Back to Journals » Therapeutics and Clinical Risk Management » Volume 15
Survival outcomes and efficacy of autologous CD19 chimeric antigen receptor-T cell therapy in the patient with diagnosed hematological malignancies: a systematic review and meta-analysis
Authors Drokow EK , Ahmed HAW , Amponsem-Boateng C , Akpabla GS , Song J , Shi M , Sun K
Received 5 February 2019
Accepted for publication 20 March 2019
Published 6 May 2019 Volume 2019:15 Pages 637—646
DOI https://doi.org/10.2147/TCRM.S203822
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Deyun Wang
Emmanuel Kwateng Drokow,1 Hafiz Abdul Waqas Ahmed,1 Cecilia Amponsem-Boateng,2 Gloria Selorm Akpabla,3 Juanjuan Song,1 Mingyue Shi,1 Kai Sun1
1Department of Hematology, Zhengzhou University People‘s Hospital & Henan Provincial People‘s Hospital Henan, Zhengzhou, People’s Republic of China; 2Department of Epidemiology and Biostatistics, College of Public Health, Zhengzhou University, Zhengzhou, People’s Republic of China; 3Department of Internal Medicine, Tianjin Medical University, Tianjin, People’s Republic of China
Purpose: Chimeric Antigen Receptor T(CAR-T) cell therapy is an immunotherapy approach used in treating cancer which has seen rapid development over the decades. It becomes the preferred treatment choice after patients have failed conventional chemotherapy.
Methods: We conducted a meta-analysis in 320 patients from 14 studies to estimate the survival outcome, response rate and toxicity of autologous CD19 CAR-T cell therapy and predict other factors associated with a better prognosis.
Results: The overall response rate was 71.88% (95% CI: 61.34–80.46%, p<0.01) and CRS toxicity was 60.15% (95% CI: 42.87–75.22%, p<0.01). Patients who received lymphodepletion was associated with a better response rate (77%, 95%CI: 67–83%; p-value =0.001) in comparison to the other patients who did not (66%, 95%CI: 41–83%).
Conclusion: Lymphodepletion regimen may play a crucial role in predicting the prognosis of patients with hematological malignancies. Lymphodepletion patients had better progression-free survival than those who did not.
Keywords: autologous, CD19, CAR-T, hematological malignancies, response rate
Introduction
Chimeric Antigen Receptor T(CAR-T) cell therapy is an immunotherapy approach used in treating cancer which has seen rapid development over the decades.1 In CAR-T cell therapy, the patient’s T cells are equipped with the ability to detect and destroy cancer cells by combining the specificity of a monoclonal antibody with the cytotoxic and memory capabilities of the T cells.2 The production of CAR-T cell therapy first involves the collection of T cells, a type of white blood cell from a patient (autologous), or a donor’s (allogeneic) blood. The collected T cells are engineered in the laboratory to produce chimeric antigen receptors on their surface. The CAR-T cells are infused into the bloodstream of the patient aiming at the identification and killing of the malignant cells that have the targeted antigen on their surface and multiply within the patient’s body.
CD19 is an IgSF surface glycoprotein of 95kDa expressed during the earliest stages of B cell development until it is lost during the differentiation of plasma cell terminal, therefore, making it a potential target against B-cell malignancies.3 Maude et al (2014) reported that about 90% of patients with relapsed or refractory acute lymphoblastic leukaemia (ALL) who received CD19 CAR-T cell therapy achieved complete remission up to 2 years.4 Less than half of the patients enrolled in Cruz et al (2013) studies attained complete and partial remission and 8 out of 11 patients diagnosed with lymphoma had up to 23 months’ remission.5 Wang et al (2014) showed that a patient achieved a complete remission up to 9 weeks after administering CAR-T cells.6 The variance in these studies may be due to several factors such as the source of T cells being either allogeneic or autologous, infused CAR-T cell doses, days of cell culture, chemotherapy regime for lymphodepletion and the production design making it difficult for comparative studies. Per our knowledge, previous studies focused on the safety and efficacy of CD20 and CD19 CAR-T cells with limited number involved.7,8 Zhang et al evaluated the efficacy of both autologous and allogeneic CD19 CAR-T cells in 131 patients.9 Zhou et al reported the safety and efficacy of CD20 and CD19 CAR-T cells in 185 patients.10 Anwer et al included allogeneic T cells, though most CAR-T cell treatment utilises autologous T cells11 and Holzinger et al studies were a narrative review, instead, of a systematic one.12 Most studies conducted so far combined both allogeneic and autologous CD19 and CD20 in their studies.
