Back to Journals » Clinical Ophthalmology » Volume 16
Surgical Outcomes of Glaucoma Drainage Device Implantation in Refractory Glaucoma Patients in Thailand
Authors Rojananuangnit K , Jiaranaisilawong P, Rattanaphaithun O, Sathim W
Received 19 October 2022
Accepted for publication 6 December 2022
Published 14 December 2022 Volume 2022:16 Pages 4163—4178
DOI https://doi.org/10.2147/OPTH.S393730
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Kulawan Rojananuangnit,1,2 Prangkhwan Jiaranaisilawong,2 Onvipa Rattanaphaithun,1 Wanwisa Sathim2
1Glaucoma Unit, Ophthalmology Department, Mettapracharak (Wat Rai Khing) Hospital, Nakhon Pathom, Thailand; 2Research and Health Technology Assessment Center, Mettapracharak (Wat Rai Khing) Hospital, Nakhon Pathom, Thailand
Correspondence: Kulawan Rojananuangnit, 52 Moo 2 Rai Khing District Sampran, Nakhon Pathom, 73210, Thailand, Email [email protected]
Purpose: To study the surgical outcomes of glaucoma drainage device (GDD) implantation in refractory glaucoma patients.
Patients and Methods: Retrospective chart review of glaucoma patients undergoing GDD implantation, Ahmed glaucoma valve (AGV), Baerveldt glaucoma implants (BGI), and Aurolab aqueous drainage implantation (AADI) from January 2012 to June 2021. Glaucoma patients were classified into two groups: primary glaucoma including: primary open angle glaucoma (POAG), primary angle closure glaucoma (PACG) and juvenile open angle glaucoma (JOAG) and secondary glaucoma including: neovascular glaucoma (NVG), ocular surgery (vitreoretinal surgery, scleral buckling procedure, postoperative extra capsular cataract extraction, scleral fixation intraocular lens, penetrating keratoplasty), intraocular trauma, uveitis glaucoma, lens-induced glaucoma, pseudoexfoliation glaucoma (PXG), iridocorneal endothelial (ICE) syndromes and Axenfeld Rieger Syndrome. Surgical outcomes were studied.
Results: Primary glaucoma included 57 eyes from 49 patients. Secondary glaucoma included 87 eyes from 85 patients. The cumulative probability of complete or qualified success of refractory glaucoma patients at five years were 53.4% (95%CI: 38.4%, 66.3%). Higher success rate was discovered in primary glaucoma at 65.8% (95%CI: 38.4%, 83.3%) than 45.2% (95%CI: 26.9%, 61.9%) in secondary glaucoma group significantly with p=0.003. While the results among success rate, adverse events and complications was not different between types of GDD. Predictors for failure were neovascular glaucoma with unadjusted hazard ratio (HR) 3.62 (95%CI: 1.45, 9.04) with p=0.006, and lens-induced glaucoma with adjusted HR 4.19 (95%CI: 1.10, 15.86) with p=0.035 in multivariable analysis. Tube malposition and occlusion were the most frequent adverse events at 11.11%, corneal decompensation at 5.5%, hypotony at 2% in the nonvalved group, and endophthalmitis at 0.69%.
Conclusion: Surgical success in refractory primary glaucoma was superior to secondary glaucoma with no difference between nonvalved and valved GDD implantation. Lens-induced glaucoma was a strong predictor for failure in GDD implantation.
Keywords: glaucoma drainage device, primary glaucoma, refractory glaucoma, secondary glaucoma
Introduction
Glaucoma drainage device (GDD) implantation was considered as a surgical procedure in medically uncontrolled intraocular pressure glaucoma in certain complicated conditions, for example, failed trabeculectomy,1 postoperative vitreoretinal surgery,2 postoperative surgery that compromised conjunctiva, postoperative corneal transplantation,3 traumatic glaucoma, and refractory neovascular glaucoma.4 GDD implants were classified into valved GDD, the Ahmed glaucoma valve (AGV), nonvalved GDD, the Baerveldt® Glaucoma Implants (BGI), and the Aurolab aqueous drainage implant (AADI). Surgical outcomes in different types of device and the various etiologies of glaucoma were not similar.5–7 Most short and intermediate outcomes with valved and nonvalved GDDs were comparable in Asian and non-Asian eyes. Except postoperative bleb encapsulation with the AGV occurred more frequently in Asian eyes and the proper size of the BGI plate area for Asian eyes were reported.8–10 Previously in Thailand, GDD was reserved for refractory glaucoma cases such as multiple ocular surgery and failed trabeculectomy. The economic reason was the price of GDD that was only reimbursed in the civil servants’ medical benefits and self-paid schemes. Consequently, GDD implantation procedures were not widely performed in Thailand. Fortunately, the GDD was added to the universal health coverage health benefit device list in 2020 and was also covered in social security scheme reimbursement in 2022. This study provided surgical outcomes of GDD implantation in uncontrolled IOP, refractory glaucoma patients in both primary glaucoma and secondary glaucoma etiologies in terms of success and failure rates in different types of GDD, factors associated with failure, and adverse events and complications. These results would be of benefit in reconsidering the application of the GDD in glaucoma practice and correctly prognostic counseling to refractory glaucoma patients.
