Back to Journals » Clinical Ophthalmology » Volume 16
Surgical Management of Peripheral Ulcerative Keratitis: Update on Surgical Techniques and Their Outcome
Authors Sabhapandit S, Murthy SI, Sharma N , Sangwan VS
Received 10 August 2022
Accepted for publication 28 September 2022
Published 19 October 2022 Volume 2022:16 Pages 3547—3557
DOI https://doi.org/10.2147/OPTH.S385782
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Swapnali Sabhapandit,1 Somasheila I Murthy,2 Namrata Sharma,3 Virender S Sangwan4
1Institute of Ophthalmic Sciences, Asian Institute of Gastroenterology Hospitals, Hyderabad, Telangana, India; 2Tej Kohli Cornea Institute, L V Prasad Eye Institute, Kallam Anji Reddy Campus, Hyderabad, Telangana, India; 3Dr Rajendra Prasad Centre for Ophthalmic Sciences, All India Institute of Medical Sciences, New Delhi, India; 4Dr Shroff’s Charity Eye Hospital, New Delhi, India
Correspondence: Swapnali Sabhapandit, Institute of Ophthalmic Sciences, Asian Institute of Gastroenterology Hospitals, Mindspace Road, Gachibowli, Hyderabad, 500032, India, Tel +91 8790622699, Email [email protected]
Abstract: Peripheral ulcerative keratitis (PUK) is an inflammatory, necrotic condition in the peripheral cornea which may end in corneal perforation and visual morbidity if not treated adequately. PUK can occur due to infectious or non-infectious causes. Early cases need medical therapy, both locally and systemically (for some cases). However, advanced PUK may necessitate surgical removal of inciting cause of the pathology and maintaining tectonic stability. Such surgical treatment, including corneal transplantations, may be used in an emergency setting or for visual rehabilitation following preliminary stabilization of the affected cornea. The outcome of these surgeries need to be analyzed to understand the long-term visual prognosis of such eyes. This is an attempt to analyze surgical modalities in the management of PUK and their outcomes.
Keywords: peripheral ulcer, tissue adhesive, amniotic membrane, keratoplasty
Introduction
Peripheral ulcerative keratitis (PUK) is an inflammatory condition in peripheral cornea with potential of devastating necrosis. It is characterized by a crescentic area of epithelial defect with stromal melting, subepithelial infiltrate at the edge and progressive corneal thinning (Figure 1). It may be unilateral or bilateral.1 PUK is sometimes associated with anterior uveitis or contiguous involvement of surrounding conjunctiva, episclera and sclera.2
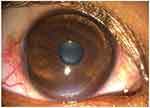 |
Figure 1 Peripheral ulcerative keratitis (PUK) with epithelial defect, stromal ulceration and peripheral corneal thinning. |
The peripheral cornea has a higher propensity for immune-related damage compared to the central cornea due to certain physiological variations.2,3 This area has less neural innervation and higher supply from the perilimbal vascular and lymphatic arcades as compared to central cornea. This causes the influx of inflammatory cells and immune mediators such as immune complexes, immunoglobulins and complement factors. This area also has higher concentration of MUC4, a cell surface plycoprotein, which causes differentiation of epithelial cells. All these factors lead to increased risk of corneal necrosis leading to PUK.
PUK can occur from systemic or local causes. Nearly 50% association of collagen vascular disease is seen in PUK with high risk of morbidity and mortality.3,4 Aggressive management of underlying systemic disease is necessary in such cases.
Ocular causes of PUK can be infective or autoimmune. Bacterial, viral, fungal, and parasitic etiology have been found in PUK.5–7 Localized PUK due to immunological reaction can manifest as Mooren’s ulcer. In this condition, there is no underlying systemic disease, hence it is diagnosed after extensively investigating and ruling out all systemic conditions. The differentiating feature of this condition is non-involvement of scleral tissue. For treating early cases of Mooren’s ulcer, immunosuppression is the first line of treatment.8,9
Medical management is the mainstay of treatment for PUK. The principle behind treatment of the systemic conditions is immunosuppression and control of inflammation.3,4,10,11 Infective etiology is managed by proper identification of microorganism from corneal scraping and appropriate antimicrobial therapy.12
Surgical intervention for PUK is required for the following conditions:
- threat to tectonic integrity of the eyeball: this can occur with localized or circumferential sterile corneal necrosis leading to descemetocele formation and corneal perforation.
- Removal of causative agent: in Mooren’s ulcer, the conjunctiva along the lesion is the primary source of antibody-producing inflammatory cells present in the ulcer.13,14 Resection of this conjunctiva with adjuvant procedures are needed to control the disease. Similarly, in infective PUK, surgical debulking may be needed in aggressive disease not responding to antimicrobial therapy.15,16
- Visual rehabilitation: once the active PUK is controlled, optical surgeries such as penetrating or lamellar keratoplasty can be undertaken for visually rehabilitating the patient.16,17
There are multiple surgical options for managing PUK, with scarce amount of scientific literature on the case selection, methodology and outcome of each technique.18 The advantages and disadvantages of each technique are summarized in Table 1. This review attempts to analyze the outcomes and benefits of different surgical techniques used for PUK management.
 |
Table 1 Comparison of the Different Techniques of Surgical Management of PUK |
Surgical Techniques
Diathermy Coagulation
Diathermy coagulation was reported in 1957 for treating Mooren’s ulcer. Somerset and Vancea reported good outcome after repeated application of light diathermy to the ulcer edges.19 However, with understanding of the pathophysiology of Mooren’s ulcer, diathermy coagulation has been discarded for managing this disease process.
Conjunctival Resection with Cryotherapy
Aviel, in 1972, reported 5 cases of Mooren’s ulcer treated with conjunctival peritomy, diathermy coagulation of leaky vessels followed by cryotherapy to the center and edges of the ulcer at −40 to −50 degrees Celsius for 20 seconds in each round of application.20 Maximum follow-up until 5 months showed improvement in the lesions.
