Back to Journals » Clinical Ophthalmology » Volume 10
Survey of intravitreal injection techniques among retina specialists in Israel
Authors Segal O, Segal-Trivitz Y, Nemet AY , Geffen N, Nesher R, Mimouni M
Received 15 September 2015
Accepted for publication 17 November 2015
Published 14 June 2016 Volume 2016:10 Pages 1111—1116
DOI https://doi.org/10.2147/OPTH.S96452
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Ori Segal,1,2 Yael Segal-Trivitz,1,3 Arie Y Nemet,1,2 Noa Geffen,1,2 Ronit Nesher,1,2 Michael Mimouni4
1Department of Ophthalmology, Meir Medical Center, Kfar Saba, 2The Sackler School of Medicine, Tel Aviv University, Tel Aviv, 3Department of Psychiatry, Geha Psychiatric Hospital, Petah Tikva, 4Department of Ophthalmology, Rambam Health Care Campus, Haifa, Israel
Purpose: The purpose of this study was to describe antivascular endothelial growth factor intravitreal injection techniques of retinal specialists in order to establish a cornerstone for future practice guidelines.
Methods: All members of the Israeli Retina Society were contacted by email to complete an anonymous, 19-question, Internet-based survey regarding their intravitreal injection techniques.
Results: Overall, 66% (52/79) completed the survey. Most (98%) do not instruct patients to discontinue anticoagulant therapy and 92% prescribe treatment for patients in the waiting room. Three quarters wear sterile gloves and prepare the patient in the supine position. A majority (71%) use sterile surgical draping. All respondents apply topical analgesics and a majority (69%) measure the distance from the limbus to the injection site. A minority (21%) displace the conjunctiva prior to injection. A majority of the survey participants use a 30-gauge needle and the most common quadrant for injection is superotemporal (33%). Less than half routinely assess postinjection optic nerve perfusion (44%). A majority (92%) apply prophylactic antibiotics immediately after the injection.
Conclusion: The majority of retina specialists perform intravitreal injections similarly. However, a relatively large minority performs this procedure differently. Due to the extremely low percentage of complications, it seems as though such differences do not increase the risk. However, more evidence-based medicine, a cornerstone for practice guidelines, is required in order to identify the intravitreal injection techniques that combine safety and efficacy while causing as little discomfort to the patients as possible.
Keywords: retina, intravitreal, injection, practices, techniques, patterns
Introduction
Intravitreal injections have become a widely accepted treatment modality for several ophthalmic diseases.1 In recent years, new indications for the recurring use of antivascular endothelial growth factor (anti-VEGF) intravitreal injections have risen.2–5 Despite the potential risks of intravitreal injections,6 there is little agreement among clinicians and researchers regarding acceptable injection techniques.7,8
Attempts have been made to draft recommendations and guidelines for clinicians,9–12 a difficult task considering the scarce amount of high-quality evidence comparing different peri-injection practices.7
The purpose of this study was to describe the personal anti-VEGF intravitreal injection techniques of retinal specialists in Israel.
Materials and methods
All members of the Israeli Retina Society were contacted by email to complete an anonymous, 19-question, Internet-based survey regarding the intravitreal injection techniques they use when injecting anti-VEGF materials (Supplementary material). In November 2013, there were 79 Israeli Retina Society members. All of the physicians listed a unique email address. Therefore, 79 surveys were emailed on November 7, 2013. Three reminder emails were sent to participants in order to maximize the response rate. Study data were collected using an online Google Form and automatically saved to a Google Spreadsheet as previously described.13 Google Inc. (Mountain View, CA, USA) has several web-based services that support data capture for research studies, providing both an intuitive interface for data entry and automated export procedures for data collection.13 All the results were tabulated on November 28, 2013. The ethic committee approval was unnecessary for this study as the questionnaire was conducted via emails.
Results
By November 28, 2013, 52 of 79 retinal specialists (66%) responded to the survey. Ninety percent (47/52) of the respondents perform intravitreal injections on a regular basis. Six of the 52 (12%) work in operating room settings, while the rest work in an outpatient retina clinic.