Material and Methods
This was a retrospective study which adhered to the tenets of the Declaration of Helsinki and was approved by the Mettapracharak (Wat Rai Khing) Research Ethics Committee in accordance with the International Conference on Harmonization of Good Clinical Practice (ICH-GCP) COA No. 008/2565. The requirement for consent had been waived by an ethics committee because all data acquired was kept anonymized. Medical records of all glaucoma patients undergoing GDD implantation from January 2012 to June 2021 were reviewed, and all privacy data that could reveal the identity of the patients including hospital number, name, and date of birth were masked and kept confidentially in compliance with the Declaration of Helsinki.
Demographic data were collected, for example, age, sex, underlying diseases, etiology, and type of glaucoma, previous medical, laser and surgical history, and type of glaucoma drainage devices. Baseline and follow-up data were gathered, such as LogMAR visual acuity, intraocular pressure (IOP), number of antiglaucoma medications, laser and surgical procedure postoperatively. Adverse events, complications, and further laser and surgical management were also recorded. Ocular parameters preoperatively and follow-up appointments were collected, for example, visual field test: mean deviation (MD), pattern standard deviation (PSD) and visual field index (VFI), optical coherence tomography (OCT) of the peripapillary retinal nerve fiber layer (RNFL) parameters, and vertical cup to disc ratio (VCDR). Following the retrospective review all data of patients were censored until they were lost to follow-up or died.
Glaucoma patients were categorized following the etiology and type of glaucoma into two2 groups; primary glaucoma including primary open angle glaucoma (POAG), primary angle closure glaucoma (PACG) and juvenile open angle glaucoma (JOAG) and secondary glaucoma including neovascular glaucoma (NVG), ocular surgery (vitreoretinal surgery, scleral buckling procedure, postoperative extra capsular cataract extraction, scleral fixation intraocular lens, penetrating keratoplasty), intraocular trauma, uveitis glaucoma, lens-induced glaucoma, pseudoexfoliation glaucoma (PXG) and other glaucoma (iridocorneal endothelial (ICE) syndromes and Axenfeld Rieger Syndrome). GDD was divided into two groups: valved GDD comprising the Ahmed glaucoma valve (AGV) (New World Medical, Inc., Rancho Cucamonga, CA) model FP7 with surface area of 184 mm2, and nonvalved GDD including Baerveldt® Glaucoma Implants (Abbott Medical Optics, Abbott Park, IL) model BG 103–250 with surface area of 250 mm2 and model BG 101–350 with surface area of 350 mm2, and the Aurolab aqueous drainage implant (AADI) (Aurolab, Madurai, India) with surface area of 350 mm2 and trimmed plate to downsize the surface area to 250 mm2.
Surgical outcomes were divided into three categories by the range of IOP postoperatively: complete success (IOP range 6–20 mmHg or IOP decrease from baseline by 30%), qualified success (IOP range 6–20 mmHg or IOP decrease from baseline by 30% with antiglaucoma medication), and failure (IOP below 6 mmHg or higher than 21 mmHg even with antiglaucoma medication and loss of light perception of vision).
Statistical Analysis
Demographic data and ocular characteristics were presented by descriptive statistics: continuous variables as the mean and standard deviation (SD), and categorical variables as frequency and percentage.