Cryotherapy as a treatment option was also reported by Comte et al and Genvert et al in Mooren’s ulcer.21 In 10 of 13 cases, conjunctival recession and suturing at 3mm behind limbus was done, while in 3 eyes, conjunctiva was resected. In a period of 1–50 months, vascularized pannus was noted in all cases, while one eye needed a repeat surgery. Recently, successful management by cryotherapy with conjunctival flap suturing over ulcer area was reported in a 70 year old male suffering from granulomatosis with polyangitis on immunosuppression by Cheng et al.22 In South West Nigeria, Fasina et al treated progressive Mooren’s ulcer using cryotherapy with conjunctival resection in 14 patients with good anatomical stability.23
Conjunctival resection with cryotherapy is not routinely used as the risk of pannus formation is high in this technique.
Conjunctival Recession or Resection
The limbal conjunctiva is believed to be the source of collagenase and other enzymes that destroy the collagen and ground matrix in PUK.14 Based on this theory, Wilson et al performed conjunctival recession with suturing around 1 mm behind limbus in 7 patients with varied etiology of PUK.24 There was positive response in 6 cases, while one case had multiple recurrences. Mondino et al performed conjunctival resection in Mooren’s ulcer cases with successful outcome in 3 of 4 unilateral ulcers and 3 of 3 bilateral non-simultaneous ulcers. However, only 2 of 15 bilateral simultaneous ulcers healed.25 This technique is nowadays popular for managing Mooren’s ulcer. The role of aggressive medical management with immunosuppressives cannot be undermined in such acute, bilateral simultaneous cases, especially in young patients.1,3,26
Conjunctival Advancement or Flap
In 1989, Portnoy et al used conjunctival flap in PUK cases for tectonic support.27 However, Tan et al reported a case of flap melt in PUK due to Crohn’s disease, needing sectorial penetrating keratoplasty.28 Similarly, Li et al reported two cases of Mooren’s ulcer, where conjunctival flap technique led to rapid deterioration of the ulcer.29 Due to such poor outcomes, conjunctival flap is seldom used nowadays in PUK.
Use of Tissue Adhesives
Synthetic cyanoacrylate glue was first used by Refojo and Webster in 1968.30,31 Two types of tissue adhesives – cyanoacrylate derivatives (butyl monomers) and biological fibrin glue – are used for extreme corneal thinning and perforation.30–35
Cyanoacrylate Glue
This compound has optimum strength and rapid polymerization; hence it is used to close corneal perforations up to 3 mm in diameter.31,33,34 However, being non-biodegradable, it may cause inflammation, papillary conjunctivitis, corneal neovascularization, infection and tissue necrosis. If glue enters the anterior chamber, it can cause iris adhesion, pupillary block or secondary glaucoma due to peripheral anterior synechiae, granulomatous reaction and cataract.34–37
Cyanoacrylate glue has been widely used in surgical management of PUK. In 1968, Webster used this glue in closing corneal perforation.31 The use of this glue in treating PUK with impending or actual perforation has been well documented in literature.33,38–41 The role of cyanoacrylate glue in healing PUK is twofold; firstly, for tectonic strength, and secondly for acting as a barrier to the influx of inflammatory cells from the conjunctiva into the adjacent corneal stroma.41–44 In Mooren’s ulcer, the standard procedure for surgery is the trimming of the overhanging necrotic ulcer lip and conjunctival resection adjacent to the ulcer followed by application of cyanoacrylate glue, with placement of bandage contact lens over the cornea. The glue remains for at least a month until it spontaneously dislodges due to underlying epithelial healing.40,41 Resolution of ulcer has been reported from 42% to 83% of cases.33,40,41
In 2013, Sharma et al reported a series of 16 eyes where PUK-related corneal perforation between 3.5 to 4.5 mm was sealed with scleral patch graft using cyanoacrylate glue. 14 eyes healed in 5–9 weeks, with 2 eyes needing repeat surgery due to loosening of the glue. 5 grafts needed few 10–0 nylon sutures. Once the graft fused with the host cornea, the glue was removed.45 A similar technique of managing PUK-related perforation was reported by Hinduik et al.46
Bernauer et al used cyanoacrylate glue in rheumatoid arthritis (RA)-related corneal perforations up to 3mm diameter without concurrent use of immunosuppressives. Though temporary closure of perforations occurred in all 6 eyes, the PUK continued due to aggressive disease, proving that systemic management of such conditions is of paramount importance.17 Similar reports were published by Weiss et al and Messmer et al as a temporary measure.33,47 Temporary closure of a perforated PUK with a 2mm size surgical drape and cyanoacrylate glue until a penetrating keratoplasty could be done 7 days later, has also been reported.48
Fibrin Glue
This biological and biodegradable adhesive is prepared from fibrinogen and thrombin component of blood.35,49 The rate of complications is very low, including granuloma and cyst formation, foreign body sensation and non-adherence of material to underlying tissue.50,51 Fibrin glue also lacks tectonic strength.
Lagoutte et al used fibrin glue to treat 8 cases of 2.5mm perforated peripheral corneal ulcers with favorable results.52 However, re-application of the glue was needed for a few cases. Due to weak tectonic strength, this adhesive is used with biological membranes or tissue grafts in PUK cases.