Preinjection practices
Ninety-eight percent (51/52) of the respondents do not instruct patients to discontinue anticoagulant or antiplatelet therapy. Preinjective treatments prescribed to the patient in the waiting room are depicted in Table 1. Briefly, 92% (48/52) prescribe at least one treatment for patients while they are in the waiting room, with topical anesthetics and mydriatics being the most common treatments. Seventy-nine percent (41/52) of the respondents wear gloves. Among those who wear gloves, 95% (39/41) wear sterile gloves and 5% (2/41) wear clean gloves. Seventy-five percent (39/52) prepare the patient in the supine position, while the rest do so in a sitting position. Table 2 depicts the characteristics of the operating field in which the injections are performed. Briefly, all but one use a speculum and 71% (37/52) use sterile surgical draping.
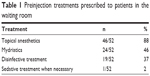  | Table 1 Preinjection treatments prescribed to patients in the waiting room |
  | Table 2 Characteristics of the operating field in which injections are performed |
Injection practices
All the survey respondents apply topical analgesics in one of the following forms: gel only (8%, 4/52), drops only (25%, 13/52), or both (67%, 35/52). Two respondents (4%) verify that the analgesic effect is sufficient before proceeding to the injection. Sixty-nine percent (36/52) measure the distance from the limbus to the injection site. Among those who measure, 56% use calipers (20/36) and 44% use another device (16/36) such as the plastic cover of a 30-gauge needle (4 mm diameter). Twenty-one percent of the respondents displace the conjunctiva prior to injection (11/52). A majority of the survey participants use a 30-gauge needle for intravitreal injections of anti-VEGF (90%, 47/52); the rest use either a 27-gauge needle (4%, 2/52) or a 31-gauge needle (4%, 2/52). The most common quadrant for injection is the superotemporal (33%, 17/52), followed by the inferotemporal (17%, 9/52), superonasal (6%, 3/52), and inferonasal (4%, 2/52), while the rest (40%, 21/52) stated that they do not prefer a specific quadrant.
Postinjection practices
Less than half of the survey respondents (44%, 23/52) routinely assess postinjection optic nerve perfusion. Among those who assess optic nerve perfusion, 48% (11/23) perform a gross visual acuity examination (finger count or hand motion assessment), 30% (7/23) visualize the optic nerve, and 26% (6/23) measure the intraocular pressure. The majority (73%, 38/52) do not perform an anterior chamber tap in order to prevent a rise in intraocular pressure (IOP), while the rest perform a tap in cases of end-stage glaucoma. Table 3 depicts the postinjection treatments prescribed immediately after the injection. Briefly, 92% (48/52) of the retinal specialists apply some form of prophylactic antibiotics immediately after the injection. A majority (77%, 40/52) apply prophylactic topical antibiotic drops immediately afterward. The majority of physicians do not prescribe prophylactic drops for the patients to continue putting in their eyes at home after receiving the injection. Half of the survey respondents (56%, 26/52) perform bilateral simultaneous intravitreal injections. Of those who do not perform bilateral injections, one half stated that they avoid doing so only in cases of steroidal injections as the chances of endophthalmitis are increased, while the other half stated that the minute chance of bilateral endophthalmitis is unacceptable.
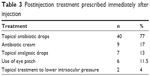  | Table 3 Postinjection treatment prescribed immediately after injection |
Discussion
In this study, we have shown the wide variety of personal intravitreal injection techniques of retina specialists based on a national survey.
The amount of intravitreal injections performed has increased to a point of being second only to cataract surgery as the most common treatment in most tertiary ophthalmic centers. It has been speculated that intravitreal injections of anti-VEGF will become the most common intraocular procedure worldwide.14 Still, despite the attempts made, well-defined guidelines and recommendations based on high-quality evidence have yet to be formulated.9–13 We herein discuss the different practices of retina specialists during the different phases of intravitreal injection (preinjection, injection, and postinjection) and compare the findings to previous reports from other countries.
Preinjection practices
In this study, all but one of the specialists instructed patients to continue anticoagulant/antiplatelet therapy. Indeed, the risk of hemorrhagic complications following intravitreal injections in such patients is extremely low, while the risk of stopping such therapy carries an increased thromboembolic risk.15 However, different diseases may have different chances of hemorrhagic complications, and therefore, each case should be treated individually. In terms of working in a sterile environment, a majority of the survey participants wear sterile gloves (39/52) for intravitreal injections. In comparison, 33% of medical retina specialists in USA7 reported wearing sterile gloves, while 90% did so in UK.8 The syringe containing bevacizumab is not sterile, as the emphasis is on the tip of the instrument which must remain sterile. Therefore, one may argue against the necessity of sterile gloves. However, one cannot entirely rule out the possibility that injecting without sterile gloves may lead to an increase in the number of infective complications. Preinjection treatment with topical antibiotics or povidone-iodine was used by approximately one-third of the survey respondents (19/52), similar to the survey conducted in USA.7 As repeated use of topical antibiotics increases the antibiotic resistance of the ocular surface flora,16 the habit of routinely instilling antibiotics should be approached with caution.