Given these impediments of past publications, along with the quick advancement of the CAR- T field, we conducted a meta-analysis study to investigate the efficacy and survival outcome of autologous CD19 CAR-T cell therapy by involving 320 patients from 14 studies and figuring out factors affecting CAR-T therapy efficacy, survival outcome, and distinguish the group of patients (Paediatric or Adult) with the better outcome.
Methods and materials
Literature searching and screening
With the search words “CD19ʹ’, “autologous”, “hematological malignancies” and “CAR-T”, we conducted a search from Pubmed, Google Scholar and Baidu Xueshu databases for the related scientific studies from May 15 to September 23, 2018. All relevant articles related to the study were incorporated.
Exclusion and inclusion criteria
The inclusion criteria included: (1) only reported clinical trials; (2) autologous CD19 CAR-T cell; (3) relapsed or refractory B-cell hematological malignancies; (4) studies conducted in humans and (5) literature published in English. The criteria for exclusion included: (1) allogeneic CD 19; (2) autologous or allogeneic CD 20; (3) studies not conducted in humans; and (4) studies not published in English.
Data extraction and quality assessment
The data we extracted from each study included the principal author, year of publishing, number of patients involved, the method of gene transfer, category of patient, type of disease, type of chemotherapy regime for lymphodepletion, costimulatory domains, time of T cell culture, infused CAR-T cell doses, response outcome and toxicity. Patients whose death was unrelated to the malignancies and lost follow-up were not included for analysis. The percentage of patients who attained partial and complete remission was used to calculate the rate of response.
For detailed analysis, lymphodepletion was assessed by “No” and “Yes”; category of patient was assessed by “Paediatric” and “Adult”; T cell culture time was assessed by “More than 14 days” and “Less than 14 days”; Total infused T cell was analyzed by “cells more than 107” and “cells less than 107”.
Statistical analysis
Of the studies examined, the total estimation of response rate and toxicity was evaluated, using the R software (version 3.5.1) and the estimation was done on the basis of a random-effects model, in which the inter-study variability was evaluated using the Der-Simonian Laird estimator. The outcomes of the overall response rate and toxicity for all the included studies were presented using forest plots. The proportion of response rate from each study was presented with exact binomial 95% confidence intervals (CIs). If heterogeneity was not present (I2 <50%), a fixed effect model was applied for analysis; otherwise, a random effect model was adopted. Funnel plots and Egger’s rank correlation test were used to assess potential publication bias. Possible publication bias is said to occur when there is a deficiency in the base of the funnel with asymmetry. We performed subgroup analysis to discover possible factors connected with efficacy and response rate. Data was transferred from an Excel spreadsheet to SPSS version 21.0 for survival analysis.
Results
A total of 320 patients with 98 being paediatric and 222 being adult from 14 clinical trials were involved in our study analysis (Figure 1). 77 were lymphoma patients, 190 ALL patients and 53 chronic lymphocytic leukaemia (CLL) patients.
 | Figure 1 Flow diagram of the selection process. |
Patient characteristics and treatment procedure
Patient characteristics were included in Tables 1 and 2.4,13–23 All patients received autologous CD19 CAR-T cells. Three patients were given CAR-T of the third-generation with the costimulatory domains CD137 and CD28. Lentivirus and gammaretrovirus were used to introduce the constructed CAR into the T cells. One hundred and ninety-one (191) patients received chemotherapy regimen for lymphodepletion. The number of CAR-T cell infused ranged between 2×105–11×108.
 | Table 1 Patients Characteristics |
 | Table 2 Response rate subgroup analyses |
Response rate and toxicity
The evaluated response rate and the 95% confidence interval (CI) from the various studies are shown in Figure 2. The total assessment of response rate from the 14 studies included in our analysis was 71.88% (95% CI: 61.34–80.46%) with substantial heterogeneity observed (I2 =65%, X2 =37.42, p<0.01). Subgroup analyses of response rate were evaluated, and the outcomes have been presented in (Table 3). We discovered that lymphodepletion patients arm group had a greater response rate (77%, 95%CI: 67–83%; p-value =0.001) in comparison to the other patients who did not (66%, 95%CI: 41–83%). The overall estimate of CRS toxicity in our analysis was 60.15% (95% CI: 42.87–75.22%) with a considerable heterogeneity detected (I2= 83%, X2 =77.85, p<0.01). Figure 3 shows the overall estimated CRS toxicity and the 95% CI from the various studies.
