Back to Journals » Clinical Ophthalmology » Volume 13
Surgical experience with a redesigned, fully preloaded, hydrophobic acrylic intraocular lens in challenging cases of pseudoexfoliation syndrome, phacodonesis, and small pupils
Authors Borkenstein AF , Borkenstein EM
Received 13 November 2018
Accepted for publication 7 January 2019
Published 22 January 2019 Volume 2019:13 Pages 199—206
DOI https://doi.org/10.2147/OPTH.S194420
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Andreas F Borkenstein, Eva-Maria Borkenstein
Borkenstein & Borkenstein, Privatklinik der Kreuzschwestern Graz, Graz, Austria
Purpose: The purpose of this study was to describe our surgical experience and evaluate safety and postoperative outcomes of a fully preloaded, monofocal, hydrophobic acrylic intraocular lens (IOL) (CT LUCIA 611P) with a newly designed optic-haptic junction in severe cases of pseudoexfoliation (PXF) syndrome, phacodonesis, and small pupils.
Setting: This study was conducted in Borkenstein & Borkenstein, private practice, Privatklinik der Kreuzschwestern Graz, Austria.
Patients and methods: This study presents outcomes of 15 eyes of 15 patients implanted with CT LUCIA 611P IOL with improved optical properties and more rigid and wider optic–haptic junction. All patients had advanced cataract and PXF syndrome, of which phacodonesis was detected in 12 cases and five cases had PXF glaucoma. All eyes had small pupils with no response to mydriatic drops, and the surgery was performed with the use of Malyugin ring. All eyes were targeted for a slight postoperative myopia (-0.25 to -0.50 D). Refractive outcomes were presented for 3 months follow-up, while adverse events were followed up for to 10 months.
Results: The mean age of the study group was 78.3 years (from 68 to 86 years). Three months postoperatively, the mean manifest spherical equivalent was -0.35 D (from 0.00 to -1.00 D) and all eyes were within ±0.50 D of their preoperative target. No significant refractive shift or refractive surprise occurred during the follow-up of 10 months. Corrected distance visual acuity (CDVA) improved from the mean value of 20/50 preoperatively to 20/20 postoperatively. No intraoperative adverse events were noted. Postoperatively, six eyes presented with a slight decentration or tilt, which did not significantly affect postoperative refraction, CDVA, or patients’ subjective visual symptoms.
Conclusion: The IOL provided good surgical performance, excellent refractive stability, and predictable outcomes in patients with PXF syndrome. Further studies are necessary to evaluate long-term stability.
Keywords: pseudoexfoliation syndrome, phacodonesis, cataract surgery, intraocular lens stability, Achilles heel of the IOL
Introduction
Pseudoexfoliation (PXF) syndrome is an age-related systemic disorder caused by progressive accumulation of extracellular material over various tissues.1 The ocular manifestations are characterized by deposition of whitish-gray protein on the lens, iris, ciliary epithelium, corneal endothelium, and in the trabecular meshwork.1–4 The prevalence of PXF syndrome varies between different populations, generally ranges between 6% and 10%, and it is more prevalent in women and older population (over 50 years).2
The disease may involve different structures and parts of the eye. Involvement of the lens, ciliary body, and zonules can lead to phacodonesis, spontaneous lens subluxation, and secondary glaucoma,5 while involvement of the iris stroma and muscle layer might cause poor pupil dilatation.5,6 Cataract surgery in eyes with PXF syndrome is therefore associated with higher rates of complications.5,7–12 During surgery, there is a relatively higher risk of posterior capsule rupture and vitreous loss due to the thinner posterior capsule and the weak zonules. Postoperative decentration or tilt of the intraocular lens (IOL) is also not uncommon because of shrinkage of the capsule or the weak zonular apparatus.