Chi-squared test and Fisher’s exact test for categorical data were performed to compare the categorical data while the unpaired t-test and Mann–Whitney U-test were used for continuous data to compare between primary glaucoma and secondary glaucoma groups and between different type of GDD. Success rates from primary and secondary glaucoma were analyzed by Kaplan–Meier Curve analysis and comparisons between groups with the log rank test. Factors associated with surgical failure were analyzed by Cox regression analysis both with univariable and multivariable analysis, and reported by hazard ratio. Factors associated with adverse effects and complications were analyzed by multiple logistic regression and were reported by odds ratio. A p-value less than 0.05 was considered statistically significant. All statistical analyses were performed with PASW Statistics (SPSS) 28.0 (SPSS Inc., Chicago, IL, USA).
Results
There were 144 eyes from 134 glaucoma patients included in this study, which were categorized into a primary glaucoma group (57 eyes from 49 patients including primary open angle glaucoma (POAG) with 41 eyes, primary angle closure glaucoma (PACG) with six eyes and juvenile open angle glaucoma (JOAG) with 10 eyes and a secondary glaucoma group (87 eyes from 85 patients, including neovascular glaucoma (NVG) with 46 eyes, ocular surgery (vitreoretinal surgery, scleral buckling procedure, postoperative extra capsular cataract extraction, scleral fixation intraocular lens, penetrating keratoplasty) with 14 eyes, intraocular trauma with 10 eyes, uveitis glaucoma with seven eyes, lens-induced glaucoma with six eyes, pseudoexfoliation glaucoma (PXG) with two eyes) and other glaucoma (iridocorneal endothelial (ICE) syndromes and Axenfeld Rieger Syndrome) with two eyes. The average age between groups was similar: the mean age was 55.6±14.2 years old in the primary glaucoma group and 52.2±52.8±14.2 in the secondary glaucoma group, p=0.277. Baseline characteristics between primary and secondary glaucoma groups were as follows: the primary glaucoma group had better mean visual acuity, 0.74±0.79 logMAR vs 1.63±0.82 logMAR, p<0.001. The secondary glaucoma group had higher preoperative mean IOP, 30.6±11.6 mmHg vs 22.9±7.4 mmHg, p<0.001. Visual field parameters were not different between groups and both groups were classified as advanced glaucoma staging following from mean deviation (MD) −18.7±11.9 dB in primary glaucoma and −20.9±10.7 db in secondary glaucoma while more advanced optic nerve damage, larger vertical cup to disc ratio (VCDR), and thinner OCT retinal nerve fiber layer thickness (RNFL) were observed in the primary glaucoma group (OCT RNFL: 64.6±13.0 vs 76.1±24.3 µm, p=0.044, VCDR: 0.87±0.13 vs 0.81±0.18, p=0.031). Diabetes mellitus as an underlying diseases was found in 39 patients (45.9%) in the secondary glaucoma group which was more than in the primary glaucoma group. Ocular underlying diseases causing secondary glaucoma were proliferative diabetic retinopathy in 34 eyes (39.1%), tractional retinal detachment in eight eyes (9.2%), rhegmatogenous retinal detachment in 13 eyes (14.9%), central retinal vein occlusion in 10 eyes (11.5%) and previous trauma in seven eyes (8.0%). Preoperative average antiglaucoma medication numbers between the two groups were not different, four types of medication, p=0.491. The primary glaucoma group had longer duration of treatment at an average of 48 months compared with 10 months in the secondary group, p<0.001. Patients in the primary glaucoma group had undergone trabeculectomy in 73.7% of cases compared with 34.5% of patients in the secondary glaucoma group, p<0.001. Pan retinal photocoagulation (PRP) and pars planar vitrectomy were performed more often in the secondary glaucoma group than in the primary glaucoma group, and were correlated with ocular diseases and glaucoma etiologies; for example, proliferative diabetic retinopathy, tractional retinal detachment, central retinal vein occlusion, neovascular glaucoma and postoperative intraocular surgery. The AGV were implanted more often in the secondary glaucoma group, at 66.7%, than in the primary glaucoma group, at 36.8%, p=0.014 (Table 1).
 |
Table 1 Baseline Characteristic and Demographic Data Between Primary and Secondary Glaucoma |
At the five-year follow-up, the cumulative probability of complete or qualified success in GDD implantation was 53.4% (95%CI: 38.4%, 66.3%) in total refractory glaucoma patients. Comparing between glaucoma etiology, primary glaucoma had significantly higher success than secondary glaucoma at 65.8% (95%CI: 38.4%, 83.3%) vs 45.2% (95%CI: 26.9%, 61.9%) with log rank test p=0.003. (Figure 1) Overall complete success, qualified success and failure in each follow-up year were following, at one year: 9.7%, 66.0% and 24.3%, at two years: 14.7%, 67.6% and 17.6%, at three years: 18.2%, 66.7% and 15.2%, at four years: 9.5%, 76.2% and 14.3%, at five years: 15.4%, 69.2% and 2%, respectively (Table 2).