 | Table 3 Response rate subgroup analyses |
 | Figure 2 Forest plot for response rate and 95% CI based on individual study and the entire study. |
 | Figure 3 Forest plot for CRS toxicity and 95% CI based on individual study and the entire study. |
Patient survival outcome
The 1-year and 6-month progression-free survival (PFS) for 163 patients were 38% and 82% respectively. The mean interval of PFS was 10 months (95% CI: 5.9–13.9), and the median interval of PFS was 7 months (95% CI: 0.16–13.8), shown in (Figure 4). The overall survival (OS) for 6 months was 65%, and the median interval of OS was 7.8 months (95% CI: 0.60–15.0). (Figure 5)
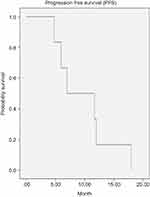 | Figure 4 Progression-free survival (PFS) curve. |
 | Figure 5 Overall survival (OS) curve. |
Heterogeneity sources
The proof of substantial bias in publication (p=0.0167) was detected by the Egger’s regression asymmetry test. Figure 6 shows the funnel plot of the response rate for publication bias. The funnel plot is used as a visualising aid in identifying systematic heterogeneity or bias. A symmetrical inverted funnel shape arises from a set of ‘ well - behaved ‘ data in which bias in publications did not occur. An asymmetric funnel shows an association between study accuracy and treatment effect estimate.
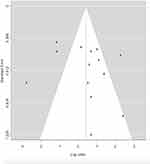 | Figure 6 Funnel plot of response rate for publication bias. |
Discussion
CAR-T cell immunotherapy has advanced rapidly and emerges as an effective way of treating patients with refractory or relapse B cell malignancies. The greatest challenge in CAR-T cell immunotherapy is how to increase and improve efficiency while reducing the toxicity related to the treatment. In our meta-analysis, the overall pooled toxicity was 60.15% (95%CI: 42.87–75.22%). CRS and on target/off-tumour effect are the significant toxicities associated with CART cell therapy. CRS is an inflammatory response syndrome caused by cytokine secretion in light of CAR-T cells activation. Three out of the 14 studies included in our meta-analysis reported grade 3 and 4 CRS incidence and severity, indicating a well-tolerated infusion of CAR-T cells in the other studies. The high toxicity in the three studies can be attributed to an increase in inflammatory cytokine. Corticosteroids and tocilizumab can be used to manage these adverse effects such as hypotension, fever, and rigours associated with CRS which mostly emerge within the first 24 hrs after CAR-T cells infusion. Two (2) studies again reported B cell aplasia. B cell aplasia is an on-target off tumour effect due to response in normal cells with the CAR-targeted antigen.9 To manage on target off tumour side effects like B cell aplasia, inducible caspase 9, a suicide gene has been integrated into CAR construction to regulate CAR-T cell persistence.23
The total pooled response rate was 71.88%(95%CI: 61.34–80.46%) higher than previous studies from Hou et al and Zhang et al. According to reports, first, second, and third generations chimeric antigen receptors (CARs) were present. Reported studies suggested that CARs of the second generation with an activation domain facilitated quick amplification, activation and the continuance of T cells in comparison to CARs of the first generation.24 More research is needed to verify the efficacy of the second generation. The third generation was not evaluated because of limited data. It remains to be explained whether chimeric antigen receptors (CARs) are better in the third generation than CARs in the second generation. The costimulatory CAR-T domains are normally used with CD137 or CD28. Which costimulatory domain has improved efficiency remains a mystery. No subgroup analysis was done between these two types of the costimulatory domain in our studies; however, some studies have shown that CD137 increased T cell expansion and persistence compared to CD2825,26 because CD137 lacks maturity and CD137 with CARs present may escalate acute toxicity and the continuance of the infused T cells. There was no test to juxtapose the effectiveness of the costimulatory signal, so both clinical and basic research are needed.