Injection practices
Similar to previous reports,7,8 nearly two-third of the participants measure the distance of the site of the injection from the limbus (36/52), with a majority of them using calipers (20/36). Previous studies have identified that sclerotomies anterior to 3.5 mm from the limbus may damage the crystalline lens,17 while those posterior to 4.5 mm may damage the peripheral retina inducing a retinal tear and consecutive development of retinal detachment.18 Therefore, precise measurement of the distance of the injection from the limbus is warranted. Only a minority of the participants (21%) reported displacing the conjunctiva prior to injection and a majority used a 30-gauge needle (47/52), a finding that is consistent with those from USA and UK.7,8 Reflux and conjunctival bleb formation is often noted at the intravitreal injection site19 and is a cause for concern among physicians because of the potential loss of drug.20 This has led to several studies evaluating factors that may influence the amount of reflux from intravitreal injections.21–24 Though vitreous prolapse may be observed after intravitreal injection with both 27- and 30-gauge needles,25 27-gauge needles require twice the force to penetrate the sclera than a 30/31-gauge needle,26 potentially leading to more discomfort during intravitreal injections.27 Though most participants in this study stated that they do not prefer a specific quadrant (21/52), the superotemporal one was the most frequent among those who did (17/52). This is interesting, considering a recent report showed that injections in the inferior half may be associated with less pain.28
Postinjection practices
Less than half of the survey respondents routinely assessed optic nerve perfusion following intravitreal injections (as opposed to three-quarters of their USA counterparts).7 A short-term increase in IOP following intravitreal injections has been well-documented,29 leading some to recommend routine assessment for ischemic optic nerve damage.9 Despite the fact that majority of the respondents did not use prophylactic topical antibiotics in the preinjection phase, an overwhelming majority (92%) did so in the postinjection phase. Despite a recent study reporting higher rates of endophthalmitis when immediate postinjection antibiotics were not instilled,30 others have shown that the addition of preinjection or postinjection topical antibiotics to povidone-iodine antisepsis offers little to no benefit31–33 while leading to the development of resistant strains of bacteria.34
This study had several limitations. First, the response rate was 66%, and therefore, response bias may have played a role in this study. Second, this study surveyed Israeli retina specialists, and therefore, its findings may differ significantly from those for the retina specialists worldwide. Due to good communication between practicing retina specialists in Israel, an impressive amount of versatility exists when performing this relatively “simple” procedure. This may imply a similar versatility in the way specialists from other countries perform this procedure as well as more complex ones. One must take into consideration that not all injection techniques are suitable for every surgeon, and therefore, diversity may, in fact, be required. These differences in techniques must be taken into consideration when performing retrospective studies in order to study the efficacy and safety of intravitreal injections. Finally, more evidence-based medicine, a cornerstone for practice guidelines, is required in order to identify the intravitreal injection techniques that combine safety and efficacy while causing as little discomfort to the patients as possible.
Disclosure
The authors report no conflicts of interest in this work.