With rising life expectancy and hence increasing numbers of PXF cases, every cataract surgeon has to face the special challenges associated with treating these patients.13,14 However, with currently available surgical devices such as iris dilators, capsular tension rings (CTRs), various ophthalmic viscosurgical devices (OVD) as well as the choice of intraocular lenses less prone to decentration or tilt, surgeons can handle PXF cataract surgeries with greater confidence and better postoperative outcomes.4,15
Previously, we published case series of patients implanted with a fully preloaded monofocal hydrophobic single piece IOL with more rigid haptics and improved optical properties and found great stability over 12 months.16 Our initial experience with this IOL in routine cataract surgery led us to believe that this lens could be a suitable option for challenging cases associated with PXF, phacodonesis, and small pupils. In this paper, we describe our own surgical technique of performing cataract surgery in patients with PXF as well as the initial outcomes of implantation of CT LUCIA 611P (Carl Zeiss Meditec AG, Jena, Germany) single-piece hydrophobic IOLs in these cases. In one of our previous studies,17 we already successfully used the predecessor of this IOL (CT LUCIA 601P) in PXF patients with acceptable clinical outcomes. The two IOLs (601P and 611P) are based on the same optical principle and asphericity concept, but in the later model, the haptics were redesigned (wider and stiffer), potentially making it an even better choice in patients where stability of the capsular bag is compromised.
Patients and methods
This retrospective study was performed in accordance with the ethical standards of the institutional research committee (approved by the ethic committee of Medical University Graz) and of the Declaration of Helsinki and its later amendments or comparable ethical standards and used only de-identified patient data. All patients signed an informed consent document.
Outcomes of 15 cases that were referred to our clinic with advanced cataract and PXF syndrome were reviewed. The cataract grade rated based on the Lens Opacities Classification System III18 scale was as follows: nine eyes had a nuclear cataract (five of NC5 and four of NC6), three eyes had a cortical cataract (C5), and three eyes had a posterior subcapsular cataract (P5). In all cases, the preoperative examination showed small pupils with absolutely no or little effect of mydriatic eye drops in increasing the size of the pupil. In five cases, PXF glaucoma was diagnosed, with characteristic damages in the optic nerve and existing visual field loss. In four cases, phacodonesis was clearly detected prior to surgery with slit lamp examination and in seven cases later on during capsulorhexis.
Preoperatively, biometry with the IOL-Master (Carl Zeiss Meditec AG) and corneal topography with Pentacam AXL (Oculus, Wetzlar, Germany) were performed in addition to standard ophthalmological examinations. Distance visual acuity was measured with a calibrated projected eye chart, recorded in Snellen meter equivalent and converted to logMAR for statistical analysis. Goldmann applanation tonometry was used for intraocular pressure (IOP) measurements. In cases where tilt or decentration of the IOL was suspected postoperatively, wavefront aberrometry was performed with iTrace aberrometer (Tracey Technologies, Houston, TX, USA).
Phacoemulsification was performed by the same surgeon (AB). The Malyugin ring (6.25 mm) was used to expand the pupil in all cases. In eight cases, the adhesions (synechiae) between the iris and the anterior lens capsule were broken with OVDs and/or spatula. In four cases, staining of the anterior capsule (use of a blue colorant) was necessary due to mature cataract formation with poor visualization and very poor fundus-light reflex.
Phacoemulsification was performed with special respect for the weak zonules and the thinner posterior lens capsule with lowest ultrasound energy and low irrigation/aspiration adjustments. To enable safe and harmless surgeries and reduce any risks of intraoperative events, the procedures were done very slowly and with most carefulness. Dispersive and cohesive OVDs were used (Arschinoff Soft-Shell technique) to coat and protect the endothelial cells (dispersive OVD) and to create space and flatten capsule (cohesive OVD). In three hyperopic eyes with very shallow anterior chamber depths (ACD, 2.4–2.9 mm) and pressure from behind (vis a tergo), the anterior chamber was refilled repeatedly with OVD to create more space to work and to protect the corneal endothelium.
The fully preloaded monofocal, aspheric, hydrophobic CT LUCIA 611P (Carl Zeiss Meditec AG) IOL was selected in all cases. Due to the fact that in PXF syndrome the IOP may rise rapidly 2–4 hours after surgery, a special postoperative therapy (2 days) with systemic acetazolamides was conducted. A cohesive viscoelastic device was used for filling the capsular bag before lens implantation based on the fact that it is completely removable at the end of surgery. Therefore, the risk of postoperative rise of IOP is lower.
