 million deaths and 5 million disabilities. It is the second-leading cause of disability and death in the people >60 years old and the fifth-leading cause of death in people aged 15–59 years.1 IS incidence varies depending on age, sex, race, and genetic factors. Epidemiological studies have revealed several factors, including age, sex, obesity, cigarette smoking, hypertension, diabetes mellitus, atherosclerosis, and dyslipidemias, contribute to the occurrence of stroke.2–5
IS, hemorrhagic stroke, and transient ischemic attacks are three main types of cerebrovascular events. Of these, IS occurs as a result of an obstruction within a blood vessel supplying blood to the brain and is the most common form of cerebrovascular disease, accounting for approximately 87% of all stroke cases. Actually, the etiology of IS affects risk of recurrence, disease prognosis, and choices for management. Therefore, the categorization of subtypes of IS based mainly on etiology was developed for the TOAST study,6 which developed five subtypes of IS: 1) large-artery atherosclerosis, 2) cardioembolism, 3) small-vessel occlusion, 4) stroke of other determined etiology, and 5) stroke of undetermined etiology.
Studies on stroke etiology have indicated that a complex interaction of genetic and environmental factors contributes to the occurrence of stroke.7,8 For genetic factors, a number of genes involved in cholesterol metabolism, inflammation, blood coagulation, homocysteine metabolism, and the renin–angiotensin system have been suggested to contribute to the development of stroke.9 Specifically, genes involved in lipid metabolism have been implicated in the etiology of stroke insofar as the concentration of low-density lipoprotein (LDL) and the oxidation of LDL represent initial events in atherogenesis by producing proatherosclerotic and proinflammatory oxidized lipids, whereas high-density lipoprotein (HDL) in functional form is an atheroprotective factor through its multifunctionality, including reverse cholesterol transport and antioxidant, anti-inflammatory, and antithrombotic effects.
Paraoxonases (PONs), a family of orphan enzymes with multiple activities, are tightly associated with the HDL surface that decreases the peroxidation of LDL and have anti-inflammatory, antioxidative, and antiatherogenic activities. PON1, PON2, and PON3 are three known members of this gene family, on the long arm of chromosome 7 between q21.3 and q22.1 in humans. These three types of PONs are antiatherogenic enzymes in terms of their antioxidant activities and inhibiting the oxidation of LDL, along with preventing oxidative modification of the cell membrane.10 Of these, PON1 is a calcium-dependent glycoprotein associated with HDL particles, and exerts a cardioprotective function through its hydrolyzing effect on LDL-oxidized phospholipids. Studies have revealed that genetic variations in PON1 can affect its concentration or activity and predict the risk of IS.11 Genetic variations in the promoter region of PON1 and the coding regions of PON1 and PON2 have been focused on for their susceptibility to IS; however, the results were not consistent,12 which may be attributed to differences in genetic background among ethnicities. To investigate the susceptibility of genetic variations in PON1 and PON2 to the risk of IS and its subtype, this case–control study was carried out on a Chinese cohort.
Methods
Study Subjects
A total of 300 patients were enrolled as cases, and all patients were diagnosed with IS on the basis of clinical symptoms, physical examination, and brain computed tomography or magnetic resonance imaging, independently assessed by a technologist and a physician. All the patients suffered a sudden onset of focal or global neurologic deficit with signs and symptoms persisting for more than 24 h. Patients with a history or occurrence of transient ischemic attacks, hemorrhagic stroke, cerebral trauma, cardiogenic thrombosis, cerebrovascular malformations, coagulation disorders, autoimmune diseases, tumors, peripheral vascular disease, or chronic infection diseases were excluded. According to the criteria and characteristics of the enrolled patients, we divided the patients into four subtypes: 1) large-artery atherosclerosis, 2) cardioembolism, 3) small-vessel occlusion, and 4) stroke of other etiology.
Healthy control subjects were recruited from the Health Medical Center of Nanjing First Hospital during the same period. All these were confirmed as healthy according to the results of routine health examination and matched to cases in terms of age and sex. For the healthy controls, those with history of tumors, autoimmune diseases, genetic diseases, liver ailments, and hematologic diseases were excluded. Demographic characteristics and clinical information — including sex, age, drinking, smoking, diastolic blood pressure (DBP), systolic blood pressure (SBP), diabetes, fasting serum levels of total plasma cholesterol, triglycerides (TGs), HDL, LDL, glucose, and homocysteine — were abstracted from medical records at our hospital. All enrolled participants were heritably unrelated ethnic Han Chinese from the same geographic region: Nanjing City, Jiangsu, China. The protocol of this study was in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Nanjing First Hospital, and written informed consent was obtained from all participants.