Amniotic Membrane Graft (AMG)
Amniotic membrane was first used in surgeries as early as 1910.53 For use as a corneal graft, AMG transplantation was attempted by De Rotth and Sorsby in the 1940s.54,55 Use of AMG on cornea gained popularity in 1997 due to the published works of Tseng et al and Dua et al.56–58
AMG causes rapid epithelialization and suppresses the expression of cytokines in damaged ocular surface epithelia.59 It also contains protease inhibitors, causing apoptosis of inflammatory cells.58,59 AMG has a high content of nerve growth factor needed for surface healing.60
Solomon et al used multiple layers of AMG for corneal perforations less than 0.5mm size with poor results in PUK due to autoimmune diseases.61
The use of AMG for treating aggressive Mooren’s ulcer is controversial.62 Motowa et al used a double layered AMG sandwich technique for a single case of Mooren’s ulcer.63 Good results have been reported in AMG use with lamellar corneal graft.64 In both cases, immunosuppressive drugs were continued. Similar satisfactory outcome was reported in cases with additional conjunctival resection or instillation of serum eye drops.65,66 However, Chen et al noted recurrence in a multilayered AMG with conjunctival autografting for Mooren’s ulcer.67 Schallenberg et al reported relapse of aggressive Mooren’s ulcer in 6 of 7 eyes which underwent AMG transplantation with conjunctival resection, even on oral immunosuppression.68
There has been mixed response to the use of AMG with fibrin glue for sealing corneal perforations in PUK. Hicks et al reported good outcome (80% success rate) in perforations less than 3mm in diameter.69 However, Hanada et al had success with multilayered AMG in a Mooren’s ulcer, but recurrence in two cases with rheumatoid arthritis.70 Similarly, Rodrigues-Ares et al reported success in 2 cases of rheumatoid arthritis with failure in an erythema multiforme major case.71 In a series of 45 eyes with perforation less than 3mm, Fan et al reported healing with good visual outcome in 6 cases with marginal ulcers.72 They filled the perforation with rolled-up AMG, covered by 3 layers of larger sized AMG and anterior chamber formation by injection of 0.3mL of 20% perfluoropropane. However, 3 cases had anterior iris synechiae postoperatively.
Liu et al conducted a meta-analysis of visual outcome and epithelial healing following cryopreserved AMG use in infectious and noninfectious corneal ulcers.73 The use of AMG was found to benefit early epithelialization and stromal healing. Multilayered AMG was found to be better for repair of deep ulcers. Freeze-dried AMG has recently been used to treat different ocular surface disorders.74,75
Corneal Patch Graft
As early as 1989, Portnoy et al used periosteal graft from anterior tibia for closing peripheral corneal perforations due to PUK.27 They also reported using crescentic or round corneal patch grafts for treating PUK.
In 1991, Kinoshita et al used donor corneal lenticules with intact epithelium along with conjunctival resection for treating 20 eyes with aggressive Mooren’s ulcer.76 Additional corneoscleral lamellar grafts were done in 5 eyes. With a follow-up period of 3.1 years, 90% eyes had complete recovery while on steroid therapy. Soong et al used free hand dissected lamellar corneal patch grafts in 31 eyes with PUK. With an average follow-up period of 1 year, 17 cases maintained stable grafts with good visual outcome. Complications noted were corneal melt necessitating repeat patch graft, cataract and need for penetrating keratoplasty for visual recovery.77
Krysik et al performed patch graft for peripheral lesions (29% of cases with total 247 eyes) with infective, autoimmune and traumatic etiology.16 Common complications noted were vascular ingrowth (50% cases), persistent epithelial defects, graft melt (mostly in autoimmune disease related eyes) and early loosening of sutures. Compared to the penetrating keratoplasty cases, visual acuity at follow-up was better in this group of cases.
In 2013, Lin et al used patch grafts from non-antigenic glycerol preserved corneas in rheumatoid arthritis related perforated PUK.78 Except one case with graft melt, all cases had stable though opaque grafts. A similar result was noted by Shi et al earlier in 8 cases of PUK.79 The same group also studied the results in 25 eyes with perforated Mooren’s ulcer who first underwent a suturing of posterior corneal donor button having intact endothelium, then covered by glycerine preserved lamellar graft.80 The immunosuppressive regime was maintained. 87.1% eyes had anatomical and visual recovery with clear grafts. The dendritic cell population was monitored in the grafts to understand the antigenic tendency and need for immunosuppression. A gradual decline of this cell population was observed in the patch grafts over 6-month period.
In 2015, Sharma et al used crescentic and circular corneal patch grafts for 4 cases of moderate level and 5 cases of severe level of involvement in PUK.81 They achieved 100% anatomical success in moderate cases with maximum follow-up of 3 years. The resolution rate was 83.3% in the severe cases.
A novel technique of suturing the lenticule obtained during small incision refractive lenticule extraction (SMILE) technique for myopia correction as donor patch graft in 14 PUK cases was reported by Jiang et al.82 In deep ulcers, multiple layers of lenticule were used. There was globe stability in all cases until around 1 year, though 3 cases needed repeat procedure.
Scleral Patch Graft
Scleral tissue from cadaveric eyeball is used for tectonic stability only in PUK cases as the tissue is opaque. Such patch grafts were used with cyanoacrylate glue by Sharma et al.45 Fasina et al used scleral patch grafts in 7 aggressive cases of Mooren’s ulcer not responsive to immunosuppression with good anatomical stability.46 Krysik et al used corneoscleral patch grafts for peripheral corneal perforations up to 5mm diameter in 117 eyes.16 There was graft vascularization in 50% cases. 16 grafts melted due to infection or uncontrolled autoimmune disease, necessitating urgent penetrating keratoplasty. Stilma et al used scleral patch grafts in 6 Mooren’s ulcer in Sierra Leone with visual and anatomical stability.83
The use of patch grafts, both corneal and scleral, is restricted by the limited availability of donor tissue and risk of graft vascularization and opacification. Hence, this technique is usually reserved for globe threatening PUK cases.