Control examinations included refraction, CDVA, IOP measurement, slit lamp examination, and Scheimpflug measurement.
Intraocular lens
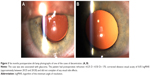
CT LUCIA 611P (CE registration number 263168 MR2) is a fully preloaded, one-piece, acrylic, hydrophobic, aspheric (aberration correcting) IOL with C-loop haptics (Figure 1). Optic diameter of the IOL is 6.00 mm and the overall diameter is 13.0 mm. Dioptric range is available between +4.0 and +34.0 D in 0.50 D increments.
The lens is manufactured from glistening-free material and incorporates a patented ZO asphericity concept (ZO:ZEISS Optics), designed to compensate for a range of aberrations arising from different corneal shapes and lens misalignments. The IOL is fully preloaded and has a heparin-coated surface for smoother injection and unfolding process. For posterior capsule opacification prevention, the lens has step-vaulted haptics to translate the optic posteriorly for better contact with the posterior capsule, and it is also equipped with a 360° square edge design (with a radius of <3 μm, including optic–haptic junction).
Statistical analyses
Data of all available cases were summarized, and the preoperative and postoperative variables were described with the mean, interquartile range (IQR), and range (minimum–maximum). Wilcoxon signed-rank test was used for the comparison of clinical parameters between preoperative and postoperative visits. All preoperative and postoperative adverse events were summarized, and their incidence was calculated. Refractive and visual outcomes were presented for the 3 months follow-up examination when all the patients were consistently refracted. Adverse events were followed for 10 months. Calculations were performed using Microsoft Office Excel 2011 program (Microsoft Corporation, Redmond, WA, USA).
Results
The study involved 15 eyes of 15 patients (eleven females and four males). The mean age of the study group was 78.3 years (IQR: 71, 81.5; range: 68–86) and the mean power of the implanted IOL was 23.5 D (IQR: 22.75, 24.75; range: 19.5–26.5).
There was an average of four lines improvement in CDVA between preoperative (mean 0.38 logMAR ≈ 20/50, IQR: 0.45, 0.25; range: 0.7–0.15) and 3 months postoperative visit (mean 0.03 logMAR ≈ 20/20, IQR 0.05, 0.00; range: 0.1 to −0.1; P<0.01). All patients were treated unilaterally and a slight myopia (between −0.25 and −0.50 D) was aimed at. The mean 3 months postoperative manifest spherical equivalent was −0.35 D (IQR: −0.5, −0.19; range: −1.0 to 0.00). Figure 2 plots the distribution of preoperative and 3 months postoperative manifest spherical equivalent. All eyes in the study were within ±0.50 D of their preoperative aim.
  | Figure 2 Preoperative and postoperative distribution of manifest spherical equivalent. |
The mean IOP reduced from 19.6 mmHg (IQR: 17.5, 21; range: 17–26) preoperatively to 17.3 mmHg (IQR: 16, 18.5, range: 14–21) at 1 month postoperatively (P<0.01). In the five cases of PXF glaucoma, the mean IOP reduced from 23 mmHg (range 20–26) to 19 mmHg (range 16–21), and this change was on the borderline of statistical significance (P=0.05).
Preoperative mean ACD was 3.2 mm (IQR: 2.9, 3.5; range: 2.4–3.7) and postoperative pseudophakic ACD at 3 months was 4.2 mm (IQR: 3.9, 4.4; range: 3.6–4.8).
Adverse events
No intraoperative adverse events were recorded in this study. Postoperatively, in six cases (40.0%), decentration or tilt was detected with either a slit lamp examination or from wavefront aberrometry (slight increase in coma). No myopic or hyperopic shifts or refractive surprises (>0.5 D) were detected over the 10 months follow-up. In three cases (20.0%), typical phimosis with shrinkage of the capsule (PXF associated) led to further decentration of the IOL. Table 1 presents refractive outcomes of each case of postoperative decentration or tilt. All eyes maintained very good CDVA with no obvious increase in postoperative refractive cylinder. Patients with decentration or tilt did not complain of any subjective visual symptoms or discomfort at any time of the follow-up. Figure 3 shows an example of IOL decentration in one of the cases. No case of spontaneous lens subluxation or dislocation was recorded over the follow-up time. At the time of the study, the surgeries had been performed minimum 10 months ago, and there was no case of postoperative subluxation or dislocation.
  | Table 1 Three months refractive outcomes of six cases with slight decentration or tilt |
Among usual early postoperative adverse events, there was no case of corneal edema that would persist for longer than 48 hours, or inflammation that would persist for longer than 5 days. During the follow-up time, there was no case of prolonged anterior uveitis or recurrence of anterior uveitis at any time of the follow-up.
