Back to Journals » Clinical Ophthalmology » Volume 14
Surgical Approaches for Implanting Xen Gel Stent without Conjunctival Dissection
Authors Vera V, Gagne S, Myers JS, Ahmed IIK
Received 9 June 2020
Accepted for publication 21 July 2020
Published 17 August 2020 Volume 2020:14 Pages 2361—2371
DOI https://doi.org/10.2147/OPTH.S265695
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Video abstract presented by Vanessa Vera.
Views: 944
Vanessa Vera,1 Sebastien Gagne,2 Jonathan S Myers,3 Iqbal Ike K Ahmed4
1Department of Ophthalmology, Unidad Oftalmologica de Caracas, Caracas, Venezuela; 2Départment d’ophthalmologie de, Université de Montréal, Montréal, Canada; 3Glaucoma Service, Wills Eye Hospital, Philadelphia, PA, USA; 4Department of Ophthalmology, University of Toronto, Toronto, Canada
Correspondence: Vanessa Vera Email [email protected]
Abstract: The XEN Gel Stent (Allergan Inc., an Abbvie company) is an implant that lowers intraocular pressure by creating a filtration pathway from the anterior chamber to the subconjunctival space, using the same pathway as trabeculectomy. While the primary method for implantation is via ab interno approach, it is also possible to implant the device ab externo. This technique paper details the surgical steps for closed conjunctival implantation of the Gel Stent and provides surgical pearls for enhancing outcomes.
Keywords: XEN Gel Stent, glaucoma, filtration surgery, ab interno, ab externo, transconjunctival implantation
Introduction
The XEN Gel Stent (Allergan Inc., an Abbvie company) is a 6 mm gelatin tube that facilitates the drainage of aqueous humor from the anterior chamber to the subconjunctival space. Multiple studies have shown a significant reduction of both intraocular pressure (IOP) and the number of IOP-lowering medications following Gel Stent implantation.1–3 As with trabeculectomy, adjunctive use of antimetabolite/anti-fibrotic therapy has been shown to improve outcomes with the Gel Stent.4 Success is generally associated with a well-functioning diffuse bleb, which indicates adequate flow from the anterior chamber into the subconjunctival space.
Three studies (Reitsamer, Gillmann and Gabbay) have reported 24-month results following Xen Gel Stent implanted ab interno without conjunctival dissection.3–5 Overall, they reported comparable IOP reduction to mid-teens (14.2 ± 3.8 to 15.2 ± 4.2 mmHg) from mean baseline IOP ranging from 19.8 ± 8.2 mmHg to 22.1 ± 6.5 mmHg across these studies. Similarly, there was a comparable reduction in the mean IOP-lowering medication count, ranging from 2.0 ± 1.3 to 2.77 ± 1.1 at baseline, to 0.4 ± 0.7 to 1.0 ± 1.0 at 24 months. The needling rates were 41.1%, 43.2% and 37.7%, as reported by Reitsamer, Gillmann and Gabbay, respectively. Safety profiles were comparable across the three studies, with numeric hypotony and hyphema being the most reported adverse events.
There is emerging evidence of the adaptation of ab externo approaches to implantation of the Gel Stent.6–9 Twelve-month outcomes have been presented at various conferences by multiple investigators including one of the authors (JM) of this paper. Overall, the results suggest comparable effectiveness and safety with ab externo to the ab interno approach.
In this technique paper, the authors, who have significant expertise with the device, describe their preferred surgical approaches to implanting the Gel Stent without dissecting the conjunctiva, with surgical pearls and tips for enhancing outcomes. This article does not report any new clinical or animal data. All images and videos presented in this manuscript have been acquired under Institutional Review Board or Ethics Committee approved protocols.