Lamellar Keratoplasty
This technique, as shown in Figure 2, first used by Paufique et al in 1947, is useful in PUK with larger area of corneal involvement without perforation.84 In a case series by Krysik et al, 11 cases with descemetocele underwent lamellar grafts using trephine or femtosecond laser with good outcome.16 Earlier, Messmer et al reported stable cornea in 3 cases of rheumatoid arthritis related PUK.47
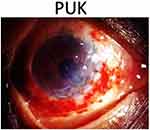 |
Figure 2 Eccentric lamellar patch graft in impending corneal perforation in peripheral ulcerative keratitis (PUK). |
Xie et al used glycerin cryopreserved limbal lamellar graft from corneoscleral rims in 4 cases of Mooren’s ulcer with tectonic instability while continuing topical immunosuppression.85 All grafts were stable with no rejection. The authors attributed this to the effect of glycerin in eliminating keratocytes, dendritic cells and other antigenic cells in the preserved tissue.80,86 Gao et al utilized a similar technique of ring shaped donor tissue in 11 eyes with PUK.87 Only one graft melted. Cheung et al used a similar technique in perforated active Fuch’s marginal keratitis case with complete recovery.88
Tavassoli reported a case of vasculopathy related perforated PUK in an asymptomatic human immunodeficiency virus (HIV) positive patient managed by deep anterior lamellar keratoplasty and systemic antiretroviral therapy.89 The graft was stable at 3 months follow-up. Recently tenon sling graft has been used in an hourglass shaped corneal melt following tuberculosis-associated PUK.90
Lamellar keratoplasty needs surgical expertise and availability of donor corneas, hence this technique is limited to tertiary level eye hospitals with access to eyebanks and proper systemic therapy by intensivists. However, with adequate management, the results of this surgery are satisfactory for visual rehabilitation of PUK cases.
Penetrating Keratoplasty (PK)
This procedure has been used for tectonic stability in PUK cases since the 1950s.91 Extreme corneal thinning or actual perforation involving extensive areas of the cornea benefit form PK (Figure 3). The surgery timing, underlying disease severity, and concurrent use of systemic immunosuppression determine the success of the PK procedure.92
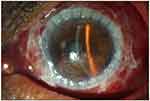 |
Figure 3 Total penetrating keratoplasty with overlying amniotic membrane placement in a large area of corneal perforation in rheumatoid arthritis with unstable ocular surface. |
Mooren’s ulcer refractory to immunosuppression and conjunctival resection may need PK for globe preservation.91 However, the survival and visual outcome are not satisfactory in most of the cases.26 Grafts done for Mooren’s ulcer had tectonic stability with systemic immunosuppression with poor visual outcome.
Riedel et al performed eccentric PK in cases with rheumatoid arthritis (21 cases) and infective PUK (47 cases) with higher complication rate and poor visual outcome.93 Messmer and Foster repeated PKs in 3 of 16 eyes with aggressive rheumatoid arthritis.47 Cases on cytotoxic drugs after surgery had clear grafts at 15–67 months of follow-up. Similar results were reported by Palay et al in 23 eyes.94 12 eyes underwent repeat PK with the survival rate at only 48% in 5 years. Bernaeur et al reported 16 eyes with rheumatoid arthritis related corneal perforation peripherally undergoing PK.17 Grafts with post-operative systemic immunosuppression had better outcome.
Tan et al reported good visual outcome in a Crohn’s disease patient undergoing PK for PUK-associated corneal perforation.28 Sharma et al performed PK in 12 eyes with advanced PUK refractory to medical management.81 2 eyes had phthisis bulbi, while the other eyes maintained tectonic stability on immunosuppression.
Cartwright et al mentioned a substantial decrease of only 16% in the surgical rate in patients using modern immunosuppressive drugs for PUK treatment.95
The outcomes documented for corneal transplantation in non-infectious PUK are worse compared to infectious cases, probably due to the underlying autoimmune pathology leading to poor tissue healing.15,96 Hence, a thorough systemic evaluation and immunosuppression is a requirement for planning corneal surgery in such cases. In case of infectious etiology, identification of the microorganism via corneal smear, culture and biopsy (as needed) helps to medically control the infection before and after surgery.
Conclusion
The quality of published literature regarding surgical management of PUK is low in scientific evidence as most of the studies are case series or case reports. There are no randomized controlled trials (RCT) published to date. Alhassan et al conducted a meta-analysis of the medical and surgical management of Mooren’s ulcer in 2014.97 They concluded that there is no scientific evidence in the form of RCT to prove the effectiveness of different treatment options in Mooren’s ulcer. With recent advances in the understanding of the pathogenesis of various autoimmune diseases and corneal inflammation, the need for well-designed RCTs to assess the methodology, benefits, timing and concurrent medical management of different surgeries for PUK is paramount.98 Amniotic membrane grafting and cyanoacrylate glue remain the mainstay for managing early cases of corneal melts in PUK. Conjunctival resection is the preferred choice for treating Mooren’s ulcer. Most cases of corneal transplantation in PUK are reported in large perforations in an emergency setting or for visual rehabilitation after the primary disease has been controlled in the cornea. Increased use of femtosecond laser in corneal grafting is another interesting avenue to explore for managing such complicated pathologies.82,99 Any surgical management has to be compounded with excellent medical therapy for controlling the underlying cause of the PUK, whether autoimmune or infectious. This improves the prognosis of visual outcome in such eyes.
This review attempted to amalgamate the published data on surgical management of PUK to date with their outcomes.
Literature Search
PubMed and MEDLINE search was done with combinations of the following search terms: peripheral ulcerative keratitis; peripheral corneal ulcers; infective peripheral ulcers; immunological peripheral ulcers; surgical procedures; complications; diagnosis; treatment and management. Relevant articles from literature search and their references when applicable were included. Articles published after 1940 and articles published in non-English languages were included if there was an English comprehensive summary of the article. Clinical studies, randomized controlled trials, review articles, case series, and case reports were included in the review.