In two cases (13.3%), despite extensive intraoperative polishing of the posterior capsule, a primary capsular fibrosis occurred and nd:YAG laser was performed 3–4 months after surgery. Twelve weeks postoperatively, the slit lamp examination and photographic analysis of the IOL (retroillumination) showed absolutely no glistenings.
Discussion
With increasing patient expectations, every cataract surgery should be performed as a refractive procedure to achieve the best postoperative results. Patients of any age rightly insist on the best technique and the newest equipment, and therefore, surgeons should not use only one monofocal intraocular lens model as a standard implant. The perfect choice should correspond with the individual needs of every case and the diagnosis of the eye not only in “refractive cases” or “premium surgeries” with additional costs. Every standard procedure should be “premium” and with the best possible conditions.
There are several aspects that should be considered when selecting an IOL in challenging cases such as PXF syndrome with small pupils and phacodonesis. First, geometric properties of the IOL play an important role. Ideally, the lens should provide long-term stability in eyes with compromised zonules that are more susceptible to lens dislocation or decentration. The IOL chosen in our case series is a one-piece IOL with an overall length of 13 mm and an optic diameter of 6 mm. We believe the size of IOL is important for perfect centration, especially in phacodonesis. IOLs with a smaller diameter (11–12.5 mm) are more likely to rotate, shift, or decenter. Compared to its predecessor (CT LUCIA 601P), the new CT LUCIA 611P was redesigned with more rigid, thicker, and wider optic–haptic junction (Figure 4).17 Due to the geometric properties of the lens, CTRs are rarely necessary even in patients with phacodonesis. In our previous study, we found great stability of this IOL over the period of 12 months with no significant refractive change between 1 and 12 months follow-up.16 Although we only present a small sample in the current study, all eyes were within ±0.50 D of their intended target at 3 months postoperatively, which is an excellent outcome considering these were difficult cases associated with phacodonesis and glaucoma. A recent study found that PXF patients have a 7.27 odds ratio of developing a refractive surprise greater than ±1.0 compared to controls.19 In our small case series, we have not seen such refractive surprises even in patients where slight decentration or tilt occurred.
  | Figure 4 Schematic illustration of the optic–haptic junction (CT LUCIA 601P and 611P) in comparison. Photograph courtesy of Carl Zeiss Meditec AG. |
Fibrotic reaction of capsular bag, zonular status, IOL edge, and haptic and optic design have an effect on the postoperative axial position of IOL and the final effective lens position, which is known to shift in patients with PXF syndrome.20 For example, in a case series involving 26 eyes, Fallah et al20 reported a backward movement of 0.08 mm of the IOL and a corresponding hyperopic shift of 0.3 D over the period of 6 months with AcrySof SA60AT IOL (Alcon Laboratories, Inc., Fort Worth, TX, USA). A similar outcome was found in the long-term study of Ostern et al: IOLs in eyes with PXF were positioned lower compared to controls 6–7 years after surgery.21 Unfortunately, our outcomes are too short to comment on long-term axial movement of this IOL.