 |
Table 2 Complete Success, Qualified Success and Failure Rate Comparing Between Ahmed Glaucoma Valve (AGV) and Nonvalved Glaucoma Drainage Devices (GDD) in Primary and Secondary Glaucoma Group |
 |
Figure 1 Kaplan–Meier survival curve showing the cumulative probability of complete or qualified success comparing between primary and secondary glaucoma. |
The cumulative probability of complete or qualified success comparing between the AGV and nonvalved GDD were not different as follows, the AGV was 40.1% (95%CI: 15.8%, 63.5%) vs nonvalved GDD was 64.0% (95%CI: 43.8%, 78.6%) with log rank test p=0.131. (Figure 2) When comparing between different types of GDD and different glaucoma etiology, in the primary glaucoma group; the AGV vs nonvalved GDD were 35.7% (95%CI: 1.5%, 77.7%) vs 76.5% (95%CI: 48.6%, 90.6%), p=0.665 and in secondary glaucoma group; the AGV vs nonvalved GDD were 47.2% (95%CI: 28.3%, 64.0%) vs 49.7% (95%CI: 21.9%, 72.4%), p=0.586, respectively (Table 3).
 |
Figure 2 Kaplan–Meier survival curve showing the cumulative probability of complete or qualified success comparing between valved and nonvalved glaucoma drainage device. |
IOP and number of antiglaucoma medications were significantly decreased from the baseline in every visit in both primary and secondary glaucoma groups, p<0.001 (Figures 3 and 4). LogMAR visual acuity was decreased from the baseline in both groups whereas the visual field parameter and structural ocular parameter were not different. Following from progression in advanced glaucomatous damage affect visual acuity, visual field testing were limited in patients with visual acuity less than 1.00 logMAR (Table 4).
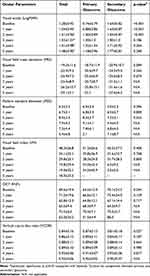 |
Table 4 Ocular Parameters Comparing Between Baseline and Follow-up |
 |
Figure 3 Mean intraocular pressure (IOP) of primary glaucoma and secondary glaucoma groups at baseline and follow-up. (*p<0.001 compared with preoperative). |
 |
Figure 4 Average number of antiglaucoma medications of primary glaucoma and secondary glaucoma groups at baseline and follow-up. (*p<0.001 compared with preoperative). |
Various factors associated with surgical failure in GDD implantation were analyzed, for example, glaucoma etiologies, age, sex, visual acuity, IOP, both visual field parameters and structural parameters, underlying generic and ocular disease, history of antiglaucoma medication, history of previous ocular laser and surgery, lens status and type of GDD. Univariate analysis for factors associated with surgical failure were neovascular glaucoma with unadjusted HR 3.62 (95%CI: 1.45, 9.04), p=0.006, and lens-induced glaucoma with unadjusted HR 4.58 (95%CI: 1.29, 16.25), p=0.018. Other glaucoma etiologies were not statistically significant associated with surgical failure for example, PACG with unadjusted HR 2.76 (95%CI: 0.55, 13.78), p=0.215, previous ocular surgery with unadjusted HR 1.98 (95%CI: 0.56, 7.01), uveitis glaucoma with unadjusted HR 2.59 (95%CI: 0.52, 12.94), p=0.247 and ICE Syndrome/Axenfeld Rieger with unadjusted HR 2.70 (95%CI: 0.32, 22.60), p=0.360 while protective factors were duration of antiglaucoma usage ≥10 months, unadjusted HR 0.45 (95%CI: 0.25, 0.82), p=0.009. The AGV was not significantly associated with failure with unadjusted HR 2.12 (95%CI: 0.51, 8.90), p=0.305. Multivariate analysis for potential factors associated with failure included glaucoma etiology, sex, proliferative diabetic retinopathy, IOP more than 23 mmHg and types of GDD implantation. The only significant factor was lens-induced glaucoma as etiology with adjusted HR 4.19 (95%CI:1.10, 15.86), p=0.035 (Table 5).