SNP Selection and Genotyping
All potential genetic variations in PON1 or PON2 associated with risk of IS were retrieved from the National Center for Biotechnology Information dbSNP database (http://www.ncbi.nlm.nih.gov/projects/SNP), and then potential genetic variations were selected: 1) positioned in exons, promoter regions, 5′UTRs, 3′UTRs, or splice sites; 2) minor-allele frequency ≥5%; and 3) had been reported to be associated with IS risk, previously. Finally, six genetic variations in PON1 and PON2 were selected (see Table 1 for details). Blood samples were collected from all participants with EDTA-coated tubes and stored in a refrigerator at −80°C. Total DNA was extracted from whole-blood samples and concentrated using a mini whole blood genomic DNA purification kit (GoldMag Xi’an, China) according to the manufacturer’s instructions, and then DNA purity was measured with spectrometry (DU530; Beckman Instruments, Fullerton, CA, US).
 |
Table 1 Enrolled Genetic Variations |
All six genetic variations selected were genotyped using the improved multiplex ligase detection–reaction technique developed by Genesky Biotech (Shanghai, China). In brief, multiplex polymerase chain reaction was performed to amplify genetic-variation loci. Secondly, amplification products were purified by nuclease and shrimp alkaline enzyme. Finally, a connection the reaction was performed to have each site containing two 5ʹ terminal allele–specific probes and a 3ʹ terminal-specific probe of fluorescent tags, and then ligation products were analyzed with an ABI 3730XL. Of all subjects, 10% were randomly selected and subjected to repeated genotyping, and reproducibility of 100% attained.
Statistical Analysis
Differences in demographic characteristics between patients and controls were compared by univariate analysis with the use of Student’s t-test. Hardy–Weinberg equilibrium in the healthy control group was tested using a goodness-of-fit χ2 test. Logistic regression was applied to calculate ORs and 95% CIs. The dominant, codominant, and additive models were tested for all genetic variations. p<0.05 was considered statistically significant.
Results
A total of 300 patients with IS and 300 age- and sex-matched healthy controls were enrolled in this population-based case–control association study, and their demographic data and clinical characteristics are summarized in Table 2. There were no significant differences with respect to age (p=0.136), sex (p=0.273), smoking (p=0.351), or drinking (p=0.854) between the two groups. For clinical characteristics, levels of DBP (p<0.001), SBP (p<0.001), TGs (p=0.024), Glu (p<0.001), and homocysteine (p=0.021) were significantly higher in patients than in controls. In contrast, levels of HDL in patients were significantly lower than in controls (p<0.001), as shown in Table 2. A total of 117 patients were identified as having large-artery atherosclerosis, 28 with cardioembolism, 56 with small-vessel occlusion, and 97 with other etiologies (Table 2).
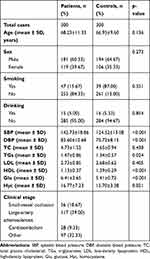 |
Table 2 Demographic Data and Clinical Characteristics of Patients with Ischemic Stroke and Controls |
Observed frequencies of all tested genotypes in controls were not derived from the Hardy–Weinberg equilibrium (data not shown). Logistic regression analysis revealed that PON1 rs662 genetic variation was associated with increased risk of IS (CT vs. TT — ORadjusted 1.79, 95% CI 1.08–2.97; p=0.025), as shown in Table 3. Subgroup analysis of patients stratified by sex revealed a significant association of PON1 rs662 with IS risk was in the male cohort (CT vs. TT — ORadjusted 2.59, 95% CI 1.29–5.21 [p=0.009; CT/CC vs TT — ORadjusted 2.03, 95% CI 1.05–3.93, [p=0.036]) but not in the of female cohort, as shown in Table 4. Further, we evaluated the susceptibility of genetic variations to risk of subtypes of IS. PON1 rs662 showed increased risk inpatients with large-artery atherosclerosis (CT/CC vs. TT — ORadjusted 2.31, 95% CI 1.09–4.91; p=0.029) but not in patients with any other type of IS, suggesting the risk of PON1 rs662 for IS is modified by its type, as shown in Table 5.
 |
Table 3 Genotype Distribution of Polymorphisms in all Participants |
 |
Table 4 Genotype Distribution of Polymorphisms in all Participants Stratified by Sex |
 |
Table 5 Associations Between Genetic Variations and Risk of Types OfIischemic Stroke |
Discussion
This population-based case–control association study with 300 paired cases and controls revealed that the PON1 rs662 genetic variation was potentially associated with increased risk of IS, especially in the male population, and that the susceptibility of PON1 rs662 to IS risk could be modified by its etiology. We observed that genotypes in controls were not derived from the Hardy–Weinberg equilibrium, indicating controls in this study were a large, randomly matching population with no selection, genetic drift, migration, or mutation, suggesting the controls we selected were reliable.