The second aspect of IOL to consider in PXF cases is the optical properties of the lens. Some postoperative tilt or decentration is inevitable in patients with PXF syndrome, and IOL used in these patients should ideally be less prone to the induction of unwanted side effects should the lens not be perfectly centered. The IOL selected in this study has an optic with a patented aspheric concept, in which the power of the IOL is higher in the center and then varies toward periphery. It was designed to benefit patients with different corneal shapes and reduce the incidence of higher order aberrations. The spherical aberration of the lens is −0.12 μm, which is optimized for corneal asphericity and designed to compensate for a range of aberrations. On the contrary, IOLs with more negative spherical aberration (IOL power decreases from center to periphery) improve contrast sensitivity, but if the optic position is not well aligned, other higher order aberrations, such as coma, could be induced. Human eye is not a perfectly symmetrical optical system, and even in routine cataract surgery in patients with unknown pathology, IOLs are often not perfectly centered. Besides, it is well known that IOLs with a low refractive index and a high Abbe number have lower chromatic aberration.16 The lens used in our case series has a relatively low refractive index of 1.49 and a high Abbe number of 51, providing improved quality of vision and less dispersion. In this study, we had six cases of slight postoperative decentration or tilt, but none of them complained about any optical side effects and all of them maintained very good postoperative CDVA. However, the effect of decentration or tilt on induction of higher order aberrations with this IOL will need to be studied further.
Another aspect of the lens to consider is the surgical manipulation and easiness of implantation, trying to avoid further trauma in eyes where corneal endothelium and zonules are already compromised. The IOL used in this study is fully preloaded and enables a good OR workflow. The injector and lens preparation are very easy to handle by the scrub nurse and surgeon alike. There is no sticking of the haptics to the optic (handshake phenomenon) even in capsular bags filled with cohesive OVD. The IOL unfolding process is facilitated with less or no manipulation (instruments, spatula, etc), and therefore, the risk of complications in eyes with small pupils, inserted Malyugin ring, and phacodonesis (weak zonules) is reduced. We used the Malyugin ring exclusively in all cases, and the haptics were not entangled with the loops of the ring.22,23 In our practice, we prefer not to use iris hooks, as we believe the more corneal incisions (wounds) the more likely we are to induce irregular astigmatism. Moreover, in PXF eyes, it is more likely that postoperative inflammation and delayed corneal wound healing (four additional incisions for the iris hooks) lead to awkward postoperative symptoms (itchy, dry eye syndrome) with discomfort. Furthermore, the ring provides balanced stretching and gentle holding of the iris tissue. In our opinion this technique is the most effective method for increasing the size of a rigid small pupil in PXF syndrome and reduces the incidence of postoperative abnormalities in pupil size and function. It is also important to note that a recent meta-analysis of postoperative lens dislocation in patients with PXF11 found that larger incisions and conventional iris hooks or retractor may potentially increase the risk of late postoperative IOL dislocation. For that reason, surgical technique as well as the choice of intraocular lens is critical in these cases. Larger three-piece lenses or more rigid polymethyl methacrylate lenses might provide better centration in the capsular bag, but generally require larger incisions. With CT LUCIA 611P IOL, when inserting in the capsular bag, the surgeon has the feeling of working with a three-piece lens but has the great advantage of being able to implant the IOL through a smaller incision. Although we find implantation of this IOL to be quite straightforward, it is also important to emphasize that the lens has a slightly different behavior during the unfolding process due to its rigid haptics, and surgeons should be made aware of this when using the IOL for the first time.16
Lastly, IOL used in PXF cases should have long-term biocompatibility and optical material clarity, as it is not advisable to revise surgery or explant IOLs in patients with week zonules and increased risk of complications. Although hydrophobic IOLs have been successfully used in PXF cases, hydrophobic material is prone to glistening, and it is still debatable whether this phenomenon affects optical performance of the IOL in long term.24,25 The IOL used in this study is made of ultra-high purity material with water content of 0.3%, and to our knowledge, there are no known reports of postoperative glistening. In one of our previous studies, we evaluated long-term outcomes of this IOL and found absolutely no glistening 1 year post implantation. Other biocompatibility aspect to consider is the uveal and capsular biocompatibility of IOL material in PXF eyes. Nowadays, the most common material used for premium intraocular lenses is hydrophobic or hydrophilic. Both have been evaluated in cases of PXF.26–29 Hydrophilic IOLs have slightly better uveal but worse capsular biocompatibility in PXF eyes, while opposite findings were reported for hydrophobic IOLs. Hydrophobic IOLs (mainly referring to AcrySof lens; Alcon Laboratories, Inc.) tend to develop higher number of foreign body giant cells in the postoperative period.26–29 In our small case series, we did not observe any significant inflammation, other than what is expected following a routine cataract surgery. This could be possibly due to heparin coating of this IOL, which was found to significantly lower inflammatory reaction.30
Our study has several limitations. It included only a small sample of patients, and long-term stability, optical performance (higher order aberration, contrast sensitivity, etc), as well as long-term postoperative adverse events should be further studied. No significant events occurred in this study during the 10 months follow-up, but the evaluation of biocompatibility and posterior capsule opacification requires much longer follow-up. Although the sample size is very small, it includes more difficult cases of PXF syndrome, with absolutely no or little effect of mydriatic eye drops, adhesions between iris and anterior lens capsule, and phacodonesis.