 |
Table 5 Univariate and Multivariate Analysis of Factors Associated with Surgical Failure in GDD Implantation |
Complication and adverse events comparing between valved and nonvalved GDD were not statistically significant different. Tube complications including tube malposition, exposure, and occlusion were the most frequent adverse events along with complications of GDD implantation, occurring in 16 eyes (11.1%), of which 13 eyes were secondary glaucoma etiology. Corneal decompensation was found in eight eyes (5.5%), three of which were diagnosed with secondary glaucoma from ICE syndrome. Hypotony was found in three eyes (2%) in nonvalved GDD, p=0.09. Endophthalmitis occurred in one eye from neovascular glaucoma etiology and the final treatment was enucleation. Further surgical treatments were performed to correct complications as follows: tube reposition, patching exposure, tube ligation, and anterior chamber irrigation and vitrectomy. Further laser and surgery treatments to control IOP from failure of GDD were second GDD implantation, trabeculectomy, needling GDD, SLT laser, micropulse laser, and LDTC (Table 6). Factors associated with adverse events and surgical complications for example, glaucoma etiologies, age, sex, visual acuity, IOP, both visual field parameters and structural parameters, underlying generic and ocular disease, history and type of antiglaucoma medication, history of previous ocular laser and surgery, lens status and type of GDD were analyzed by multiple logistic regression analysis and reported by unadjusted odds ratio. No factor was identified to be significantly associated with adverse events and surgical complications (Table 7).
 |
Table 6 Complication, Adverse Events of Glaucoma Drainage Devices Implantation and Further Laser and Surgery |
 |
Table 7 Univariate Analysis for Factors Associated with Complications and Adverse Events in GDD Implantation |
Discussion
Glaucoma drainage device implantation has become a procedure of choice in refractory glaucoma cases with comparable or higher success rates compared with conventional trabeculectomy. The efficacy and safety of GDD implantation varied along with different types of GDD model and various underlying glaucoma etiologies.6,7,11,12
The cumulative probability of complete and qualified success during five years of follow-up from the Tube Versus Trabeculectomy (TVT) study using nonvalved GDD and Baerveldt® Glaucoma Implants (BGI) was 70.2%, similar to 76.5% from our nonvalved GDD result in the primary glaucoma group. The etiologies of glaucoma participants in the TVT were similar to our primary glaucoma group including POAG, PACG and JOAG. They excluded secondary glaucoma etiology; for example, iridocorneal endothelial (ICE) syndrome, uveitis, NVG patients, and postretinal surgery. Meanwhile, a higher cumulative probability of failure during five years of follow-up was found in the trabeculectomy group at 46.9%.6 Trabeculectomy was previously performed in 73.7% of participants in our primary glaucoma group whereas a lower percentage was found in the TVT study, with 57.5% in the BGI group and 53.5% in the trabeculectomy group.
Comparing the efficacy of valved GDD implantation with conventional trabeculectomy surgery, a systematic review and meta-analysis from HaiBo et al reported the cumulative probability of complete and qualified success of the Ahmed glaucoma valve (AGV) was 51%, which was comparable with 55% from trabeculectomy. The AGV had lower adverse events than trabeculectomy at the follow-up time of two years.11 The variety of inclusion criteria from different glaucoma etiologies from this meta-analysis could affect the final result. Similar results of an AGV implantation study from Elbaklish and Gomaa were reported with decreasing IOP from 44.17 mmHg ±5.98 preoperatively to 15.18 mmHg ±2.75 at the one-year follow-up, and also reduced antiglaucoma medication from 3.89±0.31 to 2.75±1.43 postoperatively.13 Lee et al also reported that the cumulative probability of success of the AGV was 56% at the five-year follow-up.14 The cumulative probabilities of complete or qualified success of the AGV from this work was lower than previous studies at 40.1% (95%CI: 15.8%, 63.5%).