No common consensus has been reached among various researchers whether a patient should receive lymphodepletion or not. Lymphodepletion is the depletion of the receipt lymphocytes before infusing CAR-T cells. Lymphodepleting chemotherapy may improve CAR-T cell responses by getting rid of regulatory T cells, removing other immune cells which could challenge for homeostatic cytokines, and improving antigen - presenting cell activation. Before the T cell infusion, Lymphodepletion was administrated in the vast majority of the preliminaries. Our studies demonstrated that a higher and better response rate for patients who received lymphodepletion administering before T cell infusion than those who did not receive it. Furthermore, better prognosis in terms of survival analysis was also achieved in the lymphodepletion group of patients with a significant p-value.
These results indicate a correlation between lymphodepletion and better clinical outcomes; however, we did not conduct any analysis to know which type of B cell malignancies had a better response when lymphodepletion chemotherapy is administered.
Cytokines have been recognised to be useful in the expansion of T cells. The previous report showed that IL-2 promoted the expansion of T cells which affected the efficacy.27–30 We observed a similar result in our studies correlating with previous studies, but more studies are needed to clarify whether administering IL-2 to T cells or patient or not has better efficacy.
A viral vector or electroporation is the means of transducing constructed CAR to T cells. Viral transduction technique has greater transduction efficiency in comparison to the electroporation technique but also increases the chances of viral insertional oncogenesis. Viral transduction was employed by all the studies included in our analysis. More trials are needed to evaluate gene transfer efficiency between gamma retrovirus transduction and lentivirus transduction. The strength of our research lies in its sample size and also it is the first systematic review and meta-analysis which focuses on only autologous CD19 CAR-T cell therapy. Nevertheless, there are some limitations to our study. Firstly, the articles included were not entirely prospective studies. Secondly, no subgroup analysis was conducted between different ethnicities and ages.
Conclusion
In conclusion, the results of our studies demonstrated a more significant clinical response rate of autologous CD19 CAR-T cells in hematological malignancies. We again demonstrated that lymphodepletion regimen is associated with a better clinical response rate. We hope that knowledge from retrospective studies combined with advanced technology would improve the clinical efficacy of autologous CD19 CAR-T cells in treating refractory or relapse B cell malignancies.
Availability of materials
The data analysed for the current study is available with the corresponding author and can be released on reasonable request.
Acknowledgments
This study was partially supported by the National Natural Science Foundation of China (No. 81471589, No. 81273259), the Health Bureau of Henan Province, P.R. China (No. 201201005) and the foundation and frontier research grant of Henan provincial science and technology bureau, People’s Republic of China (No.112300410027, No. 132102310120).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Maher J, Brentjens RJ, Gunset G, Riviere I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta/CD28 receptor. Nat Biotechnol. 2002;20(1):70–75. doi:10.1038/nbt0102-70
2. Imai C, Mihara K, Andreansky M, et al. Chimeric receptors with 4-1BB signalling capacity provoke potent cytotoxicity against acute lymphoblastic leukaemia. Leukaemia. 2004;18(4):676–684. doi:10.1038/sj.leu.2403302
3. Rich R, Fleisher T, Shearer W, Schroeder H, Frew. A, Wevand. C. Clinical Immunology. [Miejsce nieznane]: Elsevier; 2018.
4. Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. doi:10.1056/NEJMoa1407222
5. Cruz CR, Micklethwaite KP, Savoldo B, et al. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a phase 1 study. Blood. 2013;122(17):2965–2973. doi:10.1182/blood-2013-06-506741
6. Wang Y, Zhang WY, Han QW, et al. Effective response and delayed toxicities of refractory advanced diffuse large B-cell lymphoma treated by CD20-directed chimeric antigen receptor-modified T cells. Clinical Immunology. 2014;155(2):160–175.
7. Porter DL, Levine BL, Kalos M, et al. Chimeric antigen receptor- modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi:10.1056/NEJMoa0910383
8. Yu S, Li A, Liu Q, et al. Chimeric antigen receptor T cells: a novel therapy for solid tumors. J Hematol Oncol. 2017;10(1):78.
9. Zhang T, Cao L, Xie J, et al. Efficiency of CD19 chimeric antigen receptor-modified T cells for treatment of B cell malignancies in phase I clinical trials: a meta-analysis. Oncotarget. 2015;6:33961–33971.