Conclusion
Considering severity of these cases, the IOL provided great refractive outcomes and stability, and it is definitely a viable option in challenging cases.
Author contributions
Both the authors provided substantial contributions to conception and design, data acquisition, or data analysis and interpretation. They drafted the article and critically revised it for important intellectual content. They both approved the final version to be published. The authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of the work are appropriately investigated and resolved by both the authors.
Disclosure
The authors report no conflicts of interest in this work.
References
Plateroti P, Plateroti AM, Abdolrahimzadeh S, Scuderi G. Pseudoexfoliation syndrome and pseudoexfoliation glaucoma: a review of the literature with updates on surgical management. J Ophthalmol. 2015;2015(4):1–9. | ||
Ariga M, Nivean M, Utkarsha P. Pseudoexfoliation syndrome. J Curr Glaucoma Pract. 2013;7(3):118–120. | ||
Ekström C, Botling Taube A. Pseudoexfoliation and cataract surgery: a population-based 30-year follow-up study. Acta Ophthalmol. 2015;93(8):774–777. | ||
Fontana L, Coassin M, Iovieno A, Moramarco A, Cimino L. Cataract surgery in patients with pseudoexfoliation syndrome: current updates. Clin Ophthalmol. 2017;11:1377–1383. | ||
Erkayhan GE, Dogan S. Cataract surgery and possible complications in patients with pseudoexfoliation syndrome. Eurasian J Med. 2017;49(1):22–25. | ||
Halkiadakis I, Chatziralli I, Drakos E, et al. Causes and management of small pupil in patients with cataract. Oman J Ophthalmol. 2017;10(3):220–224. | ||
Hemalatha BC, Shetty SB. Analysis of intraoperative and postoperative complications in pseudoexfoliation eyes undergoing cataract surgery. J Clin Diagn Res. 2016;10(4):NC05–NC08. | ||
Sangal N, Chen TC. Cataract surgery in pseudoexfoliation syndrome. Semin Ophthalmol. 2014;29(5–6):403–408. | ||
Shingleton BJ, Neo YN, Cvintal V, Shaikh AM, Liberman P, O’Donoghue MW. Outcome of phacoemulsification and intraocular lens implantation in eyes with pseudoexfoliation and weak zonules. Acta Ophthalmol. 2017;95(2):182–187. | ||
Turalba A, Cakiner-Egilmez T, Payal AR, et al. Outcomes after cataract surgery in eyes with pseudoexfoliation: results from the Veterans Affairs ophthalmic surgery outcomes data project. Can J Ophthalmol. 2017;52(1):61–68. | ||
Vazquez-Ferreiro P, Carrera-Hueso FJ, Fikri-Benbrahim N, Barreiro-Rodriguez L, Diaz-Rey M, Ramón Barrios MA. Intraocular lens dislocation in pseudoexfoliation: a systematic review and meta-analysis. Acta Ophthalmol. 2017;95(3):e164–e169. | ||
Vazquez-Ferreiro P, Carrera-Hueso FJ, Poquet Jornet JE, Fikri-Benbrahim N, Diaz-Rey M, Sanjuan-Cerveró R. Intraoperative complications of phacoemulsification in pseudoexfoliation: Metaanalysis. J Cataract Refract Surg. 2016;42(11):1666–1675. | ||
Schweitzer C. [Pseudoexfoliation syndrome and pseudoexfoliation glaucoma]. J Fr Ophtalmol. 2018;41(1):78–90. French. | ||
Hietanen J, Kivelä T, Vesti E, Tarkkanen A. Exfoliation syndrome in patients scheduled for cataract surgery. Acta Ophthalmol. 1992;70(4):440–446. | ||