Acknowledgments
The author acknowledges the contribution of S Banu, librarian of L V Prasad Eye Institute, Hyderabad, in the access, collection and reassessment of all references mentioned in the article.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Galor A, Thorne JE. Scleritis and peripheral ulcerative keratitis. Rheum Dis Clin N Am. 2007;33:835–854. doi:10.1016/j.rdc.2007.08.002
2. Robin JB, Schanzlin DJ, Verity SM, et al. Peripheral corneal disorders. Surv Ophthalmol. 1986;31:1–36. doi:10.1016/0039-6257(86)90049-4
3. Tauber J, Sainz de la Maza M, Hoang-Xuan T, et al. An analysis of therapeutic decision making regarding immunosuppressive chemotherapy for peripheral ulcerative keratitis. Cornea. 1990;9(1):66–73. doi:10.1097/00003226-199001000-00013
4. Ladas JG, Mondino BJ. Systemic disorders associated with peripheral corneal ulceration. Curr Opin Ophthalmol. 2000;11:468–471. doi:10.1097/00055735-200012000-00014
5. Asbell P, Stenson S. Ulcerative keratitis: survey of 30 years’ laboratory experience. Arch Ophthalmol. 1982;100:77e80. doi:10.1001/archopht.1982.01030030079005
6. Baum J, Fedulowica HB, Jordan A. A survey of Moraxella corneal ulcers in a derelict population. Am J Ophthalmol. 1980;90:476e80. doi:10.1016/S0002-9394(14)75014-7
7. Polack FM, Kaufman HE, Newmark E. Keratomycosis: medical and surgical treatment. Arch Ophthalmol. 1971;85:41e6. doi:10.1001/archopht.1971.00990050412003
8. Srinivasan M, Zegans ME, Zelefsky JR, et al. Clinical characteristics of Mooren’s ulcer in South India. Br J Ophthalmol. 2007;91:570–575. doi:10.1136/bjo.2006.105452
9. Garg P, Sangwan VS. Mooren’s ulcer. In: Krachmer JH, Mannis MJ, Holland EJ, editors. Cornea: Fundamentals, Diagnostic, Management.
10. Galor A, Jabs DA, Leder HA, et al. Comparison of antimetabolite drugs as corticosteroid-sparing therapy for noninfectious ocular inflammation. Ophthalmology. 2008;115:1826–1832. doi:10.1016/j.ophtha.2008.04.026
11. Senolt L, Vencovský J, Pavelka K, et al. Prospective new biological therapies for rheumatoid arthritis. Autoimmun Rev. 2009;9(2):102–107. doi:10.1016/j.autrev.2009.03.010
12. Chung G. Phlyctenular keratoconjunctivitis and marginal staphylococcal keratitis. In: Krachmer JH, Mannis MJ, Holland EJ, editors. Cornea: Fundamentals, Diagnostic, Management.
13. Sangwan VS, Zafirakis P, Foster CS. Mooren’s ulcer: current concepts in management. Indian J Ophthalmol. 1997;45:7–17.
14. Brown S. Mooren’s ulcer. Treatment by conjunctival excision. Br J Ophthalmol. 1975;59:675–682. doi:10.1136/bjo.59.11.675
15. Yokogawa H, Kobayashi A, Yamazaki N, et al. Surgical therapies for corneal perforations: 10 years of cases in a tertiary referral hospital. Clin Ophthalmo. 2014;8:2165–2170. doi:10.2147/OPTH.S71102
16. Krysik K, Dobrowolski D, Lyssek-Boron A, et al. Differences in surgical management of corneal perforations, measured over six years. J Ophthalmol. 2017;2017:1582532. doi:10.1155/2017/1582532
17. Bernauer W, Ficker LA, Watson PG, Dart JKG. The management of corneal perforations associated with rheumatoid arthritis: an analysis of 32 eyes. Ophthalmology. 1995;102(9):1325–1337. doi:10.1016/S0161-6420(95)30867-6
18. de Farias CC, Norma Allemann JÁ, Gomes P. Randomized trial comparing amniotic membrane transplantation with lamellar corneal graft for the treatment of corneal thinning. Cornea. 2016;35:438–444. doi:10.1097/ICO.0000000000000754
19. Somerset EJ. Mooren’s ulcer treated by diathermy coagulation. Br J Ophthalmol. 1957;41(9):570–573. doi:10.1136/bjo.41.9.570
20. Avieal E. Combined cryoapplications and peritomy in Mooren’s ulcer. Br J Ophthalmol. 1972;56:48. doi:10.1136/bjo.56.1.48
21. Genvert G, Sakauve CM, Arentsen JJ. Treatment of marginal corneal ulcers with cryotherapy and conjunctival recession or resection. Cornea. 1984;3(4):256–261. doi:10.1097/00003226-198404000-00005
22. Cheng-Wei L, Zhou -D-D, Wan J, Hao J-L. Surgical treatment of peripheral ulcerative keratitis and necrotizing scleritis in granulomatosis with polyangiitis. Saudi Med J. 2016;37(2):205–207. doi:10.15537/smj.2016.2.13390
23. Fasina O, Ogundipe AO, Ezichi EI. Mooren’s ulcer in Ibadan, Southwest Nigeria. J West Afr Coll Surg. 2013;3(3):102–119.