Preoperative Optimization
Comorbid conditions that cause inflammation can increase the risk of postoperative fibrosis, directly impacting outcomes of glaucoma filtering surgery.10 Preoperatively, attention should be given to managing ocular surface and lid margin diseases to optimize the ocular surface in the weeks preceding a glaucoma filtering procedure. The use of topical IOP-lowering medications has also been shown to increase the recruitment of inflammatory cells.11,12 When appropriate, stopping any offending topical IOP-lowering medications 1 to 4 weeks prior to surgery is recommended to allow optimization of the ocular surface. In addition, the use of routine preoperative topical steroids and lubricants may be used to help reduce the rate of postoperative needling.10,13
Ab Interno Placement Technique
An ab interno placement technique is what has been used in the majority of published studies of the Gel Stent to date. It results in the least amount of conjunctival tissue disruption and has an excellent safety profile. Another benefit of an ab interno approach is the ability to precisely place the implant in the angle with an injector that is designed and optimized for this approach. This approach does require a corneal incision and the routine use of viscoelastic (Figure 1A and B).
Exposure, visibility, and maneuverability are keys to successful ab interno delivery of the Gel Stent. Without optimizing these factors, predictable Gel Stent placement and success may be limited. For exposure, some authors prefer a 3-post speculum, like the Brown speculum (Rhein Medical), as it allows for excellent visibility of the superior bulbar conjunctiva.
The Gel Stent is implanted using a handheld disposable injector. A subconjunctival injection of mitomycin C (MMC) may be performed prior to or after implantation of the Gel Stent, as preferred. The injector needle must traverse the anterior chamber, enter the angle (Figure 1C), and penetrate the sclera to achieve placement into the subconjunctival space, as previously described in detail.10 The authors’ experiences have provided the following learnings:
- Avoid placing the implant too far nasally, as this risks erosion and/or bleb dysesthesia.
- A somewhat shorter scleral track (exit 2 mm from limbus—Figure 1D) is preferable to ensure enough of the needle bevel (and therefore the implant) is placed above or in superficial Tenon’s. Tenon’s is more adherent to sclera where it inserts, approximately 1.5 mm from limbus. Thus, the needle may be able to penetrate Tenon’s here more easily, as it is well anchored to the sclera.
- It is critical to clearly visualize the full bevel of the needle once it exits the sclera, as this ensures the implant’s distal end is located in a superficial position and not embedded in Tenon’s. It has been found that Tenon’s capsule plays an important role in resistance beyond the device. Gel Stents that are implanted deeper within Tenon’s or embedded into this tissue are at greater risk for failure due to increased interstitial resistance.14
There is a virtual space between the conjunctiva and Tenon’s capsule, but often there are small adhesions present (which can be separated). It is in this “supra-Tenon’s” space that we aim to place the Gel Stent. The endpoint we look for is that the needle bevel has fully emerged through sclera and is clearly seen with the metallic stippling easily visible (Figure 2). To do this, the entire injector must have enough maneuverability outside the eye to move in a “heel down, (needle) tip up” to push the needle as superficial as possible in the subconjunctival tissue. As an aside, there is also a virtual space between Tenon’s capsule and sclera; however, adhesions are often present even beyond 2 mm from the limbus. Considering that the globe is curved and the needle is straight emerging through the sclera, it would be very difficult to ensure the implant is perfectly placed sub-Tenon’s using an ab interno approach. This point was emphasized by Lenzhofer and colleges who reported performing primary needling in all cases during XEN implantation to ensure that the distal end of the stent was completely free and mobile in its layer of implantation.15 Furthermore, attempting sub-Tenon’s placement risks bleeding from the episcleral vessels and intra-Tenon’s placement.
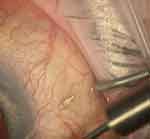 |
Figure 2 Needle in the “supra-Tenon’s space”, bevel fully emerged from sclera and metallic stippling clearly visible. Figure courtesy of Iqbal “Ike” Ahmed, MD, FRCSC. |
Given the above, the authors of this paper advocate a more temporal incision in the cornea to ensure the needle of the injector enters the angle at, or close to, 12 o’clock. This tangential approach reduces the potential of the injector to hit or be limited by the inferior orbital rim and/or speculum. It allows for an enhanced vertical range of the injector to permit superficial positioning of the Gel Stent.