PON1 rs662 is a genetic variation in the coding region of PON1, causing a missense substitution at position 192 (192Gln [Q]/Arg [R]). Studies have reported that this genetic variation is the major determining factor leading to PON1 activity in that the 192R variant can hydrolyze paraoxonase faster than the 192Q variant.13–14 Therefore, PON1 rs662 has been regarded as a risk factor of cardiovascular disease15 and IS, although results have often been conflicting.11 This study based on a Chinese population also reported the PON1 rs662 R(G) allele was a potential risk factor for IS, consistent with pooled results of published data verifying the association between PON1 rs662 and stroke risk,16,17 especially in Asian populations.12 In addition, we observed that the risk of PON1 rs662 in IS was more obvious in the male subcohort than the female one, indicating the interaction of sex and PON1 rs662 contributed to different risk of IS18,119 and that males with the PON1 rs662 C allele are at higher risk of IS. Consistently, sex differences, including dyslipidemia, are regarded as predictors of IS,20 which may contribute to the sex difference in susceptibility of PON1 rs662 to IS. Despite the limited sample size, we observed the risk of PON1 rs662 for IS was more obvious in patients with large-artery atherosclerosis, consistent with the result of previous report.21 Actually, large-artery atherosclerosisshares a similar etiology with atherosclerosis,6 and PON1 rs662 genetic variation presents a risk of atherosclerosis.22 Therefore, the contradiction of published data regarding the susceptibility of PON1 rs662 to IS risk may be due to lack of classification of stroke subtypes, which should be verified by further larger studies. To date, few studies have actually discussed the association between PON1 rs662 polymorphism and risk of IS subtypes. The novelty of this study was that we firstly reported that PON1 rs662 polymorphism was associated with risk of large-artery atherosclerosis in a Chinese population, although the sample was relatively small.
Three genetic variations in the promoter and one in 3′UTR of PON1 were also investigated in this study, and no significant association was observed. Although these genetic variations have been reported to regulate PON1 expression11 and contribute to susceptibility to IS,23,24 in this study we failed to find any association of them to risk of IS, which should be confirmed by further large-sample studies. For PON1 rs854571, the results of this study are consistent with previous reports.23–25 The PON2 rs7493 genetic variation causes a substitution (C311S) on exon 9 and has been reported not to be associated with IS risk in Chinese population.23–26 Pooled results of published data have also revealed such an association27 consistent with the results of this study.
In short, this study suggests that PON1 rs662 is a potential risk of IS, especially for males, and this association is heightened in large-artery atherosclerosis.
Data-Sharing Statement
The data that support the findings of this study are available from the corresponding author Yanping Mei upon reasonable request.
Ethics Statement and Consent
The protocol of this study was in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the Nanjing First Hospital, and written informed consent was obtained from all the participants.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Innovation Team of Jiangsu Provincial Health-Strengthening Engineering by Science and Education (CXTDB2017008), Jiangsu Youth Medical Talents Training Project grants to BH (QNRC2016066) and YP (QNRC2016074), and grants from Key Project of Science and Technology Development of Nanjing Medicine (ZKX18030).
Disclosure
The authors report no conflicts of interest for this work.
References
1. Johnson W, Onuma O, Owolabi M, Sachdev S. Stroke: a global response is needed. Bull World Health Organ. 2016;94(9):634–634A. doi:10.2471/BLT.16.181636
2. Price AJ, Wright FL, Green J, et al. Differences in risk factors for 3 types of stroke: UK prospective study and meta-analyses. Neurology. 2018;90(4):e298–e306. doi:10.1212/WNL.0000000000004856
3. Pastuszak Z, Kozniewska E, Stepien A, Piusinska-Macoch A, Czernicki Z, Koszewski W. Importance rating of risk factors of ischemic stroke in patients over 85 years old in the polish population. Neurol Neurochir Pol. 2018;52(1):88–93. doi:10.1016/j.pjnns.2017.11.007
4. Alloubani A, Saleh A, Abdelhafiz I. Hypertension and diabetes mellitus as a predictive risk factors for stroke. Diabetes MetabSyndr. 2018.