24. Wilson FM, Grayson M, Ellis FD. Treatment of peripheral corneal ulcers by limbal conjunctivectomy. Br J Ophthalmol. 1976;60:713. doi:10.1136/bjo.60.10.713
25. Bartly J, Mondino BJ. Inflammatory diseases of the peripheral cornea. Ophthalmology. 1988;95:463–472. doi:10.1016/S0161-6420(88)33164-7
26. Chow C, Foster CS. Mooren’s ulcer. Int Ophthalmol Clin. 1996;36:1–13. doi:10.1097/00004397-199603610-00003
27. Portney SL, Insler MS, Kaufman HE. Surgical management of corneal ulceration and perforation. Surv Ophthalmol. 1989;34(1):47–58. doi:10.1016/0039-6257(89)90129-X
28. Tan MH, Chen SD, Rubinstein A, et al. Corneal perforation due to severe peripheral ulcerative keratitis in Crohn disease. Cornea. 2006;25:628–630. doi:10.1097/01.ico.0000214206.29823.2d
29. Saiqun L, Deng Y, Caiyuan D. Rapid deterioration of Mooren’s ulcers after conjunctival flap: a review of 2 cases. BMC Ophthalmol. 2017;17:93. doi:10.1186/s12886-017-0488-1
30. Refojo MF, Dohlman CH, Ahmad B, et al. Evaluation of adhesives for corneal surgery. Arch Ophthalmol. 1968;80(5):645–656. doi:10.1001/archopht.1968.00980050647013
31. Webster RG, Slansky HH, Refojo MF, et al. The use of adhesive for the closure of corneal perforations: report of two cases. Arch Ophthalmol. 1968;80:705–709. doi:10.1001/archopht.1968.00980050707004
32. Setlik DE, Seldomridge DL, Adelman RA, et al. The effectiveness of isobutyl cyanoacrylate tissue adhesive for the treatment of corneal perforations. Am J Ophthalmol. 2005;140(5):920–921. doi:10.1016/j.ajo.2005.04.062
33. Weiss JL, Williams P, Lindstrom RL, et al. The use of tissue adhesive in corneal perforations. Ophthalmology. 1983;90(6):610–615. doi:10.1016/S0161-6420(83)34508-5
34. Leahey AB, Gottsch JD, Stark WJ. Clinical experience with N-butyl cyanoacrylate (Nexacryl) tissue adhesive. Ophthalmology. 1993;100:173–180. doi:10.1016/S0161-6420(93)31674-X
35. Radosevich M, Goubran HI, Burnouf T. Fibrin sealant: scientific rationale, production methods, properties, and current clinical use. Vox Sang. 1997;72:133–143. doi:10.1159/000461980
36. Sridhar MS, Mandal AK, Garg P, et al. Pupillary block glaucoma after tissue adhesive application and anterior chamber reformation with air [letter]. Cornea. 2000;19(2):250–251. doi:10.1097/00003226-200003000-00026
37. Rohrbach JM, Wohlrab TM, Nolle B, et al. Cyanoacrylate injuries of the eye. Ophthalmologe. 2000;97:878–880. German. doi:10.1007/s003470070013
38. Golubovic S, Parunovic A. Cyanoacrylate glue in the treatment of corneal ulcerations. Fortschr Ophthalmol. 1990;87:378–381.
39. Bodaghi B, Levy C, Votan P, et al. Value of cyanoacrylate tissue adhesives in peripheral corneal ulcers of inflammatory origin. J Fr Ophtalmol. 1996;19(2):127–132.
40. Agrawal V, Kumar A, Sangwan V, et al. Cyanoacrylate adhesive with conjunctival resection and superficial keratectomy in Mooren’s ulcer. Indian J Ophthalmol. 1996;44(1):23–27.
41. Lal I, Shivanagari SB, Ali MH, et al. Efficacy of conjunctival resection with cyanoacrylate glue application in preventing recurrences of Mooren’s ulcer. Br J Ophthalmol. 2016;100(7):971–975. doi:10.1136/bjophthalmol-2015-307350
42. Fogle JA, Kenyon KR, Foster CS. Tissue adhesive arrests stromal melting in the human cornea. Am J Ophthal. 1980;89:795–802. doi:10.1016/0002-9394(80)90168-3
43. Berkavitz PT, Arentsen JJ, Felberg NT, et al. Presence of circulating immune complexes in patients with peripheral corneal diseases. Arch Ophthalmol. 1983;101:242–245. doi:10.1001/archopht.1983.01040010244012
44. Schaap OL, FeltKamp TEW, Bregtoart AC. Circulating antibodies to corneal tissue in a patient suffering from Mooren’s ulcer (ulcus rodens corneac). Clin Exp Immunol. 1969;5:365–370.
45. Sharma A, Mohan K, Sharma R, Nirankari VS. Scleral patch graft augmented cyanoacrylate tissue adhesive for treatment of moderate-sized noninfectious corneal perforations (3.5–4.5 mm). Cornea. 2013;32:1326–1330. doi:10.1097/ICO.0b013e31829cb625
46. Hyndiuk RA, Hull DS, Kinyoun JL. Free tissue patch and cyanoacrylate in corneal perforations. Ophthalmic Surg. 1974;5:50–55.
47. Messmer EM, Foster CS. Destructive corneal and scleral disease associated with rheumatoid arthritis. Medical and surgical management. Cornea. 1995;14(4):408–417. doi:10.1097/00003226-199507000-00010
48. Yousuf M, Bailony MR, Bloomer MM, Killingsworth D, Jeng BH. Management of nontraumatic corneal perforation with tectonic drape patch and cyanoacrylate glue. Cornea. 2010;29(10):1173–1175. doi:10.1097/ICO.0b013e3181d5d996
49. Spotnitz WD, Mintz PD, Avery N, et al. Fibrin glue from stored human plasma. An inexpensive and efficient method for local blood bank preparation. Am Surg. 1987;53:460–462.