After corneal incisions are made, a cohesive viscoelastic is used to fill the anterior chamber. A double-mirror, direct-view goniolens is preferred, but an indirect gonioscope may also be suitable to visualize the angle structures and place the injector needle just above the pigmented trabecular meshwork (anterior to Schlemm’s canal). Placement just anterior to the pigmented trabecular meshwork avoids reflux bleeding from the canal. Advance through the sclera using a second instrument at the side port or a corneal traction suture.
One of the authors (IA) has transitioned from locating the side port position from superotemporal to superonasal. This avoids the second instrument from getting in the way of the tangential approach of the injector when a temporal incision is made. Also, this allows the second instrument to incyclotort the eye further, which allows easier access to 12 o’clock and ensures that the injector is clear of the inferior orbital rim and/or speculum.
After anchoring the needle in the angle, push the needle forward, and aim for an optimal scleral track, emerging 2 mm from the limbus where Tenon’s is the thinnest and most adherent to sclera. As previously mentioned, this allows penetration of the needle to reach the supra-Tenon’s space, and ensures enough length of the implant, so the tip is not caught in the tissue.
Visualize the needle and needle tip bevel directly under the conjunctiva (Figure 2), pushing up superficially. It is advisable to pause for a moment and relax the hand to carefully control the injector forces during implant deployment to avoid unintended vertical or horizontal bend force on the needle. Deploy the implant, and the needle will retract into the sleeve. If a small heme occurs at the exit site of implantation, applying pressure for 30 seconds will aid in hemostasis. Verify that the implant is in the angle, anterior to the trabecular meshwork with about 1 mm in the anterior chamber, using a gonio mirror. Approximately 2 to 3 mm of implant should be visible in the subconjunctival space, with the tip free and mobile. Once implant placement has been verified, remove viscoelastic. Hydrate the incisions and pressurize the anterior chamber with balanced salt solution, which will prime the implant and form the bleb. A low and diffuse bleb will form, and the bleb will be easily visible through a translucent conjunctiva. Leave the anterior chamber deep and stable (Video 1).
An ideally positioned Gel Stent is approximately 1 mm in the anterior chamber, 2 mm in the scleral tunnel, and 3 mm in the subconjunctival space to permit outflow as posterior as possible to the Tenon’s insertion point. Avoid iris and trabecular meshwork trauma. If incorrectly positioned, the device can be adjusted, reloaded, or exchanged. Many surgeons prefer to carefully remove the implant through the anterior chamber with micrograsper forceps (microinstruments usually used for anterior segment or retina surgery), and if the implant looks intact after inspection under the microscope, reloading the implant in the XEN injector is an acceptable option. To reload the implant, the blue sliding button on the injector has to be manually pulled all the way back (to the starting position) in order to expose the bevel and the needle all the way out of the sleeve.
Described below are two approaches to ensure a superficial placement of the Gel Stent, one of which (XEN “air”) can be performed before implantation for eyes where a thick tenon is anticipated, and the other (primary needling10) for those eyes where superficial subconjunctival placement was not achieved.
XEN “Air” Technique
The procedure was developed by one of the authors (IA) as a method to provide a more consistent and reliable placement of the XEN Gel Stent above Tenon’s, which is intended to reduce resistance and needling rates. This method creates a pneumatic and viscoelastic dissection of the conjunctiva from Tenon’s prior to placement of the Gel Stent. A short piece of IV tubing connects the viscoelastic syringe to a 30-gauge needle (Figure 3A). This creates an airlock within the tubing, and it is then primed, so that half of the tubing is filled with viscoelastic prior to injection (Figure 3B). A traction suture may be used to pull the eye down to allow visualization. One hand is used to place the needle under the conjunctiva while the other hand handles the syringe, maintaining a finger on the plunger (Figure 3C) (Video 2).