There were two multicenter randomized controlled trials comparing surgical success rates between valved and nonvalved GDD; the Ahmed Baerveldt Comparison (ABC) study and the Ahmed Versus Baerveldt (AVB) study which compared both efficacy and safety between the AGV and the BGI. The two studies shared similar baseline characteristics to our study. For example, they included postoperative fail trabeculectomy in the primary glaucoma group and included secondary glaucoma etiologies such as neovascular glaucoma and uveitis glaucoma. Our surgical success rates between valved and nonvalved GDD comparing between primary and secondary glaucoma were not different, which was similar to the ABC study. The ABC reported no statistically significant difference in surgical success rates between the AGV and the BGI at five years with the cumulative probability of success of 55.3% in the AGV group and 60.6% in the BGI group, p=0.65, while failure from safety issues such as hypotony, explantation, and loss of light perception was found more in the BGI than in the AGV, at 16.5% vs 7.7%.15 While the AVB study reported differently, the cumulative complete or qualified success rate was significantly higher in the BGI than in the AGV group at 60% vs 47%, p=0.04, with lower postoperative IOP and a lower number of antiglaucoma medications in every visit, while postoperative hypotony was found at 4% in only the BGI group, p=0.02,16 which was twofold to the 2% from nonvalved GDD from our study.
Criteria for success were varied between studies, thus the cumulative probability rate of complete success or qualified success base on without antiglaucoma medication at five years were following, the AVB study: 12% in the AGV vs 19% in the BGI, the TVT study: 25% in the BGI vs 29% in the trabeculectomy, the ABC study: 8% in the AGV vs 14% in the BGI.
The BGI has been discontinued for distribution in Thailand since 2019; therefore Aurolab aqueous drainage implantation (AADI) was replaced for nonvalved GDD implantation since then. The AADI was an economized GDD which was invented by a manufacturing division of the Aravind Eye Institute in India and had gained the European conformity (CE) mark and also Thai food and drug administration approval. For efficacy of the AADI, the cumulative success rates were 66.2% in adults and 77.2% in children at the two-year follow-up visit.17 The meta-analysis conducted by Hong et al reported that the pooled odds ratios comparing the AADI with the AGV were 3.68 (95%CI: 2.58–5.25) for complete success rate and 1.72 (95%CI: 1.24 to 2.39) for qualified success rate in refractory glaucoma patients.18 In contrast, the result from Khan et al showed an equivalent cumulative probability of success at the two-year follow-up between AADI and the AGV in children of 69.9% (95%CI: 45.9%, 84.9%) for the AADI vs 66.8% (95%CI: 53.4%, 77.1%) for the AGV, respectively.12 Following from our study which included a small number of children, comparison with those studies were limited.
Glaucoma etiology could be the predictor for surgical success or failure in GDD implantation as primary glaucoma had a significantly higher success rate than secondary glaucoma, whereas neovascular glaucoma and lens-induced glaucoma were significantly associated with failure in our study while other identifiable predictors were not statistically significant. Similar results were identified, for example, Lee et al found uveitis glaucoma and severe postoperative complication associated with failure,14 Christakis et al reported neovascular glaucoma and not having a prior trabeculectomy as the risk for failure in univariate analysis model but did not show different in multivariate analysis model.16 Similarly with our finding as protective factor for failure was previous trabeculectomy with unadjusted HR 0.66. Other baseline demographic data and ocular parameters between failure and success reported as unadjusted hazard ratio were not statistically different. Higher uncontrolled preoperative IOP in the secondary glaucoma group (30.6±11.8 vs 23.1±7.00 mmHg, p<0.001) was the factor to consider whether treatment involved valved or nonvalved GDD implantation; therefore the AGV was implanted more in secondary glaucoma than in primary glaucoma at 65.9% vs 39%, p=0.014. For that reason, the AGV showed more benefit than nonvalved GDD in terms of immediate lowering of IOP postoperatively, while in nonvalved GDD it needed 4–6 weeks of longer waiting time until the capsule fibrosis around the plate was formed to function. While protective factor was duration of antiglaucoma mediation shorter than 10 months prior to GDD implantation with unadjusted HR 0.45 (95%CI: 0.25, 0.82), p=0.009, which was implied that the longer duration of antiglaucoma treatment the more failure in GDD implantation similar to the report of associate factors for failure outcome in trabeculectomy by Wong et al.19 Corneal complications such as endothelial cell loss leading to corneal decompensation in our study were comparable with the results reported by Beatson et al by 5% (79 from 1610 eyes) and risk factors were as follows: older age, postoperative hypotony, tube-cornea touch, Fuchs' dystrophy, iridocorneal endothelial (ICE) syndrome, and higher number of previous glaucoma surgeries.20 Because the anterior chamber location of the tube placement was one of the identifiable risks of corneal decompensation, the drawback of tube placement in the sulcus or in pars planar21 when possible instead could lead to more incidents of tube occlusion or tube malposition, that occurred at a rate of 11.1% in this study. Hypotony occurred 2% of nonvalved GDD cases which was less than other studies.15,16
These following procedures were used to attempt to control IOP in failure of GDD: implantation of the second GDD, which was reported to have a cumulative failure rate at three years of 32.5%,22 transscleral cyclophotocoagulation procedure which was equally effective as the second GDD with cumulative success rates at one year of 88% and 79%, respectively23 and needling revision of the glaucoma drainage device filtering bleb which were reported as 43% to 72.2% cumulatively for both complete and qualified success at 24 months.24–26
Limitations of this study were losing a number of patients to complete five years and limited number of patients were included prior to year 2020. As a referral center, referring patients back to their local general hospital when glaucoma disease were under-controlled to minimize transportation cost and indirect cost for patients. The price of GDD was only reimbursed in the civil servants’ medical benefits and self-paid schemes before 2020, implantation of GDD was limited by this economic reason. Furthermore, our precise ocular parameters both structural and functional test for following-up progression of disease were overlooked due to advanced glaucomatous damage and visually impairment at baseline.