50. Dal Pizzol MM, Roggia MF, Kwitko S, et al. Use of fibrin glue in ocular surgery. Arq Bras Oftalmol. 2009;72(3):308–312. doi:10.1590/s0004-27492009000300006
51. Cagatay HH, Gokçe G, Mete A, et al. Non-recurrence complications of fibrin glue use in pterygium surgery: prevention and management. Open Ophthalmol J. 2015;9(1):159–163. doi:10.2174/1874364101509010159
52. Lagoutte FM, Gauthier L, Comte PR. A fibrin sealant for perforated and preperforated corneal ulcers. Br J Ophthalmol. 1989;73:757–761. doi:10.1136/bjo.73.9.757
53. Davis JW. Skin transplantation with a review of 550 cases at the Johns Hopkins Hospital. Johns Hopkins Med J. 1910;15:307–396.
54. DeRötth A. Plastic repair of conjunctival defects with fetal membranes. Arch Ophthalmol. 1940;23(3):522–525. doi:10.1001/archopht.1940.00860130586006
55. Sorsby A, Haythorne J, Reed H. Further experience with amniotic membrane grafts in caustic burns of the eye. Br J Ophthalmol. 1947;31(7):409–418. doi:10.1136/bjo.31.7.409
56. Tseng SC, Prabhasawat P, Lee SH. Amniotic membrane transplantation for conjunctival surface reconstruction. Am J Ophthalmol. 1997;124(6):765–774. doi:10.1016/S0002-9394(14)71693-9
57. Lee SH, Tseng SC. Amniotic membrane transplantation for persistent epithelial defects with ulceration. Am J Ophthalmol. 1997;123(3):303–312. doi:10.1016/S0002-9394(14)70125-4
58. Dua HS. Amniotic membrane transplantation. Br J Ophthalmol. 1999;83(6):748–752. doi:10.1136/bjo.83.6.748
59. Solomon A, Rosenblatt M, Monroy D, et al. Suppression of interleukin 1 and interleukin 1 in human limbal epithelial cells cultured on the amniotic membrane stromal matrix. Br J Ophthalmol. 2001;85:444–449. doi:10.1136/bjo.85.4.444
60. Touhami A, Grueterich M, Tseng SCG. The role of NGF signaling in human limbal epithelium expanded by amniotic membrane culture. Invest Ophthalmol Vis Sci. 2002;43:987–994.
61. Solomon A, Meller D, Prabhasawat P. Amniotic membrane grafts for nontraumatic corneal perforations, descemetoceles, and deep ulcers. Ophthalmology. 2002;109:694–703. doi:10.1016/S0161-6420(01)01032-6
62. Ngan ND, Chau HT. Amniotic membrane transplantation for Mooren’s ulcer. Clin Experiment Ophthalmol. 2011;13(5):386–392. doi:10.1111/j.1442-9071.2010.02479.x
63. Motowa SA, Zobidi MA. Amniotic membrane transplant with a special technique (Motowa’s Sandwich Technique) in Mooren’s ulcer. Middle East Afr J Ophthalmol. 2015;22(3):386–388. doi:10.4103/0974-9233.159776
64. Bhandari V, Siddharthan KS. Bilateral Mooren’s ulcer – customised corneal graft with additional amniotic membrane graft. Saudi J Ophthalmol. 2015;29:235–237. doi:10.1016/j.sjopt.2014.12.005
65. Lambiase A, Sacchetti M, Sgrulletta R, et al. Amniotic membrane transplantation associated with conjunctival peritomy in the management of Mooren’s ulcer: a case report. Eur J Ophthalmol. 2005;15(2):274–276. doi:10.1177/112067210501500217
66. Lavaju P, Sharma M, Sharma A, et al. Use of amniotic membrane and autologous serum eye drops in Mooren’s ulcer. Nepal J Ophthalmol. 2013;5(1):120–123. doi:10.3126/nepjoph.v5i1.7839
67. Chen KH, Hsu WM, Liang CK. Relapsing Mooren’s ulcer after amniotic membrane transplantation combined with conjunctival autografting. Ophthalmology. 2004;111:792–795. doi:10.1016/j.ophtha.2003.06.024
68. Schallenberg M, Westekemper H, Steuhl KP, Meller D. Amniotic membrane transplantation ineffective as additional therapy in patients with aggressive Mooren’s ulcer. BMC Ophthalmol. 2013;13:81. doi:10.1186/1471-2415-13-81
69. Hick S, Demers PE, Brunette I, et al. Amniotic membrane transplantation and fibrin glue in the management of corneal ulcers and perforations: a review of 33 cases. Cornea. 2005;24(4):369–377. doi:10.1097/01.ico.0000151547.08113.d1
70. Hanada K, Shimazaki J, Shimmura S, et al. Multilayered amniotic membrane transplantation for severe ulceration of the cornea and sclera. Am J Ophthalmol. 2001;131(3):324–331. doi:10.1016/S0002-9394(00)00825-4
71. Rodríguez-Ares MT, Touriño R, López-Valladares MJ, Gude F. Multilayer amniotic membrane transplantation in the treatment of corneal perforations. Cornea. 2004;23(6):577–583. doi:10.1097/01.ico.0000121709.58571.12
72. Fan J, Wang M, Zhong F. Improvement of amniotic membrane method for the treatment of corneal perforation. Biomed Res Int. 2016;2016:1693815. doi:10.1155/2016/1693815
73. Liu J, Li L, Li X. Effectiveness of cryopreserved amniotic membrane transplantation in corneal ulceration: a meta-analysis. Cornea. 2019;38(4):454–462. doi:10.1097/ICO.0000000000001866
74. Nakamura T, Yoshitani M, Rigby H. Sterilized, freeze-dried amniotic membrane: a useful substrate for ocular surface reconstruction. Invest Ophthalmol Vis Sci. 2004;45(1):93–99. doi:10.1167/iovs.03-0752
75. Allen CL, Clare G, Stewart EA. Augmented dried versus cryopreserved amniotic membrane as an ocular surface dressing. PLoS One. 2013;8(10):e78441. doi:10.1371/journal.pone.0078441
76. Kinoshita S, Ohashi Y, Ohji M, et al. Long-term results of keratoepithelioplasty in Mooren’s ulcer. Ophthalmology. 1991;98:438–445. doi:10.1016/S0161-6420(91)32272-3
77. Soong HK, Farjo AA, Katz D, Meyer RF, Sugar A. Lamellar corneal patch grafts in the management of corneal melting. Cornea. 2000;19(2):126–134. doi:10.1097/00003226-200003000-00002
78. Lin HC, Lee YS, Chia JH. Management of rheumatoid arthritis-related peripheral ulcerative keratitis using glycerol-preserved corneas. Asia Pac J Ophthalmol. 2013;2:291–294. doi:10.1097/APO.0b013e318299868e
79. Shi W, Liu M, Gao H, Li S, Wang T, Xie L. Penetrating keratoplasty with small-diameter and glycerin-cryopreserved grafts for eccentric corneal perforations. Cornea. 2009;28(6):631–637. doi:10.1097/ICO.0b013e318191b857
80. Liu J, Shi W, Li S, Gao H, Wang T. Modified lamellar keratoplasty and immunosuppressive therapy guided by in vivo confocal microscopy for perforated Mooren’s ulcer. Br J Ophthalmol. 2015;99:778–783. doi:10.1136/bjophthalmol-2014-306012
81. Sharma N, Sinha G, Shekhar H, et al. Demographic profile, clinical features and outcome of peripheral ulcerative keratitis: a prospective study. Br J Ophthalmol. 2015;99:1–6.