Starting at the 12 o’clock position, about 4 to 6 mm posterior to the limbus, the needle is passed toward the superonasal quadrant, placed very superficially into the plane beneath the conjunctiva and above Tenon’s, with the bevel up (Figure 4A). Air has a high surface tension, which will not penetrate into Tenon’s and will create a virtual space between the tissues. As the air is injected, macro bubbles will form showing that the conjunctiva has been pneumatically dissected from Tenon’s (Figure 4B). This is followed by the viscoelastic, and together they create a pocket in the area of the superonasal quadrant, creating a space where the Gel Stent can be easily placed (Figure 4C). Less than 0.05 cc of viscoelastic is injected, and the needle tract is long enough to prevent postoperative leakage. A cotton tip can be used to massage some of the viscoelastic and air away from the limbus (Figure 4D).
With the conjunctiva elevated, the surgeon can proceed with the implantation of the Gel Stent as previously described. A temporal incision will allow for a radial pass in a tangential manner through the anterior chamber (Figure 4E). The needle will emerge from the sclera in the space created by the air and viscoelastic (Figure 4F). Because the pocket has been created, there is no risk of the Gel Stent getting stuck in Tenon’s or below Tenon’s, positions that likely create more fibrosis and resistance. If the Gel Stent is stuck in Tenon’s, it is likely to appear twisted or curled. If it is below Tenon’s, it will appear straight, but the tip will not be free and mobile. The well-positioned Gel Stent can be visualized in the subconjunctival space, will be elevated slightly within the pocket that has been created, and the tip will be free and mobile (Figure 4G). MMC can then be injected within the viscoelastic space to control fibrosis during wound healing (Figure 4H). Balanced Salt Solution is then injected into the anterior chamber, and it is possible to visualize the priming of the bleb as a fairly large bleb is formed. A small amount of priming is ideal as not to dilute the MMC (Figure 4I) (Video 3).
Primary Needling (Intraoperative)
During surgery, if the implant is immobile or excessively curled (ie, “pig tailed”) or when interstitial resistance from intra-Tenon’s placement is suspected, primary needling is recommended.5 Currently, many surgeons are incorporating primary needling as part of their routine intraoperative procedure despite the concern that this step may be associated with an increased risk of subconjunctival bleeding, as ensuring supra-Tenon’s placement may outweigh the risk of bleeding.
One of the authors (IA) performs primary needling during the MMC injection by making a few swipes around the distal tip of the microstent to clear Tenon’s before the MMC has been delivered (Video 1).
Ab Externo Approach
In cases where previous surgery or challenging facial anatomy makes it difficult to place the implant ab interno or where the surgeon does not prefer that approach, a transconjunctival ab externo implantation is an alternative option.
This approach has several other benefits: corneal incisions are not required, the use of viscoelastic is optional, intraocular maneuvers are minimized or absent, and there is possibly a decreased likelihood of the distal end of the Gel Stent becoming embedded in Tenon’s. Additionally, glaucoma surgeons are generally accustomed to this approach, as is it very similar to how tube shunts are placed and to the maneuvers used to needle failed trabeculectomies, which may help to shorten the learning curve of the procedure.
The surgical technique recommended by some of the authors (VV, JM) is described below:
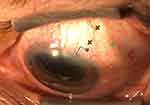
Patients need a well-exposed superior or superotemporal quadrant. Some surgeons may choose to place a corneal traction suture to aid in exposure and for counter traction (Figure 5A). Alternatively, non-toothed forceps can be used for this purpose. A subconjunctival injection of MMC may be performed either prior to or after implantation of the Gel Stent. When done prior to XEN implantation, lidocaine 2% + epi (0.05 cc) is added to the MMC volume (0.1 cc) to provide excellent anesthesia during Gel Stent implantation. Generally, MMC is kept posteriorly closer to the area where the tip of the Gel Stent will release fluid and away from the limbus. Use calipers to place marks at 8 mm (Figure 5B) and 2.5 mm (Figure 5C) from the limbus for surgical reference. The point of conjunctival entry can range from 7 to 10 mm; and between 2 and 2.5 mm for the scleral entry as per surgeon preference.