5. Wassertheil-Smoller S, Qi Q, Dave T, et al. Polygenic risk for depression increases risk of ischemic stroke: from the stroke genetics network study. Stroke. 2018;49(3):543–548. doi:10.1161/STROKEAHA.117.018857
6. Adams HP
7. Hankey GJ. Stroke. Lancet. 2017;389(10069):641–654. doi:10.1016/S0140-6736(16)30962-X
8. Humphries SE, Morgan L. Genetic risk factors for stroke and carotid atherosclerosis: insights into pathophysiology from candidate gene approaches. Lancet Neurol. 2004;3(4):227–235. doi:10.1016/S1474-4422(04)00708-2
9. Gulcher JR, Gretarsdottir S, Helgadottir A, Stefansson K. Genes contributing to risk for common forms of stroke. Trends Mol Med. 2005;11(5):217–224. doi:10.1016/j.molmed.2005.03.001
10. Deakin SP, Bioletto S, Bochaton-Piallat ML, James RW. HDL-associated paraoxonase-1 can redistribute to cell membranes and influence sensitivity to oxidative stress. Free Radic Biol Med. 2011;50(1):102–109. doi:10.1016/j.freeradbiomed.2010.09.002
11. Tajbakhsh A, Rezaee M, Rivandi M, Forouzanfar F, Afzaljavan F, Pasdar A. Paraoxonase 1 (PON1) and stroke; the dilemma of genetic variation. Clin Biochem. 2017;50(18):1298–1305. doi:10.1016/j.clinbiochem.2017.08.001
12. Rodriguez-Esparragon F, Lopez-Fernandez JC, Buset-Rios N, et al. Paraoxonase 1 and 2 gene variants and the ischemic stroke risk in Gran Canaria population: an association study and meta-analysis. Int J Neurosci. 2017;127(3):191–198. doi:10.3109/00207454.2016.1165675
13. Humbert R, Adler DA, Disteche CM, Hassett C, Omiecinski CJ, Furlong CE. The molecular basis of the human serum paraoxonase activity polymorphism. Nat Genet. 1993;3(1):73–76. doi:10.1038/ng0193-73
14. Mackness B, Mackness MI, Arrol S, Turkie W, Durrington PN. Effect of the molecular polymorphisms of human paraoxonase (PON1) on the rate of hydrolysis of paraoxon. Br J Pharmacol. 1997;122(2):265–268. doi:10.1038/sj.bjp.0701390
15. Bhattacharyya T, Nicholls SJ, Topol EJ, et al. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA. 2008;299(11):1265–1276. doi:10.1001/jama.299.11.1265
16. Banerjee I. Relationship between Paraoxonase 1 (PON1) gene polymorphisms and susceptibility of stroke: a meta-analysis. Eur J Epidemiol. 2010;25(7):449–458.
17. Dahabreh IJ, Kitsios GD, Kent DM, Trikalinos TA. Paraoxonase 1 polymorphisms and ischemic stroke risk: A systematic review and meta-analysis. Genet Med. 2010;12(10):606–615. doi:10.1097/GIM.0b013e3181ee81c6
18. Spychala MS, Honarpisheh P, McCullough LD. Sex differences in neuroinflammation and neuroprotection in ischemic stroke. J Neurosci Res. 2017;95(1–2):462–471. doi:10.1002/jnr.23962
19. Madsen TE, Khoury JC, Alwell KA, et al. Sex differences in cardiovascular risk profiles of ischemic stroke patients with diabetes in the Greater Cincinnati/Northern Kentucky Stroke Study. J Diabetes. 2017.
20. Samai AA, Martin-Schild S. Sex differences in predictors of ischemic stroke: current perspectives. Vasc Health Risk Manag. 2015;11:427–436.
21. Juan J, Jiang X, Tang X, et al. Joint effects of PON1 polymorphisms and vegetable intake on ischemic stroke: a family-based case control study. Int J Mol Sci. 2017;18(12):2652. doi:10.3390/ijms18122652
22. Cozzi L, Campolo J, Parolini M, et al. Paraoxonase 1 L55M, Q192R and paraoxonase 2 S311C alleles in atherothrombosis. Mol Cell Biochem. 2013;374(1–2):233–238. doi:10.1007/s11010-012-1525-2
23. Zhang G, Li W, Li Z, et al. Association between paraoxonase gene and stroke in the Han Chinese population. BMC Med Genet. 2013;14:16. doi:10.1186/1471-2350-14-16
24. Liu ME, Liao YC, Lin RT, et al. A functional polymorphism of PON1 interferes with microRNA binding to increase the risk of ischemic stroke and carotid atherosclerosis. Atherosclerosis. 2013;228(1):161–167. doi:10.1016/j.atherosclerosis.2013.01.036
25. Voetsch B, Benke KS, Panhuysen CI, Damasceno BP, Loscalzo J. The combined effect of paraoxonase promoter and coding region polymorphisms on the risk of arterial ischemic stroke among young adults. Arch Neurol. 2004;61(3):351–356. doi:10.1001/archneur.61.3.351
26. Xu HW, Yuan N, Zhao Z, et al. Study of the relationship between gene polymorphisms of paraoxonase 2 and stroke in a Chinese population. Cerebrovasc Dis. 2008;25(1–2):87–94. doi:10.1159/000111996
27. Li BH, Zhang LL, Yin YW, et al. Association between paraoxonase 2 Ser311Cys polymorphism and ischemic stroke risk: a meta-analysis involving 5008 subjects. Mol Biol Rep. 2012;39(5):5623–5630. doi:10.1007/s11033-011-1367-0
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