82. Jiang Y, Li Y, Liu XW, Novel Tectonic A. Keratoplasty with femtosecond laser intrastromal lenticule for corneal ulcer and perforation. Chin Med J. 2016;129:
83. Stilma JS. Conjunctival excision or lamellar scleral autograft in 38 Mooren’s ulcers from Sierra Leone. Br J Ophthalmol. 1983;67:475–478. doi:10.1136/bjo.67.7.475
84. Paufique L, Sourdille GP, Guy O. Les Greffes de la Cornee. Tr Am Ophth Soc. 1948;46:127–152.
85. Xie HT, Li J, Liu Y, et al. Cryopreserved limbal lamellar keratoplasty for peripheral corneal and limbal reconstruction. Int J Ophthalmol. 2018;11(4):699–702.
86. Li J, Yu L, Deng Z, et al. Deep anterior lamellar keratoplasty using acellular corneal tissue for prevention of allograft rejection in high-risk corneas. Am J Ophthalmol. 2011;152(5):762–770.e3. doi:10.1016/j.ajo.2011.05.002
87. Gao H, Wang X, Echegaray JJ, et al. Partial lamellar keratoplasty for peripheral corneal disease using a graft from the glycerin-preserved corneoscleral rim. Graefes Arch Clin Exp Ophthalmol. 2014;252(6):963–968. doi:10.1007/s00417-014-2642-2
88. Cheung AY, Sarnicola E, Kurji KH, et al. Three hundred sixty-degree fuchs superficial marginal keratitis managed with annular lamellar keratoplasty. Cornea. 2017;37:1–3.
89. Tavassoli S, Gunn D, Tole D, et al. Peripheral ulcerative keratitis with corneal melt as the primary presentation in a case of human immunodeficiency virus. BMJ Case Rep. 2019;12(2):e226936. doi:10.1136/bcr-2018-226936
90. Anitha V, Ghorpade A, Ravindran M. A modified Tenons sling annular graft for advanced peripheral ulcerative keratitis with an hourglass cornea. Indian J Ophthalmol. 2022;70:655–657. doi:10.4103/ijo.IJO_2035_21
91. Grana PC. Therapeutic keratoplasty in Mooren’s ulcer. Arch Ophthalmol. 1959;62(3):414–418. doi:10.1001/archopht.1959.04220030070010
92. Brown SI, Mondino BJ. Penetrating keratoplasty in Mooren’s ulcer. Am J Ophthalmol. 1980;89(2):255–258. doi:10.1016/0002-9394(80)90120-8
93. Tobias R, Berthold S, Achim L, et al. Visual acuity and astigmatism after eccentric penetrating keratoplasty - a retrospective study on 117 patients. Klin Monatsbl Augenheilkd. 2002;219:40–45. doi:10.1055/s-2002-23499
94. Palay DA, Stulting RD, Waring GO, et al. Penetrating keratoplasty in patients with rheumatoid arthritis. Ophthalmology. 1992;99(4):622–627. doi:10.1016/S0161-6420(92)31927-X
95. Cartwright Knox NE, Tole DM, Georgoudis P, et al. Peripheral ulcerative keratitis and corneal melt: a 10-year single center review with historical comparison. Cornea. 2014;33(1):27–31. doi:10.1097/ICO.0000000000000008
96. Rush SW, Rush RB. Outcomes of infectious versus sterile perforated corneal ulcers after therapeutic penetrating keratoplasty in the United States. J Ophthalmol. 2016;2016:1–6. doi:10.1155/2016/6284595
97. Alhassan MB, Rabiu M, Agbabiaka IO. Interventions for Mooren’s ulcer. Cochrane Database Syst Rev. 2014. doi:10.1002/14651858.CD006131.pub3
98. Sabhapandit S, Murthy SI. Peripheral ulcerative keratitis: clinical syndromes, classifications, and differential diagnosis. In: Tandon R, Galor A, Sangwan V, editors. Peripheral Ulcerative Keratitis: A Comprehensive Guide. Springer; 2017: 61–81.
99. Mian SI, Soong HK, Patel SV, et al. In vivo femtosecond laser- assisted posterior lamellar keratoplasty in rabbits. Cornea. 2006;25(10):1205e9. doi:10.1097/01.ico.0000231491.95377.0b
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