Ring forceps or nontoothed forceps are used to lift the conjunctiva >7 mm posterior to the limbus (Figure 6A). This is done so the XEN injector needle is introduced into the subconjunctival space and advanced anteriorly just under the conjunctiva, while staying parallel in this plane, avoiding blood vessels, and ensuring no micro-perforations are created during subconjunctival path (Figure 6B).
The injector enters the sclera approximately 2.5 mm behind the limbus (Figure 6C) to create a scleral track. At the surgical limbus, the needle approach may be slightly steepened to enter the anterior chamber over the iris plane, away from the iris (to reduce obstruction risk) and the cornea (to protect endothelial cells). The corneal traction suture can be held in the opposite hand and used to help create the scleral track (Figure 6D) and visualize needle entrance in the anterior chamber (Figure 6E). Once the needle position is confirmed in the anterior chamber, counter traction is released and the Gel Stent is deployed (Figure 6F). After deployment, confirm implant length in the anterior chamber (Figure 7A) and subconjunctival space (Figure 7B). A gonioscopy lens could be used to confirm the position in the angle. As the injector was not originally designed for ab externo implantation, adjustments may be required to avoid placement of the stent too long in the anterior chamber and too short in the subconjunctival space. One adjustment is to slowly and progressively pull back the injector as the Gel Stent is being deployed to adjust the length in the anterior chamber and subconjunctival space. Alternatively, the Gel Stent can be grasped after deployment with blunt forceps through the conjunctiva to adjust the subconjunctival portion as needed (Figure 7C) until an ideally positioned stent approximately 1 mm in the anterior chamber, 2 mm in the scleral tunnel, and 3 mm in the subconjunctiva is achieved (Figure 7D). Sliding conjunctiva forward before grasping the Gel Stent from outside the conjunctiva and pulling back minimizes forces on the tauter anterior conjunctiva.
Once the Gel Stent is in position, an anti-metabolite injection may be performed, if it was not done prior to implantation (Video 4). Finally, consider Seidel testing to rule out an active leak from an inadvertent conjunctival tear or micro-perforations from the needle entry or track. If necessary, place a 10–0 nylon or vicryl suture to close the leak.
The corneal traction suture can be used to infraduct the eye for exposure while creating the subconjunctival path, then to supraduct the eye to create the scleral track and enter anterior chamber, and finally to elevate the eye during implant delivery (Video 5).
One of our authors (SG) is credited as the first person to do an ab externo implantation of XEN Gel Stent without opening the conjunctiva (transconjunctival implantation) at the slit lamp in fall of 2016. Slit Lamp XEN (« SLX ») implantation technique requires an exposed superotemporal quadrant and a very cooperative patient. The technique is described below.
After topical anesthesia with tetracaine, the eye is prepared with 5% povidone iodine drops. A subconjunctival injection of a mixture of antimetabolite (MMC volume 0.1 cc) and lidocaine 2% + epinephrine (volume 0.05 cc) for a total volume of 0.15 cc is performed at the slit lamp in the target area at least 10 mm posterior to the limbus. Attention is taken to ensure the subconjunctival volume is guided posteriorly, either with a cotton tip or massaged posteriorly using the surgeon’s thumb over the patient’s superior eyelid.
The ideal window to perform the implantation is 5 to 7 minutes after the injection described above, as this will allow MMC to bind to the tissue, as well as achieve the desired anesthetic effect. Implanting too early may increase the risk of inadvertent entry of MMC into the anterior chamber, while later implantation will make it difficult to find the hydrodissected subconjunctival tissue plane.