Conclusion
This study shows cumulative probability of success of GDD implantation both valved and nonvalved GDD in refractory glaucoma patients from primary and secondary glaucoma etiology. Primary glaucoma had a significantly better success rate than secondary glaucoma. Neovascular glaucoma and lens-induced glaucoma were predictors for failure while less than 10 months duration of antiglaucoma treatment was a protective factor for failure. Comparing between different type of GDD, success and failure rate, complications and adverse events were comparable between valved and nonvalved GDD. These results would be of benefit in both prognostic counseling and considering GDD implantation in refractory glaucoma patients.
Acknowledgments
Glaucoma staff and fellows in glaucoma at Mettapracharak (Wat Rai Khing) Hospital.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Dawson EF, Rosenberg NC, Meyer AM, et al. Comparison of outcomes of glaucoma drainage implant surgery with or without prior failed trabeculectomy. J Glaucoma. 2021;30(7):585–595. doi:10.1097/ijg.0000000000001852
2. Kornmann HL, Gedde SJ. Glaucoma management after vitreoretinal surgeries. Curr Opin Ophthalmol. 2016;27(2):125–131. doi:10.1097/icu.0000000000000238
3. Knape RM, Szymarek TN, Tuli SS, Driebe WT, Sherwood MB, Smith MF. Five-year outcomes of eyes with glaucoma drainage device and penetrating keratoplasty. J Glaucoma. 2012;21(9):608–614. doi:10.1097/IJG.0b013e31821db3e5
4. Yang H, Yu X, Sun X. Neovascular glaucoma: handling in the future. Taiwan J Ophthalmol. 2018;8(2):60–66. doi:10.4103/tjo.tjo_39_18
5. Clement CI, Goldberg I. The management of complicated glaucoma. Indian J Ophthalmol. 2011;59:S141–7. doi:10.4103/0301-4738.73686
6. Gedde SJ, Schiffman JC, Feuer WJ, Herndon LW, Brandt JD, Budenz DL. Treatment outcomes in the Tube Versus Trabeculectomy (TVT) study after five years of follow-up. Am J Ophthalmol. 2012;153(5):789–803.e2. doi:10.1016/j.ajo.2011.10.026
7. Alasbali T, Alghamdi AA, Khandekar R. Outcomes of Ahmed valve surgery for refractory glaucoma in Dhahran, Saudi Arabia. Int J Ophthalmol. 2015;8(3):560–564. doi:10.3980/j.issn.2222-3959.2015.03.22
8. Aung T, Seah SK. Glaucoma drainage implants in Asian eyes. Ophthalmology. 1998;105(11):2117–2122. doi:10.1016/s0161-6420(98)91136-8
9. Lai JSM, Poon ASY, Chua JKH, Tham CCY, Leung ATS, Lam DSC. Efficacy and safety of the Ahmed glaucoma valve implant in Chinese eyes with complicated glaucoma. Br J Ophthalmol. 2000;84:718–721. doi:10.1136/bjo.84.7.718
10. Seah SK, Gazzard G, Aung T. Intermediate-term outcome of Baerveldt glaucoma implants in Asian eyes. Ophthalmology. 2003;110(5):888–894. doi:10.1016/s0161-6420(03)00088-5
11. HaiBo T, Xin K, ShiHeng L, Lin L. Comparison of Ahmed glaucoma valve implantation and trabeculectomy for glaucoma: a systematic review and meta-analysis. PLoS One. 2015;10(2):e0118142. doi:10.1371/journal.pone.0118142
12. Khan AM, Ahmad K, Alarfaj M, Alotaibi H, AlJaloud A, Malik R. Surgical outcomes of the Aurolab aqueous drainage implant (AADI) versus the Ahmed glaucoma valve for refractory paediatric glaucoma in Middle Eastern children. BMJ Open Ophthalmol. 2021;6:e000831. doi:10.1136/bmjophth-2021-000831