Dwivedi NR, Dubey AK, Shankar PR. Intraoperative and immediate postoperative outcomes of cataract surgery using phacoemulsification in eyes with and without pseudoexfoliation syndrome. J Clin Diagn Res. 2014;8(12):VC01–VC05. | ||
Borkenstein AF, Borkenstein EM. Long-term clinical results and scanning electron microscopic analysis of the aspheric, hydrophobic, acrylic intraocular lens CT LUCIA 611P(Y). Clin Ophthalmol. 2018;12:1219–1227. | ||
Borkenstein AF, Borkenstein EM. Patient and surgeon satisfaction levels after using an acrylic, hydrophobic, monofocal IOL and the Malyugin ring in pseudoexfoliation syndrome patients. J Ophthalmol. 2018;2018(4):1–5. | ||
Chylack LT, Wolfe JK, Singer DM, et al. The lens opacities classification system III. The longitudinal study of cataract Study Group. Arch Ophthalmol. 1993;111(6):831–836. | ||
Manoharan N, Patnaik JL, Bonnell LN, et al. Refractive outcomes of phacoemulsification cataract surgery in glaucoma patients. J Cataract Refract Surg. 2018;44(3):348–354. | ||
Fallah Tafti MR, Abdollah Beiki H, Mohammadi SF, Latifi G, Ashrafi E, Fallah Tafti Z. Anterior chamber depth change following cataract surgery in pseudoexfoliation syndrome; a preliminary study. J Ophthalmic Vis Res. 2017;12(2):165–169. | ||
Ostern AE, Sandvik GF, Drolsum L. Positioning of the posterior intraocular lens in the longer term following cataract surgery in eyes with and without pseudoexfoliation syndrome. Acta Ophthalmol. 2014;92(3):253–258. | ||
Malyugin BE. Recent advances in small pupil cataract surgery. Curr Opin Ophthalmol. 2018;29(1):40–47. | ||
Malyugin B. Small pupil phaco surgery: a new technique. Ann Ophthalmol. 2007;39(3):185–193. | ||
Kohnen T. Impact of glistenings on visual quality. J Cataract Refract Surg. 2015;41(6):1129–1130. | ||
Kawai K, Hayakawa K, Suzuki T. Simulation of 20-year deterioration of acrylic IOLs using severe accelerated deterioration tests. Tokai J Exp Clin Med. 2012;37(3):62–65. | ||
Abela-Formanek C, Amon M, Kahraman G, Schauersberger J, Dunavoelgyi R. Biocompatibility of hydrophilic acrylic, hydrophobic acrylic, and silicone intraocular lenses in eyes with uveitis having cataract surgery: long-term follow-up. J Cataract Refract Surg. 2011;37(1):104–112. | ||
Richter-Mueksch S, Kahraman G, Amon M, Schild-Burggasser G, Schauersberger J, Abela-Formanek C. Uveal and capsular biocompatibility after implantation of sharp-edged hydrophilic acrylic, hydrophobic acrylic, and silicone intraocular lenses in eyes with pseudoexfoliation syndrome. J Cataract Refract Surg. 2007;33(8):1414–1418. | ||
Abela-Formanek C, Amon M, Schauersberger J, et al. Uveal and capsular biocompatibility of 2 foldable acrylic intraocular lenses in patients with uveitis or pseudoexfoliation syndrome: comparison to a control group. J Cataract Refract Surg. 2002;28(7):1160–1172. | ||
Abela-Formanek C, Amon M, Schild G, Schauersberger J, Heinze G, Kruger A. Uveal and capsular biocompatibility of hydrophilic acrylic, hydrophobic acrylic, and silicone intraocular lenses. J Cataract Refract Surg. 2002;28(1):50–61. | ||
Krall EM, Arlt EM, Jell G, et al. Intraindividual aqueous flare comparison after implantation of hydrophobic intraocular lenses with or without a heparin-coated surface. J Cataract Refract Surg. 2014;40(8):1363–1370. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.