The next step is to find the target entry site for conjunctival and scleral insertion, avoiding blood vessels. For the scleral entry point, use the reticle of the slit lamp to measure 2 to 2.5 mm from the limbus. Conjunctival entry should be off set one clock hour from the scleral entry site and 6 to 7 mm behind the limbus (Figure 8). Entering the conjunctiva too close to where the distal end of the implant will lay may lead to bleb leak and potential XEN exposure; entering the conjunctiva too far back may lead to the sleeve or collar (Figure 9) of the injector being caught in the conjunctiva, preventing further advancement or causing a conjunctival tear. A 10–0 or 9–0 vicryl or nylon suture can be used to close any conjunctival defects or leaks, especially if they persist.
 |
Figure 9 The injector sleeve or collar. Image courtesy of Sebastien Gagne, MD. |
Enter with the XEN needle under the conjunctiva and one clock hour away from the targeted scleral entry, horizontally (bevel en face) first to prevent the XEN Gel Stent from falling out of the injector (Figure 10A). Once the needle is in the subconjunctival space, direct the injector toward the scleral point of entry 2 to 2.5 mm from the limbus (Figure 10B). Enter the sclera with the needle aimed directly toward the anterior chamber and create a scleral track until the tip of the needle is visualized in the anterior chamber (Figure 10C). Do not deploy the implant if the needle tip is not visible in the AC, partially retract the needle and create a new path with a steeper approach to enter the AC.
The blue slider on the injector is then advanced forward; when the XEN Gel Sent is visualized in the anterior chamber slowly and simultaneously pull back the injector as the implant is released (Video 6).
Avoid performing SLX in patients on blood thinners. Ensure that there is quick and easy access to an OR in case of any unexpected complications.
Troubleshooting During Gel Stent Implantation
The most common intraoperative challenges with ab interno Gel Stent implantation and how to address the same have been well summarized in a prior publication.10 Interestingly, many of these apply to ab externo placement as well, including:
- Proximal end of implant short or not visible in the angle: with non-toothed forceps, grab the implant at the scleral exit site and push the implant back into the AC.
- Distal end of implant short under the conjunctiva: when small adjustments (~0.5 to 1 mm) are needed, use non-toothed forceps to gently pull the implant out into the subconjunctival space. Slide the conjunctiva forward before grabbing, holding, and pulling the implant.
- Distal end not visible under the conjunctiva, implant long in the AC or suboptimal angle placement (ie too close to iris or cornea): create a paracentesis and fill the anterior chamber with balanced salt solution or viscoelastic. Using microforceps (ie, vitrectomy forceps, anterior chamber microinstruments, etc.), grasp the implant close to where it enters the angle and gently pull the device away. If the implant looks intact, it can then be reloaded back in the injector, and a second implantation attempted. Alternatively, a new implant can be used.
Modulation of Wound Healing
Antimetabolite agents like MMC and fluorouracil (5-FU) can help modulate the wound healing process following subconjunctival filtering surgery.1,10
An MMC concentration of 0.2 to 0.4 mg/mL administered in a volume of 0.1 cc + lidocaine 2% with epinephrine volume 0.05 cc (total volume of 0.15 cc) is considered by the authors to be appropriate for XEN surgery. Dosing and timing of perioperative MMC administration can be customized based on the patient’s needs and the technique that best suits the surgeon’s preference:
- Injection of MMC prior to Gel Stent implantation: an intra-Tenon’s/subconjunctival injection of MMC 6 to 8 mm posterior to the limbus is done to ensure a more posterior bleb in the superior quadrant, where XEN is intended to be implanted. A moist cotton tip is used to keep the MMC away from the limbus following injection. MMC binds quickly, but the surface of the eye should be thoroughly irrigated prior to any incisions. If done in combination with phacoemulsification, MMC is administered, phacoemulsification is performed, the IOL is placed in the capsular bag and then the Gel Stent is implanted.
- Injection of MMC after Gel Stent implantation: the technique of injection is similar to the previously described, but it is necessary to inject at least 8 mm posterior to the limbus and keep the MMC posterior and intra-Tenon’s to reduce risk of reflux into the anterior chamber. Some surgeons prefer doing the MMC injection prior to evacuating the viscoelastic from the anterior chamber while others perform it with a fully formed and stable anterior chamber at the end of the surgery.