References
Fagan XJ, Al-Qureshi S. Intravitreal injections: a review of the evidence for best practice. Clin Experiment Ophthalmol. 2013;41(5):500–507. | ||
Azad R, Chandra P. Intravitreal bevacizumab in aggressive posterior retinopathy of prematurity. Indian J Ophthalmol. 2007;55(4):319; author reply 320. | ||
Nepomuceno AB, Takaki E, Paes de Almeida FP, et al. A prospective randomized trial of intravitreal bevacizumab versus ranibizumab for the management of diabetic macular edema. Am J Ophthalmol. 2013;156(3):502–510. e502. | ||
Tonello M, Costa RA, Almeida FP, Barbosa JC, Scott IU, Jorge R. Panretinal photocoagulation versus PRP plus intravitreal bevacizumab for high-risk proliferative diabetic retinopathy (IBeHi study). Acta Ophthalmol. 2008;86(4):385–389. | ||
Yazdani S, Hendi K, Pakravan M, Mahdavi M, Yaseri M. Intravitreal bevacizumab for neovascular glaucoma: a randomized controlled trial. J Glaucoma. 2009;18(8):632–637. | ||
Jager RD, Aiello LP, Patel SC, Cunningham ET Jr. Risks of intravitreous injection: a comprehensive review. Retina. 2004;24(5):676–698. | ||
Green-Simms AE, Ekdawi NS, Bakri SJ. Survey of intravitreal injection techniques among retinal specialists in the United States. Am J Ophthalmol. 2011;151(2):329–332. | ||
Anijeet DR, Hanson RJ, Bhagey J, Bates RA. National survey of the technique of intravitreal triamcinolone injection in the United Kingdom. Eye (Lond). 2007;21(4):480–486. | ||
Aiello LP, Brucker AJ, Chang S, et al. Evolving guidelines for intravitreous injections. Retina. 2004;24(5 Suppl):S3–S19. | ||
Korobelnik JF, Weber M, Cohen SY, groupe dE. [Recommendations for carrying out intravitreal injections]. J Fr Ophtalmol. 2009;32(4):288–289. French. | ||
Gomez-Ulla F, Basauri E, Arias L, Martinez-Sanz F. [Management of intravitreal injections]. Arch Soc Esp Oftalmol. 2009;84(8):377–388. French. | ||
Avery RL, Bakri SJ, Blumenkranz MS, et al. Intravitreal injection technique and monitoring: updated guidelines of an expert panel. Retina. 2014;34 Suppl 12:S1–S18. | ||
Rayhan RU, Zheng Y, Uddin E, Timbol C, Adewuyi O, Baraniuk JN. Administer and collect medical questionnaires with Google documents: a simple, safe, and free system. Appl Med Inform. 2013;33(3):12–21. | ||
Williams GA. Intravitreal injections: health policy implications. Rev Ophthalmol. 2014;21:62–64. | ||
Mason JO, 3rd, Frederick PA, Neimkin MG, et al. Incidence of hemorrhagic complications after intravitreal bevacizumab (avastin) or ranibizumab (lucentis) injections on systemically anticoagulated patients. Retina. 2010;30(9):1386–1389. | ||
Yin VT, Weisbrod DJ, Eng KT, et al. Antibiotic resistance of ocular surface flora with repeated use of a topical antibiotic after intravitreal injection. JAMA Ophthalmol. 2013;131(4):456–461. | ||
Smiddy WE, Michels RG, Green WR. Lens and peripheral retinal relationships during vitrectomy. Retina. 1991;11(2):199–203. | ||
Wimpissinger B, Binder S. Entry-site-related retinal detachment after pars plana vitrectomy. Acta Ophthalmol Scand. 2007;85(7):782–785. | ||
Benz MS, Albini TA, Holz ER, et al. Short-term course of intraocular pressure after intravitreal injection of triamcinolone acetonide. Ophthalmology. 2006;113(7):1174–1178. | ||
Brodie FL, Ruggiero J, Ghodasra DH, et al. A novel method for the measurement of reflux from intravitreal injections: data from 20 porcine eyes. Current Eye Res. 2014;39(7):752–757. | ||
Cortez RT, Ramirez G, Collet L, Thakuria P, Giuliari GP. Intravitreous bevacizumab injection: an experimental study in New Zealand white rabbits. Arch Ophthalmol. 2010;128(7):884–887. | ||
Rodrigues EB, Grumann A Jr, Penha FM, et al. Effect of needle type and injection technique on pain level and vitreal reflux in intravitreal injection. J Ocul Pharmacol Ther. 2011;27(2):197–203. | ||
Turgut B, Demir T, Celiker U. The effects of injection site on the reflux following intravitreal injections. J Clin Med Res. 2009;1(5):280–284. | ||
Rodrigues EB, Meyer CH, Grumann A Jr, Shiroma H, Aguni JS, Farah ME. Tunneled scleral incision to prevent vitreal reflux after intravitreal injection. Am J Ophthalmol. 2007;143(6):1035–1037. | ||