13. Elbaklish KH, Gomaa WA. A one-year follow-up of two Ahmed glaucoma valve models (S2 and FP7) for refractory glaucoma: a prospective randomized trial. Clin Ophthalmol. 2020;14:693–705. doi:10.2147/OPTH.S224653
14. Lee CK, Ma KT, Hong YJ, Kim CY. Long-term clinical outcomes of Ahmed valve implantation in patients with refractory glaucoma. PLoS One. 2017;12(11):e0187533. doi:10.1371/journal.pone.0187533
15. Budenz DL, Barton K, Gedde SJ, et al. Five-year treatment outcomes in the Ahmed Baerveldt comparison study. Ophthalmology. 2015;122(2):308–316. doi:10.1016/j.ophtha.2014.08.043
16. Christakis PG, Kalenak JW, Tsai JC, et al. The Ahmed versus baerveldt study: five-year treatment outcomes. Ophthalmology. 2016;123(10):2093–2102. doi:10.1016/j.ophtha.2016.06.035
17. Puthuran GV, Palmberg P, Wijesinghe HK, Srivastav KS, Krishnadas SR, Lee Robin A. Aurolab aqueous drainage implant with and without scleral patch graft in refractory adult and pediatric glaucomas: a comparative study. Am J Ophthalmol. 2020;216:226–236. doi:10.1016/j.ajo.2020.03.022
18. Hong M, Peng Y, Lai Y, Hong C. Comparison of Aurolab Aqueous Drainage Implant with Ahmed Glaucoma Valve for refractory glaucoma: a meta-analysis; 2022.
19. Wong JKW, Leung TK, Lai JS, Chan JC. Evaluation of adverse effects of topical glaucoma medications on trabeculectomy outcomes using the glaucoma medications intensity index. Ophthalmol Ther. 2022;11(1):387–401. doi:10.1007/s40123-021-00447-x
20. Beatson B, Wang J, Boland MV, et al. Corneal edema and keratoplasty: risk factors in eyes with previous glaucoma drainage devices. Am J Ophthalmol. 2022;238:27–35. doi:10.1016/j.ajo.2021.12.017
21. Chang EK, Gupta S, Chachanidze M, Miller JB, Chang TC, Solá-Del Valle DA. Combined pars plana glaucoma drainage device placement and vitrectomy using a vitrectomy sclerotomy site for tube placement: a case series. BMC Ophthalmol. 2021;21(1):106. doi:10.1186/s12886-021-01872-z
22. Ong SC, Aquino MC, Chew P, Koh V. Surgical outcomes of a second Ahmed glaucoma valve implantation in asian eyes with refractory glaucoma. J Ophthalmol. 2020;2020:8741301. doi:10.1155/2020/8741301
23. Feldman RM, Chuang AZ, Mansberger SL, et al. Outcomes of the second aqueous shunt implant versus transscleral cyclophotocoagulation treatment study: a randomized comparative trial. J Glaucoma. 2022;31(9):701–709. doi:10.1097/IJG.0000000000002079
24. Chen PP, Palmberg PF. Needling revision of glaucoma drainage device filtering blebs. Ophthalmology. 1997;104(6):1004–1010. doi:10.1016/s0161-6420(97)30194-8
25. Quaranta L, Floriani I, Hollander L, Poli D, Katsanos A, Konstas AG. Needle revision with 5-fluorouracil for the treatment of Ahmed glaucoma valve filtering blebs: 5-fluorouracil needling revision can be a useful and safe tool in the management of failing Ahmed glaucoma valve filtering blebs. J Glaucoma. 2016;25(4):e367–71. doi:10.1097/IJG.0000000000000366
26. Erdem B, Imamoglu S, Ercalik NY. Needling with 5-fluorouracil for encapsulated blebs after Ahmed glaucoma valve implantation. Cutan Ocul Toxicol. 2019;38(4):395–400. doi:10.1080/15569527.2019.1650060
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