While many surgeons continue to successfully inject MMC prior to Gel Stent implantation, two of the authors of this paper (IA, JM) are now routinely injecting MMC after implantation. In fact, they prefer no pre-implantation injections to keep the tissue planes as natural as possible, using topical anesthesia for all cases. In their view, any subconjunctival injection of fluid prior to implantation is less likely to create dissection of tissue, but rather hydroexpand it, very much like a sponge. Hydrating Tenon’s makes finding the subconjunctival plane more difficult. On the other hand, using air or gas, which have a higher surface tension, allows more effective separation of tissue planes, which makes the XEN Air technique a good alternative for surgeons who find it difficult to get the implant in the subconjunctival plane.
Discussion
The option of having both ab interno and ab externo approaches for XEN Gel Stent implantation offers increased flexibility (Table 1), allowing surgeons to better optimize the surgery depending on the individual needs of the patients (eg conjunctival status, facial anatomy, etc.) and also allowing customization of the surgery to better fit the surgeon’s preference and comfort with a specific technique.
 |
Table 1 Comparison of Ab Interno and Ab Externo Closed Conjunctiva Approaches to XEN Gel Stent Implantation |
The surgical technique and approaches to Gel Stent have evolved significantly since it became available to surgeons. As detailed earlier, the preparation of the ocular surface may play a key role in improving outcomes overall. Many of the early studies did not report a streamlined pre-operative preparatory regimen.
Changes to the surgical technique may hold the key to better outcomes, starting with customizing the dosing of perioperative MMC (lower volumes and higher concentrations), the time of application of MMC (eg before, during or after surgery) and the awareness of the role of Tenon’s capsule in creating increased interstitial resistance and thereby a higher risk of failure, if the distal end of the implant is embedded in Tenon’s. Meticulous attention to ensure supra-Tenon’s (with techniques such as XEN “air”) or sub-Tenon’s implant placement based on the surgeon’s preference, and the use of primary needling after implantation to minimize tissue obstruction of the distal end of the implant, may also contribute to an overall positive impact on the outcomes.3,4,10,16,17
Conclusion
As surgeons gain experience with the XEN Gel Stent, continued innovation has led to the development of new and varied techniques to further customize the procedure with the objectives to make the surgery more predictable and to improve outcomes. Studies are underway to further assess the newer approaches and better understand their outcomes over the long term.
The varied approaches detailed here allow for accommodation of the needs of both patient and surgeon, leave the conjunctiva intact, and preserve invaluable conjunctival “real estate” that might be necessary later for a more invasive traditional glaucoma surgery as the disease progresses over the patient’s lifetime.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and no study with human participants or animals was performed by the authors for this manuscript.
Acknowledgments
Article-processing charges were funded by Allergan PLC (Irvine, CA). The authors participated in a consensus panel on this topic for which they were reimbursed. However, they were not provided honoraria for any time spent developing, reviewing, or editing this manuscript or for contributing videos.
The authors thank Won Kim, MD, and Oluwatosin U. Smith, MD, who provided images and videos that are included with this manuscript. Editorial assistance in the preparation of the manuscript was provided by Adrianne Resek, MA. Support for this assistance was funded by Allergan, PLC (Irvine, CA).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception and execution, including drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
All of the authors are consultants for Allergan, PLC (Irvine, CA). Sebastien Gagne, MD; Iqbal “Ike” Ahmed, MD, FRCSC; and Jonathan Myers, MD, also receive research support from Allergan. Vanessa Vera reports writing support via grant to Bryn Mawr Communications from Allergan, during the conduct of the study; personal fees, consulting fees, travel assistance, and non-financial support from Allergan, outside the submitted work. In addition, Dr Vanessa Vera has multiple patents associated with Gel stent and injector; but no licenses or royalties to report. Jonathan S Myers reports grants, personal fees from Aerie, Allergan, Glaukos, and Olleyes, and grants from Diopsys, Haag Streit, Nicox, and Santen, outside the submitted work. Iqbal Ahmed reports grants and personal fees from Allergan, outside the submitted work. The authors report no other potential conflicts of interest for this work.