Chen SD, Mohammed Q, Bowling B, Patel CK. Vitreous wick syndrome – a potential cause of endophthalmitis after intravitreal injection of triamcinolone through the pars plana. Am J Ophthalmol. 2004;137(6):1159–1160; author reply 1160–1161. | ||
Pulido JS, Zobitz ME, An KN. Scleral penetration force requirements for commonly used intravitreal needles. Eye (Lond). 2007;21(9):1210–1211. | ||
Pulido JS, Pulido CM, Bakri SJ, McCannel CA, Cameron JD. The use of 31-gauge needles and syringes for intraocular injections. Eye (Lond). 2007;21(6):829–830. | ||
Moisseiev E, Regenbogen M, Bartfeld Y, Barak A. Evaluation of pain in intravitreal bevacizumab injections. Curr Eye Res. 2012;37(9):813–817. | ||
Kim JE, Mantravadi AV, Hur EY, Covert DJ. Short-term intraocular pressure changes immediately after intravitreal injections of anti-vascular endothelial growth factor agents. Am J Ophthalmol. 2008;146(6):930–934. e931. | ||
Lyall DA, Tey A, Foot B, et al. Post-intravitreal anti-VEGF endophthalmitis in the United Kingdom: incidence, features, risk factors, and outcomes. Eye (Lond). 2012;26(12):1517–1526. | ||
Bhatt SS, Stepien KE, Joshi K. Prophylactic antibiotic use after intravitreal injection: effect on endophthalmitis rate. Retina. 2011;31(10):2032–2036. | ||
Moss JM, Sanislo SR, Ta CN. A prospective randomized evaluation of topical gatifloxacin on conjunctival flora in patients undergoing intravitreal injections. Ophthalmology. 2009;116(8):1498–1501. | ||
Cheung CS, Wong AW, Lui A, Kertes PJ, Devenyi RG, Lam WC. Incidence of endophthalmitis and use of antibiotic prophylaxis after intravitreal injections. Ophthalmology. 2012;119(8):1609–1614. | ||
Kim SJ, Toma HS. Antimicrobial resistance and ophthalmic antibiotics: 1-year results of a longitudinal controlled study of patients undergoing intravitreal injections. Arch Ophthalmol. 2011;129(9):1180–1188. |
Supplementary material
Questionnaire regarding intravitreal injection of anti-vascular endothelial growth factor (VEGF)
- Do you regularly perform intravitreal injections of anti-VEGF?
- No
- Yes
- Do you provide instructions to the patient before they arrive to the clinic for the injection?
- No
- Yes
- Does the patient receive any treatment while waiting to receive the injection?
- No
- Topical anesthetics
- Topical mydriasis
- Where do you perform injections?
- Office-based clinic
- Operating room
- How do you position the patient while injecting?
- Sitting
- Supine
- How do you prepare a sterile field before the injection?
- Speculum – yes/no
- Sterile draping – yes/no
- Hair cover for surgeon – yes/no
- Mouth cover for surgeon – yes/no
- Do you use gloves when performing injections?
- No
- Regular gloves
- Sterile surgical gloves
- Which anesthetic do you add before performing the injection?
- Topical drops
- Topical gel
- Both
- None
- Do you perform a test for sufficient anesthesia before the injection?
- No
- Yes
- How do you measure and mark the distance from the limbus before the injection?
- None
- Caliper
- Another method
- At which site do you perform the injection itself?
- No specific site
- Inferior temporal
- Inferior nasal
- Superior temporal
- Superior nasal
- What is the gauge of the needle you use?
- Do you displace the conjunctiva before inserting the needle?
- No
- Yes
- How do you verify adequate optic nerve perfusion after the injection?
- I do not
- Check tonus of the globe
- Check visual acuity for finger count
- Pulsation of the optic nerve
- Do you perform paracentesis of the anterior chamber in order to prevent a rise in intraocular pressure?
- I do not as the danger outweighs the benefits
- I do in cases of end-stage glaucoma
- Do you perform bilateral injections at the same visit?
- No
- Yes
- What treatment do you provide immediately after the injection itself?
- Topical antibiotic drops – yes/no
- Topical antibiotic ointment – yes/no
- Topical anesthetics – yes/no
- Dressing eye with bandage – yes/no
- Do you prescribe topical antibiotics for use at home afterwards?
- No
- Yes
- Do you have any general comments or ideas that you would like to suggest?
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