10. Hui Zhou, Yuling Luo, Sha Zhu et al. The efficacy and safety of anti-CD19/20 chimeric antigen receptor – T cells immunotherapy in relapsed r refractory B-cell malignancies: a meta-analysis. BMC Cancer. 2018;18:929. doi:10.1186/s12885-018-4242-8
11. Anwer F, Shaukat AA, Zahid U, et al. Donor origin CAR T cells: graft versus malignancy effect without GVHD, a systematic review. Immunotherapy. 2017;9:123–130. doi:10.2217/imt-2017-0004
12. Holzinger A, Barden M, Abken H. The growing world of CAR T cell trials: a systematic review. Cancer Immunol Immunother. 2016;65:1433–1450. doi:10.1007/s00262-016-1895-5
13. Turtle CJ, Hanafi L-A, Berger C, et al. Addition of fludarabine to cyclophosphamide lymphodepletion improves in vivo expansion of CD19 chimeric antigen receptor-modified T cells and clinical outcome in adults with B cell acute lymphoblastic leukemia. Blood. 2015;126(23):3773.
14. Porter DL, Hwang WT, Frey NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7(303):303ra139. doi:10.1126/scitranslmed.aad3106
15. Porter DL, Frey NV, Melenhorst JJ, et al. Randomized, phase II dose optimization study of chimeric antigen receptor modified T cells directed against CD19 (CTL019) in patients with relapsed, refractory CLL. Blood. 2014;124(21):1982.
16. Schuster SJ, Svoboda J, Nasta SD, et al. Sustained remissions following chimeric antigen receptor modified T cells directed against CD19 (CTL019) in patients with relapsed or refractory CD19+ Lymphomas. Blood. 2015;126(23):183.
17. Grupp SA, Maude SL, Shaw PA, et al. Durable remissions in children with relapsed/refractory ALL treated with T cells engineered with a CD19-targeted chimeric antigen receptor (CTL019). Blood. 2015;126(23):681.
18. Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517–528. doi:10.1016/S0140-6736(14)61403-3
19. Kochenderfer JN, Dudley ME, Feldman SA, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119(12):2709–2720. doi:10.1182/blood-2011-10-384388
20. Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33(6):540–549. doi:10.1200/JCO.2014.56.2025
21. Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5(177):177ra138. doi:10.1126/scitranslmed.3005930
22. Savoldo B, Ramos CA, Liu E, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011;121(5):1822–1826. doi:10.1172/JCI46110
23. Kalos M, Levine BL, Porter DL, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukaemia. Sci Transl Med. 2011;3(95):95ra73. doi:10.1126/scitranslmed.3002842
24. Gargett T, Brown MP. The inducible caspase-9 suicide gene system as a “safety switch” to limit on-target, off-tumor toxicities of chimeric antigen receptor T cells. Front Pharmacol. 2014;5:235. doi:10.3389/fphar.2014.00235
25. Forman SJ, Rowe JM. The myth of the second remission of acute leukaemia in the adult. Blood. 2013;121:1077–1082. doi:10.1182/blood-2012-08-234492
26. Milone MC, Fish JD, Carpenito C, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther. 2009;17(8):1453–1464. doi:10.1038/mt.2009.83
27. Song DG, Ye Q, Carpenito C, et al. In vivo persistence, tumor localization, and antitumor activity of CAR-engineered T cells is enhanced by costimulatory signalling through CD137 (4-1BB). Cancer Res. 2011;71(13):4617–4627. doi:10.1158/0008-5472.CAN-11-0422
28. Caserta S, Alessi P, Basso V, Mondino A. IL-7 is superior to IL-2 for ex vivo expansion of tumour-specific CD4(+) T cells. Eur J Immunol. 2010;40(2):470–479. doi:10.1002/eji.200939801
29. Xu XJ, Zhao HZ, Tang YM. Efficacy and safety of adoptive immunotherapy using anti-CD19 chimeric antigen receptor transduced T-cells: a systematic review of phase I clinical trials. Leuk Lymphoma. 2013;54(2):255–260. doi:10.3109/10428194.2013.796052
30. Zhu Y, Tan Y, Ou R, et al. Anti-CD19 chimeric antigen receptor-modified T cells for B-cell malignancies: a systematic review of efficacy and safety in clinical trials. Eur J Haematol. 2016;96(4):389–396. doi:10.1111/ejh.12602
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