References
1. Mansouri K, Guidotti J, Rao HL, et al. Prospective evaluation of standalone XEN gel implant and combined phacoemulsification-XEN gel implant surgery: 1-year results. J Glaucoma. 2018;27(2):140–147. doi:10.1097/IJG.0000000000000858
2. Lenzhofer M, Kersten-Gomez I, Sheybani A, et al. Four-year results of a minimally invasive transscleral glaucoma get stent implantation in a prospective multi-centre study. Clin Exp Ophthalmol. 2019;47(5):581–587. doi:10.1111/ceo.13463
3. Gillmann K, Bravetti GE, Mermoud A, et al. XEN gel stent in pseudoexfoliative glaucoma: 2-year results of a prospective evaluation. J Glaucoma. 2019;28(8):676–684. doi:10.1097/IJG.0000000000001295
4. Reitsamer H, Sng C, Vera V, et al. Two-year results of a multicenter study of the ab interno gelatin implant in medically uncontrolled primary open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol. 2019;257:983–996. doi:10.1007/s00417-019-04251-z
5. Gabbay IE, Allen F, Morley C, et al. Efficacy and safety data for the XEN45 implant at 2 years: a retrospective analysis. Br J Ophthalmol. 2020;104:1125–1130. doi:10.1136/bjophthalmol-2019-313870
6. Yuan L, Lai G, Raiciulescu S, Kim W. Outcomes of ab interno placement versus ab externo transconjunctival placement of Xen 45 gel stents.
7. Gallardo M, Porter M, Vincent L, et al. Outcomes following implantation of an anterior segment drainage device (Xen45 Gel Stent) via an ab-interno or ab-externo approach in patients with uncontrolled open angle glaucoma.
8. Purgert RJ, Lin MM, Mehren N, et al. Outcomes of ab interno versus ab externo XEN gel stent implantation.
9. Gagne S, Yuen D, Cohen S Slit lamp implantation of a Gel Microstent.
10. Vera V, Ahmed IK, Stalmans I, Reitsamer H. Gel stent implantation – recommendations for preoperative assessment, surgical technique, and postoperative management. US Ophthalmic Rev. 2018;11(1):38–46. doi:10.17925/USOR.2018.11.1.38
11. Sherwood MB, Grierson I, Milgar L, Hitchings RA. Long-term morphologic effects of antiglaucoma drugs on the conjunctiva and Tenon’s capsule in glaucomatous patients. Ophthalmology. 1989;96:327–335. doi:10.1016/S0161-6420(89)32888-0
12. Baudouin C, Liang H, Hamard P, et al. The ocular surface of glaucoma patients treated over the long term expresses inflammatory markers related to both T-helper 1 and T-helper 2 pathways. Ophthalmology. 2008;115:109–115. doi:10.1016/j.ophtha.2007.01.036
13. Breusegem C, Spielberg L, Van Ginderdeuren R, et al. Preoperative nonsteroidal anti-inflammatory drug or steroid and outcomes after trabeculectomy: a randomized controlled trial. Ophthalmology. 2010;117(7):1324–1330. doi:10.1016/j.ophtha.2009.11.038
14. Ong JA, Wu P, Ahmed IIK. Outcomes of ab interno gelatin microstent with MMC using targeted supra-Tenon’s placement.
15. Lenzhofer M, Strohmaier C, Sperl P, et al. Effect of the outer stent position on efficacy after minimally invasive transscleral glaucoma gel stent implantation. Acta Ophthalmol. 2019;97(8):e1105–1111. doi:10.1111/aos.14167
16. Vera V, Sheybani A, Lindfield D, Stalmans I, Ahmed IIK. Recommendations for the management of elevated intraocular pressure due to bleb fibrosis after XEN gel stent implantation. Clin Ophthalmol. 2019;13:685–694. doi:10.2147/OPTH.S195457
17. Figueroa-Vercellino JP, Peraza-Nieves JE, Milla E, Pazos M. Relationship between the location of the XEN stent glaucoma implant (with respect to the Tenon capsule) and the intraocular pressure. J Fr Ophtalmol. 2019;42(4):421–422. doi:10.1016/j.jfo.2018.10.009
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.